Abstract
Scalp psoriasis is a frequent expression of the common skin disease psoriasis, and scaling and itching are the two major complaints. Topical treatments are the mainstay of the treatment of psoriasis of the scalp, with the vehicle as well as the active ingredient relevant to efficacy, tolerability and compliance. Vehicles can be shampoos, lotions, gels, foams, creams and more greasy ointments. Active ingredients are keratolytics, coal tar (liquor carbonis detergens), dithranol, corti-costeroids and vitamin D3 analogues. Some effect has also been described from topical or systemic imidazole derivatives.
Topical corticosteroids remain the mainstay in the treatment of scalp psoriasis. The effects are rapid, the formulations are patient friendly and the adverse effects seem limited, although no data are available to support safety during prolonged use (more than 4 weeks).
Topical vitamin D3 analogues have been available for the treatment of psoriasis since 1992. In the lotion formulation in particular, vitamin D3 analogues are a patient friendly, tolerable and effective alternative to corticosteroids, although the effects are optimal after 8 weeks, in contrast to 2–3 weeks for topical corticosteroids. Facial irritation (often temporary) can also be a disadvantage of vitamin D3 analogues, although only a small proportion of patients stop treatment for this reason.
All other treatment options for psoriasis, such as tazarotene, phototherapy and systemic treatment with methotrexate, acitretin and cyclosporin are often not indicated or not suitable for treatment of the scalp.
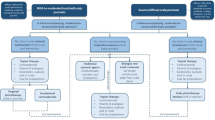
In daily practice, to make a choice from the available therapeutic arsenal for psoriasis, each patient should be examined individually. Deteriorating factors have to be excluded. For scaling, keratolysis is the first step. Subsequently, active treatment can be chosen depending on the clinical picture. When the psoriatic lesions are mainly characterised by inflammation, anti-inflammatory drugs such as topical corticosteroids are indicated. When scaling is the more important clinical feature, vitamin D3 analogues are indicated.
Generally, intermittently used topical corticosteroids alternating with vitamin D3 derivatives either combined or not with liquor carbonis detergens containing shampoo is the most suitable treatment for most patients. Because psoriasis capitis is a chronic disease, long term treatment should, in addition to medical advice, also provide patient support and motivation.

Similar content being viewed by others
References
Farber EM, Nail L. Natural history and treatment of scalp psoriasis. Cutis 1992; 49: 396–400
Moon CM, Schissel DJ. Pityriasis amiantacea. Cutis 1999; 63: 169–70
Runne U, Kroneisen-Wiersma P. Psoriatic alopecia: acute and chronic hair loss in 47 patients with scalp psoriasis. Dermatology 1992; 185: 82–7
Rosenberg EW, Noah PW, Skinner Jr RB. Microorganisms and psoriasis. J Natl Med Assoc 1994; 86: 305–10
Rosenberg EW, Noah PW, Skinner Jr RB, et al. Microbial associations of 167 patients with psoriasis. Acta Derm Venereol Suppl (Stockh) 1989; 146: 72–4
Langner A, Wolska H, Hebborn P. Treatment of psoriasis of the scalp with coal tar gel and shampoo preparations. Cutis 1983; 32: 290–6
Arnold WP. Tar. In: van de Kerkhof PCM, editor. The management of psoriasis. Clin Dermatol 1997; 15: 739–44
Jongeneelen FJ, Bos RP, Anzion RB, et al. Biological monitoring of polycyclic aromatic hydrocarbons. Metabolites in urine. Scand J Work Environ Health 1986; 12: 137–43
Farr PM, Krause LB, Marks JM, et al. Response of scalp psoriasis to oral ketoconazole. Lancet 1985; II: 921–2
Jury CS, Hugh M, Shankland GS, et al. Arandomized, placebo-controlled trial of oral itraconazole in scalp psoriasis. J Dermatol Treat 2000; 11: 85–9
Rosenberg EW, Belew PW, Skinner Jr RB. Treatment of psoriasis with antimicrobial agents. Semin Dermatol 1985; 4: 307–11
Ashton RE, Andre P, Lowe NJ, et al. Anthralin: historical and current perspectives. J Am Acad Dermatol 1983; 9: 173–92
Galewsky E. Ueber Cignolin, ein Ersatzpraparat des Chrysarobins. Dermatol Wschr 1916; 62: 113–5
Mahrle G, Bonnekoh B, Wevers A, et al. Anthralin: how does it act and are there more favourable derivatives? Acta Derm Venereol (Stockh) 1994; Suppl. 86: 83–4
Engel DJ, Marx AF, Rekker RF, et al. Topically active cortico-steroids. A quantitative evaluation of McKenzie’s skin-blanching test. Arch Dermatol 1974; 109: 863–5
Maibach HI, Stoughton RB. Topical corticosteroids. Med Clin North Am 1973; 57: 1253–64
Olsen EA, Cram DL, Ellis CN, et al. A double-blind, vehicle-controlled study of clobetasol propionate 0.05% (Temovate) scalp application in the treatment of moderate to severe scalp psoriasis. J Am Acad Dermatol 1991; 24: 443–7
van de Kerkhof PC, de Hoop D, de Korte J, et al. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology 1998; 197: 326–34
Kragballe K, Wildfang IL. Calcipotriol (MC 903), a novel vitamin D3 analogue stimulates terminal differentiation and inhibits proliferation of cultured human keratinocytes. Arch Dermatol Res 1990; 282: 164–7
Berth-Jones J, Bourke JF, Iqbal SJ, et al. Urine calcium excretion during treatment of psoriasis with topical calcipotriol. Br J Dermatol 1993; 129: 411–4
Ramsay CA, Berth-Jones J, Brundin G, et al. Long-term use of topical calcipotriol in chronic plaque psoriasis. Dermatology 1994; 189: 260–4
Klaber MR, Hutchinson PE, Pedvis-Leftick A, et al. Comparative effects of calcipotriol solution (50 micrograms/ml) and betamethasone 17-valerate solution (1 mg/ml) in the treatment of scalp psoriasis. Br J Dermatol 1994; 131: 678–83
Caccialanza M, Piccinno R, Cappio F, et al. Phototherapy of psoriasis of the scalp. Results in 21 patients treated with a special portable ultraviolet rays lamp. G Ital Dermatol Venereol 1989; 124: LXI–LXV
Braun R, Dotterud LK, Falk ES. Comparison of betamethasone valerate solution with phototherapy (UVB comb) in scalp psoriasis treatment. Acta Derm Venereol 1998; 78: 385
Foster RH, Brogden RN, Benfield P. Tazarotene. Drugs 1998; 55: 705–11
Gollnick HP, Finzi AF, Marks R, et al. Optimising the use of tazarotene in clinical practice: consensus statement from the European advisory panel for tazarotene (Zorac TM). Dermatology 1999; 199: 40–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Vleuten, C.J., van de Kerkhof, P.C.M. Management of Scalp Psoriasis. Drugs 61, 1593–1598 (2001). https://doi.org/10.2165/00003495-200161110-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161110-00006