Abstract
This review provides current information on the use of antigen-binding fragments (Fab) from cleaved antibodies to treat poisoning with digoxin and other potent, low formula mass poisons, such as colchicine and tricyclic antidepressants. Anti-digoxin Fab fragments have been used successfully for many years in the management of severe poisoning with digoxin, digitoxin, and a range of other structurally related compounds, including cardiotoxins from Nerium and Thevetia sp. (oleander) and Bufo sp. (toads). However, their main use remains treating digoxin poisoning.
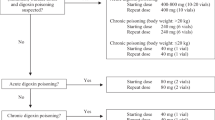
Equimolar doses of anti-digoxin Fab fragments completely bind digoxin in vivo. The approximate dose of Fab fragments (mg) is 80 times the digoxin body burden (mg). If neither the dose ingested nor the plasma digoxin/digitoxin concentration is known, in an adult 380mg of anti-digoxin Fab fragments should be given. The dose for elderly patients or those with renal impairment should be similar to that for those with normal renal function. Fab fragments have a plasma half-life of 12–20 hours, but this can be prolonged in patients with renal impairment. Analysis of serum ultrafiltrate using an immunoassay shown not to have matrix bias remains the most accurate approach to measuring free digoxin in the presence of anti-digoxin Fab fragments.
The antibody fragments are given intravenously over 15–30 minutes after dilution to at least 250mL with plasma protein solution, 0.9% (w/v) sodium chloride, or deionised water, except in infants where the volume infused can be reduced. Factors limiting the efficacy of Fab fragments are the dose, the duration of the infusion and any delay in administration. Guidelines for Fab fragment administration in children include (i) dilution to a final Fab concentration of 10 g/L in either 5% (w/v) dextrose or 0.9% (w/v) sodium chloride; (ii) infusion through a 0.22μm filter; (iii) administration of the total dose over a minimum of 30 minutes; and (iv) avoiding coadministration of other drugs and/or electrolyte solutions. Fab fragments are generally well tolerated. Adverse effects attributable to Fab treatment include hypokalaemia and exacerbation of congestive cardiac failure; renal function could be impaired in some patients.
Fab fragment preparations for treating acute colchicine and tricyclic antidepressant poisoning have been developed, but are not available commercially. Colchicine poisoning is rare in Western countries, and pharmacological management together with supportive care is usually effective even in severe tricyclic antidepressant overdosage. Attempts have been made to produce anti-paraquat antibodies capable of enhancing paraquat elimination from the lung, but thus far all such attempts have proved unsuccessful.












Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Flanagan RJ, Jones A. Antidotes. London: Taylor & Francis, 2001
Sullivan JB. Immunotherapy in the poisoned patient: overview of present applications and future trends. Med Toxicol 1986; 1: 47–60
Howland MA, Smilkstein MJ. Primer on immunology with applications to toxicology. In: Hoffman RS, Goldfrank LR, editors. Contemporary management in critical care. Vol. 1 (Pt3): Critical Care Toxicology. New York: Churchill Livingstone, 1991: 109–46
Bowden CA, Krenzelok EP. Clinical applications of commonly used contemporary antidotes: a US perspective. Drug Saf 1997; 16: 9–47
Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med 2002; 347: 347–56
Sabouraud A, Scherrmann JM. Immunotherapy of drug poisoning [in French]. Therapie 1994; 49: 41–8
Butler VP, Smith TW, Schmidt DH, et al. Immunological reversal of the effects of digoxin. Fed Proc 1977; 36: 2235–41
Scherrmann JM, Terrien N, Urtizberea M, et al. Immunotoxicotherapy: present status and future trends. J Toxicol Clin Toxicol 1989; 27: 1–35
