Abstract
Background: Donepezil is licensed for the treatment of mild-to-moderate Alzheimer’s disease (AD) at doses of 5–10 mg/day and has recently been approved in the US for severe AD. Multiple studies have suggested that donepezil 10 mg/day provides additional cognitive and functional benefits over the 5 mg/day dose. Higher doses of donepezil, if safe and well tolerated, might provide further benefits for patients with AD.
Objective: To evaluate the safety and tolerability of donepezil at doses of 15 and 20 mg/day.
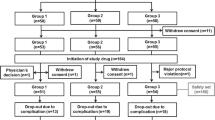
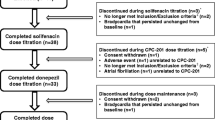
Method: A 24-week, randomized, double-blind, placebo-controlled, pilot study conducted at two investigational sites in the US. Enrolled patients (male and female; aged 50–86 years) had a diagnosis of probable AD at the mild-to-moderate stage (Mini-Mental State Examination [MMSE] score 10–26). All patients had been treated with donepezil 10 mg/day for 12–30 months prior to enrolment. Patients (n = 31) were randomized 1 : 1 to receive either a standard dose of donepezil (donepezil 10 mg/day plus placebo 5 mg/day for weeks 1–12; donepezil 10 mg/day plus placebo 10 mg/day for weeks 13–24) or a higher dose of donepezil (donepezil 15 mg/day for weeks 1–12; donepezil 20 mg/day for weeks 13–24). Primary outcome measures were tolerability (as determined by monitoring of discontinuations, dose modifications and adverse events) and safety (as determined by adverse event monitoring, physical examinations, clinical laboratory tests and ECGs). Psychometric measures (Alzheimer’s Disease Assessment Scale-Cognitive Subscale [ADAS-cog], MMSE and Clinician’s Interview-Based Impression of Change with caregiver information [CIBIC+]) and pharmacokinetic/pharmacodynamic parameters were secondary outcomes.
Results: No patients withdrew from the study and there were no serious adverse events or deaths. By week 24, 15 of 16 patients in the higher-dose group tolerated the maximum 20 mg/day dose; one patient had a permanent dose reduction to donepezil 15 mg/day. In the standard-dose group, 14 of 15 patients tolerated donepezil 10 mg/day plus placebo 10 mg/day by the end of the study; one patient had a permanent dose reduction to donepezil 10 mg/day plus placebo 5 mg/day. Temporary dose reductions occurred in two patients (one from each group). Adverse events reported were as expected for donepezil and were all mild to moderate in intensity. Adverse events considered to be possibly or probably related to treatment were reported for three patients in the standard-dose group and six patients in the higher-dose group. One patient in the higher-dose group had weight loss reported as possibly or probably treatment related. Mean changes on ECGs were not clinically significant in either group, and the incidence of bradycardia was comparable. No treatment difference on any of the psychometric measures was observed between the groups. Pharmacokinetic analyses showed that an increased donepezil dose was associated with an increase in donepezil plasma concentrations from baseline.
Conclusion: In this small pilot study of patients with mild-to-moderate AD already stabilized on donepezil 10 mg/day, doses of 15 and 20 mg/day of donepezil appeared safe and well tolerated. These results justify initiation of larger clinical trials designed to investigate the efficacy and safety of doses of donepezil higher than 10 mg/day in patients with AD.







Similar content being viewed by others
Notes
Use of donepezil 15 and 20 mg/day for treatment of AD in this pilot study is up to twice the dosage approved by the US FDA and publication of this study does not imply endorsement of use of this dose as ‘usual care’.