Dart RC, McNally J. Efficacy, safety, and use of snake antivenoms in the United States. Ann Emerg Med 2001; 37: 181–8
Huston JS, Levinson D, Mudgett-Hunter M, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A 1988; 85: 5879–83
Glockshuber R, Malia M, Pfitzinger I, et al. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry 1990; 29: 1362–7
Hudson PJ, Souriau C. Engineered antibodies. Nat Med 2003; 9: 129–34
Kabat EA. Antibody diversity versus antibody complementarity. Pharmacol Rev 1982; 34: 23–38
Bismuth C, Borron SW, Baud FJ, et al. Immunotoxicotherapy: successes, disappointments and hopes. Hum Exp Toxicol 1997; 16: 602–8
Scherrmann JM. Antibody treatment of toxin poisoning: recent advances. J Toxicol Clin Toxicol 1994; 32: 363–75
Seifert SA, Boyer LV. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies. Ann Emerg Med 2001; 37: 189–95
Schaumann W, Kaufmann B, Neubert P, et al. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur J Clin Pharmacol 1986; 30: 527–33
Schmidt DH, Butler VP. Reversal of digoxin toxicity with specific antibodies. J Clin Invest 1971; 50: 1738–44
Smith TW, Haber E, Yeatmen L, et al. Reversal of advanced digoxin intoxication with Fab fragments of digoxin-specific antibodies. N Engl J Med 1976; 294: 797–800
Smith TW, Butler VP, Haber E, et al. Treatment of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments: experience in 26 cases. N Engl J Med 1982; 307: 1357–62
Smith TW, Lloyd BL, Spicer N, et al. Immunogenicity and kinetics of distribution and elimination of sheep digoxin-specific IgG and Fab fragments in the rabbit and baboon. Clin Exp Immunol 1979; 36: 384–96
Butler VP, Schmidt DH, Smith TW, et al. Effects of sheep digoxin-specific antibodies and their Fab fragments on digoxin pharmacokinetics in dogs. J Clin Ivest 1977; 59: 345–59
Ochs HR, Vatner SF, Smith TW. Reversal of inotropic effects of digoxin by specific antibodies and their Fab fragments in the conscious dog. J Pharmacol Exp Ther 1978; 207: 64–71
Antman EM, Wenger TL, Butler VP, et al. Treatment of 150 cases of life-threatening digitalis intoxication with digoxin-specific Fab antibody fragments: final report of a multicenter study. Circulation 1990; 81: 1744–52
Smith TW. New advances in the assessment and treatment of digitalis toxicity. J Clin Pharmacol 1985; 25: 522–8
Martiny SS, Phelps SJ, Massey KL. Treatment of severe digitalis intoxication with digoxin-specific antibody fragments: a clinical review. Crit Care Med 1988; 16: 629–35
Stolshek BS, Osterhout SK, Dunham G. The role of digoxin-specific antibodies in the treatment of digitalis poisoning. Med Toxicol Adverse Drug Exp 1988; 3: 167–71
Woolf A. Digitalis intoxication: therapy with digoxin-specific antibody fragments. Clin Immunother 1995; 4: 312–30
Smolarz A, Roesch E, Lenz E, et al. Digoxin specific antibody (Fab) fragments in 34 cases of severe digitalis intoxication. J Toxicol Clin Toxicol 1985; 23: 327–40
Wenger TL, Butler VP, Haber E, et al. Treatment of 63 severely digitalis-toxic patients with digoxin-specific antibody fragments. J Am Coll Cardiol 1985; 5Suppl. A: 118A–23A
Hess T, Riesen W, Scholtysik G, et al. Digitoxin intoxication with severe thrombocytopenia: reversal by digoxin-specific antibodies. Eur J Clin Invest 1983; 13: 159–63
Schmitt K, Tulzer G, Hackel F, et al. Massive digitoxin intoxication treated with digoxin-specific antibodies in a child. Pediatr Cardiol 1994; 15: 48–9
Hess T, Stucki P, Barandun S, et al. Treatment of a case of lanatoside C intoxication with digoxin-specific F(ab’)2 antibody fragments. Am Heart J 1979; 98: 767–71
Ma G, Brady WJ, Pollack M, et al. Electrocardiographic manifestations: digitalis toxicity. J Emerg Med 2001; 20: 145–52
Baud FJ, Brouard A, Haddad P. Fragments Fab d’anticorps spécifiques anti-digitaliques. In: Baud F, Barriot P, Riou B, editors. Les antidotes. Paris: Masson, 1992: 127–38