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Pfizer Inc. Aricept [package insert]. New York: Pfizer Inc., 2005
Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 1998 May 11; 158(9): 1021–31
Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease: Donepezil Study Group. Neurology 1998 Jan; 50(1): 136–45
Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer’s disease: results from a multinational trial. Dement Geriatr Cogn Disord 1999 May-Jun; 10(3): 237–44
Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001 Aug 14; 57(3): 489–95
Whitehead A, Perdomo C, Pratt RD, et al. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer’s disease: a meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry 2004 Jul; 19(7): 624–33
Mohs RC, Doody RS, Morris JC, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients [published erratum appears in Neurology 2001 Nov 27; 57 (10): 1942]. Neurology 2001 Aug 14; 57(3): 481–8
Gauthier S, Feldman H, Hecker J, et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int Psychogeriatr 2002 Dec; 14(4): 389–404
Cummings JL, McRae T, Zhang R, et al. Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry 2006 Jul; 14(7): 605–12
Hager K, Calabrese P, Frolich L, et al. An observational clinical study of the efficacy and tolerability of donepezil in the treatment of Alzheimer’s disease. Dement Geriatr Cogn Disord 2003; 15(4): 189–98
Relkin NR, Reichman WE, Orazem J, et al. A large, community-based, open-label trial of donepezil in the treatment of Alzheimer’s disease. Dement Geriatr Cogn Disord 2003; 16(1): 15–24
Boada-Rovira M, Brodaty H, Cras P, et al. Efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a global, multinational, clinical experience study. Drugs Aging 2004; 21(1): 43–53
Pratt RD, Perdomo CA, Surick IW, et al. Donepezil: tolerability and safety in Alzheimer’s disease. Int J Clin Pract 2002 Nov; 56(9): 710–7
Rogers SL, Friedhoff LT, Donepezil Study Group. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia 1996 Nov–Dec; 7(6): 293–303
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994
McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984 Jul; 34(7): 939–44
Matthews HP, Korbey J, Wilkinson DG, et al. Donepezil in Alzheimer’s disease: eighteen month results from Southampton Memory Clinic. Int J Geriatr Psychiatry 2000 Aug; 15(8): 713–20
Birks J. Cholinesterase inhibitors for Alzheimer’s disease [online]. Available from URL: http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD005593/pdf_fs.html [Accessed 2007 Jan 9]
Greenberg SM, Tennis MK, Brown LB, et al. Donepezil therapy in clinical practice: a randomized crossover study. Arch Neurol 2000 Jan; 57(1): 94–9
White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc 1998 Oct; 46(10): 1223–7
Feldman H, Gauthier S, Hecker J, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease [published erratum appears in Neurology 2001 Dec 11; 57 (11): 2153]. Neurology 2001 Aug 28; 57(4): 613–20
Tariot PN, Cummings JL, Katz IR, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer’s disease in the nursing home setting. J Am Geriatr Soc 2001 Dec; 49(12): 1590–9
Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 2003 Aug 26; 61(4): 479–86
Black S, Roman GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 2003 Oct; 34(10): 2323–30
Tiseo PJ, Foley K, Friedhoff LT. An evaluation of the pharmacokinetics of donepezil HCl in patients with moderately to severely impaired renal function. Br J Clin Pharmacol 1998 Nov; 46 (1 Suppl.): 56S–60S
VanDenBerg CM, Kazmi Y, Jann MW. Cholinesterase inhibitors for the treatment of Alzheimer’s disease in the elderly. Drugs Aging 2000 Feb; 16(2): 123–38
Lam YW, Gaedigk A, Ereshefsky L, et al. CYP2D6 inhibition by selective serotonin reuptake inhibitors: analysis of achievable steady-state plasma concentrations and the effect of ultrarapid metabolism at CYP2D6. Pharmacotherapy 2002 Aug; 22(8): 1001–6
Nagy CF, Kumar D, Perdomo CA, et al. Concurrent administration of donepezil HCl and sertraline HCl in healthy volunteers: assessment of pharmacokinetic changes and safety following single and multiple oral doses. Br J Clin Pharmacol 2004 Nov; 58 (1 Suppl.): 25S–33S
Ritchie CW, Ames D, Clayton T, et al. Meta-analysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psychiatry 2004 Jul–Aug; 12(4): 358–69
Acknowledgements
Activities related to study design, data collection and data analysis were funded by Eisai and Pfizer. Editorial support was provided by R. Daniel, PhD, of PAREXEL and was funded by Pfizer. The help of Nisith Kumar and Mindy Sovel throughout the trial is also gratefully acknowledged.
Honglan Li and John Ieni are employees of Eisai. Richard Zhang and Rachel Schindler are employees and stock owners of Pfizer. Rachelle Doody has received honoraria from Eisai, Pfizer, Janssen, Novartis, Merz, Forest and Lundbeck for consulting and/or lecturing, and has been a principal investigator on multiple clinical drug trials sponsored by various pharmaceutical companies, including Eisai and Pfizer. Jody Corey-Bloom has been a principal investigator on multiple clinical drug trials sponsored by various pharmaceutical companies, including Eisai and Pfizer, and received compensation through her institution to conduct the current study.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doody, R.S., Corey-Bloom, J., Zhang, R. et al. Safety and Tolerability of Donepezil at Doses up to 20 mg/day. Drugs Aging 25, 163–174 (2008). https://doi.org/10.2165/00002512-200825020-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200825020-00008