Ujhelyi MR, Robert S. Pharmacokinetic aspects of digoxin-specific Fab therapy in the management of digitalis toxicity. Clin Pharmacokinet 1995; 28: 483–93
Woolf AD, Wenger T, Smith TW, et al. The use of digoxin-specific Fab fragments for severe digitalis intoxication in children. N Engl J Med 1992; 326: 1739–44
Spiegel A, Marchlinski FE. Time course for reversal of digoxin toxicity with digoxin-specific antibody fragments. Am Heart J 1985; 109: 1397–9
Thanh-Barthet CV, Urtizberea M, Sabouraud AE, et al. Development of a sensitive radioimmunoassay for Fab fragments: application to Fab parmacokinetics in humans. Pharm Res 1993; 10: 692–6
Grene-Lerouge NAM, Bazin-Redureau MI, Debray M, et al. Interspecies scaling of clearance and volume of distribution for digoxin-specific Fab. Toxicol Appl Pharmacol 1996; 138: 84–9
Hickey AR, Wenger TL, Carpenter VP, et al. Digoxin immune Fab therapy in the management of digitalis intoxication: safety and efficacy results of an observational surveillance study. J Am Coll Cardiol 1991; 17: 590–8
Eddleston M, Rajapakse S, Rajakanthan S, et al. Anti-digoxin Fab fragments in cardiotoxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet 2000; 355: 967–72
Bosse GM, Pope TM. Recurrent digoxin overdose and treatment with digoxin-specific Fab antibody fragments. J Emerg Med 1994; 12: 179–85
Smith TW. Review of clinical experience with digoxin immune Fab (ovine). Am J Emerg Med 1991; 9Suppl. 1: 1–6
Timsina MP, Hewick DS. Digoxin-specific Fab fragments impair renal function in the rabbit. J Pharm Pharmacol 1992; 44: 867–9
Wenger TL. Experience with digoxin immune Fab (ovine) in patients with renal impairment. Am J Emerg Med 1991; 9(2 Suppl. 1): 21–3
Chillet P, Korach JM, Petitpas D, et al. Digoxin poisoning and anuric acute renal failure: efficiency of the treatment associating digoxin-specific antibodies (Fab) and plasma exchanges. Int J Artif Organs 2002; 25: 538–41
Zdunek M, Mitra A, Mokrzycki MH. Plasma exchange for the removal of digoxin-specific antibody fragments in renal failure: timing is important for maximizing clearance. Am J Kidney Dis 2000; 36: 177–83
Ward SB, Sjostrom L, Ujhelyi MR. Comparison of the pharmacokinetics and in vivo bioaffinity of DigiTAb versus Digibind. Ther Drug Monit 2000; 22: 599–607
Renard C, Grene-Lerouge N, Beau N, et al. Pharmacokinetics of digoxin-specific Fab: effects of decreased renal function and age. Br J Clin Pharmacol 1997; 44: 135–8
Amma Z, Kis E, Jozan-Jilling M, et al. A specific antidote for the treatment of digitalis poisoning in uremic patients [in Hungarian]. Orv Hetil 1997; 138: 1859–61
Renard C, Weinling E, Pau B, et al. Time- and dose-dependent digoxin redistribution by digoxin-specific antigen binding fragments in a rat model. Toxicology 1999; 137: 117–27
Wells TG, Young RA, Kearns GL. Age-related differences in digoxin toxicity and its treatment. Drug Saf 1992; 7: 135–51
Gibb I, Adams PC, Parnham AJ, et al. Plasma digoxin: assay anomalies in Fab-treated patients. Br J Clin Pharmacol 1983; 16: 445–7
Gibb I, Adams PC. Digoxin assay modifications to eliminate interference following immunotherapy for toxicity [abstract]. Ann Biol Clin 1985; 43: 696
Hursting MJ, Raisys VA, Opheim KE, et al. Determination of free digoxin concentrations in serum for monitoring Fab treatment of digoxin overdose. Clin Chem 1987; 33: 1652–5
Sinclair AJ, Hewick DS, Johnston PC, et al. Kinetics of digoxin and anti-digoxin antibody fragments during treatment of digoxin toxicity. Br J Clin Pharmacol 1989; 28: 352–6
George S, Braithwaite RA, Hughes EA. Digoxin measurements following plasma ultrafiltration in two patients with digoxin toxicity treated with specific Fab Fragments. Ann Clin Biochem 1994; 31: 380–2
Valdes R, Jortani SA. Monitoring of unbound digoxin in patients treated with anti-digoxin antigen-binding fragments: a model for the future? Clin Chem 1998; 44: 1883–5
Jortani SA, Pinar A, Johnson NA, et al. Validity of unbound digoxin measurements by immunoassays in presence of antidote (Digibind®). Clin Chim Acta 1999; 283: 159–69
Ujhelyi MR, Green PJ, Cummings DM, et al. Determination of free serum digoxin concentrations in digoxin toxic patients after administration of digoxin Fab antibodies. Ther Drug Monit 1992; 14: 147–54
Miller JJ, Straub RW, Valdes R. Analytical performance of a monoclonal digoxin assay with increased specificity on the ACS: 180. Ther Drug Monit 1996; 18: 65–72
McMillin GA, Owen WE, Lambert TL, et al. Comparable effects of DIGIBIND and DigiFab in thirteen digoxin immunoassays. Clin Chem 2002; 48: 1580–4
Tzou MC, Reuning RH, Sams RA. Quantitation of interference in digoxin immunoassay in renal, hepatic, and diabetic disease. Clin Pharmacol Ther 1997; 61: 429–41
Steimer W, Muller C, Eber B. Digoxin assays: frequent, substantial, and potentially dangerous interference by spironolactone, canrenone, and other steroids. Clin Chem 2002; 48: 507–16
Cummins RO, Haulman J, Quan L, et al. Near-fatal yew berry intoxication treated with external cardiac pacing and digoxin-specific FAB antibody fragments. Ann Emerg Med 1990; 19: 38–43
Rich SA, Libera JM, Locke RJ. Treatment of foxglove extract poisoning with digoxin-specific Fab fragments. Ann Emerg Med 1993; 22: 1904–7
Bania T, Hoffman RS, Howland MA, et al. Accidental Indian hemp (Apocyneacea cannabinum) cardiac glycoside toxicity [abstract]. Vet Hum Toxicol 1993; 35: 328
Clark RF, Selden BS, Curry SC. Digoxin-specific Fab fragments in the treatment of oleander toxicity in a canine model. Ann Emerg Med 1991; 20: 1073–7
Safadi R, Levy I, Amitai Y, et al. Beneficial effect of digoxin-specific Fab antibody fragments in oleander intoxication. Arch Intern Med 1995; 155: 2121–5
Dasgupta A, Hart AP. Rapid detection of oleander poisoning using fluorescence polarization immunoassay for digitoxin: effect of treatment with digoxin-specific Fab antibody fragment (ovine). Am J Clin Pathol 1997; 108: 411–6
Eddleston M, Warrell DA. Management of acute yellow oleander poisoning. Q J Med 1999; 92: 483–5
Barrueto F, Jortani SA, Valdes R, et al. Cardioactive steroid poisoning from an herbal cleansing preparation. Ann Emerg Med 2003; 41: 396–9
Slifman NR, Obermeyer WR, Aloi BK, et al. Contamination of botanical dietary supplements by Digitalis lanata. N Engl J Med 1998; 339: 806–11
Dasgupta A, Emerson L. Neutralization of cardiac toxins oleandrin, oleandrigenin, bufalin, and cinobufotalin by digibind: monitoring the effect by measuring free digitoxin concentrations. Life Sci 1998; 63: 781–8
Brubacher JR, Hoffman RS, Kile T. Toad venom poisoning: failure of a monoclonal digoxin immunoassay to cross-react with the cardioactive steroids. J Toxicol Clin Toxicol 1996; 34: 529–30
Brubacher JR, Ravikumar PR, Bania T, et al. Treatment of toad venom poisoning with digoxin-specific Fab fragments. Chest 1996; 110: 1282–8
Eddleston M, Senarathna L, Mohammed F, et al. Deaths due to lack of an affordable antitoxin for plant poisoning. Lancet 2003; 362: 1041–4
Pantanowitz L, Naude TW, Leisewitz A. Noxious toads and frogs of South Africa. S Afr Med J 1998; 88: 1408–14
Brubacher JR, Lachmanen D, Ravikumar PR, et al. Efficacy of digoxin specific Fab fragments (Digibind®) in the treatment of toad venom poisoning. Toxicon 1999; 37: 931–42
Dasgupta A, Lopez AE, Wells A, et al. The Fab fragment of anti-digoxin antibody (digibind) binds digitoxin-like immunoreactive components of Chinese medicine Chan Su: monitoring the effect by measuring free digitoxin. Clin Chim Acta 2001; 309: 91–5
Sabouraud A, Urtizberea M, Cano N, et al. Specific anti-digoxin Fab fragments: an available antidote for proscillaridin and scilliroside poisoning. Hum Exp Toxicol 1990; 9: 191–3
Owens SM, Mayersohn M. Phencyclidine-specific Fab fragments alter phencyclidine disposition in dogs. Drug Metab Dispos 1986; 14: 52–8
Hursting MJ, Opheim KE, Raisys VA, et al. Tricyclic antidepressant-specific Fab fragments alter the distribution and elimination of desipramine in the rabbit: a model for overdose treatment. J Toxicol Clin Toxicol 1989; 27: 53–66
Sabouraud AE, Urtizberea M, Benmoussa K, et al. Fab-bound colchicine appears to adopt Fab fragment disposition in rats. J Pharm Pharmacol 1992; 44: 1015–9
Sabouraud AE, Urtizberea M, Cano NJ, et al. Colchicine-specific Fab fragments alter colchicine disposition in rabbits. J Pharmacol Exp Ther 1992; 260: 1214–9
McClurkan MB, Valentine JL, Arnold L, et al. Disposition of a monoclonal anti-phencyclidine Fab fragment of immunoglobulin G in rats. J Pharmacol Exp Ther 1993; 266: 1439–45
Valentine JL, Arnold LW, Owens SM. Anti-phencyclidine monoclonal Fab fragments markedly alter phencyclidine pharmacokinetics in rats. J Pharmacol Exp Ther 1994; 269: 1079–85
Hill RE, Heard K, Bogdan GM, et al. Attenuation of verapamilinduced myocardial toxicity in an ex-vivo rat model using a verapamil-specific ovine immunoglobin. Acad Emerg Med 2001; 8: 950–5
Bonnel RA, Villalba ML, Karwoski CB, et al. Deaths associated with inappropriate intravenous colchicine administration. J Emerg Med 2002; 22: 385–7
Putterman C, Ben-Chetrit E, Caraco Y, et al. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum 1991; 21: 143–55
Stern N, Kupferschmidt H, Meier-Abt PJ. Follow-up and therapy of acute colchicine poisoning [in German]. Schweiz Rundsch Med Prax 1997; 86: 952–6
Nagaratnam N, De Silva DP, De Silva N. Colchicine poisoning following ingestion of Gloriosa superba tubers. Trop Geogr Med 1973; 25: 15–7
Mendis S. Colchicine cardiotoxicity following ingestion of Gloriosa superba tubers. Postgrad Med J 1989; 65: 752–5
Critchley JA, Critchley LA, Yeung EA, et al. Granulocyte-colony stimulating factor in the treatment of colchicine poisoning. Hum Exp Toxicol 1997; 16: 229–32
Wolff J, Capraro H-G, Brossi A, et al. Colchicine binding to antibodies. J Biol Chem 1980; 255: 7144–8
Terrien N, Urtizberea M, Scherrmann JM. Reversal of advanced colchicine toxicity in mice with goat colchicine-specific antibodies. Toxicol Appl Pharmacol 1990; 104: 504–10
Urtizberea M, Sabouraud A, Cano N, et al. Reversal of murine colchicine toxicity by colchicine-specific Fab fragments. Toxicol Lett 1991; 58: 193–8
Sabouraud A, Urtizberea M, Grandgeorge M, et al. Dose-dependent reversal of acute murine colchicine poisoning by goat colchicine-specific Fab fragments. Toxicology 1991; 68: 121–32
Scherrmann JM, Urtizberea M, Pierson P, et al. The effect of colchicine-specific active immunization on colchicine toxicity and disposition in the rabbit. Toxicology 1989; 56: 213–22
Scherrmann JM, Sabouraud A, Urtizberea M, et al. Clinical use of colchicine specific Fab fragments in colchicine poisoning [abstract]. Vet Hum Toxicol 1992; 34: 334
Baud FJ, Sabouraud A, Vicaut E, et al. Treatment of severe colchicine overdose with colchicine-specific Fab fragments. N Engl J Med 1995; 332: 642–5
Eddleston M, Persson H. Acute plant poisoning and antitoxin antibodies. J Toxicol Clin Toxicol 2003; 41: 309–15
Singh N, Singh HK, Khan IA. Serial electrocardiographic changes as a predictor of cardiovascular toxicity in acute tricyclic antidepressant overdose. Am J Ther 2002; 9: 75–9
Bowles M, Johnston SC, Schoof DD, et al. Large scale production and purification of paraquat and desipramine monoclonal antibodies and their Fab fragments. Int J Immunopharmacol 1988; 10: 537–45
Pentel PR, Scarlett W, Ross CA, et al. Reduction of desipramine cardiotoxicity and prolongation of survival in rats with the use of polyclonal drug-specific antibody Fab fragments. Ann Emerg Med 1995; 26: 334–41
Pentel PR, Keyler DE. Drug-specific antibodies as antidotes for tricyclic antidepressant overdose. Toxicol Lett 1995; 82/3: 801–6
Brunn GJ, Keyler DE, Pond SM, et al. Reversal of desipramine toxicity in rats using drug-specific antibody Fab’ fragment: effects on hypotension and interaction with sodium bicarbonate. J Pharmacol Exp Ther 1992; 260: 1392–9
Dart RC, Sidki A, Sullivan JB, et al. Ovine desipramine antibody fragments reverse desipramine cardiovascular toxicity in the rat. Ann Emerg Med 1996; 27: 309–15
Lin G, Pentel PR, Shelver WL, et al. Bacterial expression and characterization of an anti-desipramine single-chain antibody fragment. Int J Immunopharmacol 1996; 18: 729–38
Shelver WL, Keyler DE, Lin G, et al. Effects of recombinant drug-specific single chain antibody Fv fragment on [3H]-desipramine distribution in rats. Biochem Pharmacol 1996; 51: 531–7
Ragusi C, Boschi G, Rips R, et al. Facilitation of imipramine efflux from the brain by systemic specific antibodies. Br J Pharmacol 1996; 118: 2152–6
Ragusi C, Boschi G, Risède P, et al. Influence of various combinations of specific antibody dose and affinity on tissue imipramine redistribution. Br J Pharmacol 1998; 125: 35–40
Ragusi C, Scherrmann J-M, Harrison K, et al. Redistribution of imipramine from regions of the brain under the influence of circulating specific antibodies. J Neurochem 1998; 70: 2099–105
Heard K, O’Malley GF, Dart RC. Treatment of amitriptyline poisoning with ovine antibody to tricyclic antidepressants. Lancet 1999; 354: 1614–5
Protherics financials [online]. Available from URL: http://www.protherics.com/financials/detailed.htm [Accessed 2003 Mar]
Smith LL. The toxicity of paraquat. Adverse Drug React Acute Poisoning Rev 1988; 1: 1–17
Johnston SC, Bowles M, Winzor DJ, et al. Comparison of paraquat-specific murine monoclonal antibodies produced by in vitro and in vivo immunization. Fundam Appl Toxicol 1988; 11: 261–7
Wright AF, Green TP, Robson RT, et al. Specific polyclonal and monoclonal antibody prevents paraquat accumulation into rat lung slices. Biochem Pharmacol 1987; 36: 1325–31
Chen N, Bowles MR, Pond SM. Prevention of paraquat toxicity in suspensions of alveolar type II cells by paraquat-specific antibodies. Hum Exp Toxicol 1994; 13: 551–7
Nagao M, Takatori T, Wu B, et al. Immunotherapy for the treatment of acute paraquat poisoning. Hum Toxicol 1989; 8: 121–3
Cadot R, Descotes J, Grenot C, et al. Increased plasma paraquat levels in intoxicated mice following antiparaquat F(ab’)2 treatment. J Immunopharmacol 1985; 7: 467–77
Devlin CM, Bowles MR, Gordon RB, et al. Production of a paraquat-specific murine single chain Fv fragment. J Biochem 1995; 118: 480–7
Bowles MR, Mulhern TD, Gordon RB, et al. Bound Tris confounds the identification of binding site residues in a paraquat single chain antibody. J Biochem (Tokyo) 1997; 122: 101–8
Protherics products [online]. Available from URL: http://www.protherics.com/products/default.htm. [Accessed 2004 Sep]
Mauskopf JA, Wenger TL. Cost-effectiveness analysis of the use of digoxin immune Fab (ovine) for treatment of digoxin toxicity. Am J Cardiol 1991; 68: 1709–14
DiDomenico RJ, Walton SM, Sanoski CA, et al. Analysis of the use of digoxin immune Fab for the treatment of non-life-threatening digoxin toxicity. J Cardiovasc Pharmacol Ther 2000; 5: 77–85
Theakston RD, Warrell DA. Crisis in snake antivenom supply for Africa [letter]. Lancet 2000; 356: 2104
Acknowledgements
We thank Ms Debbie Shaw, Medical Toxicology Unit, for help with table II and Dr M. Eddleston (Colombo) for critical review of the manuscript. No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flanagan, R.J., Jones, A.L. Fab Antibody Fragments. Drug-Safety 27, 1115–1133 (2004). https://doi.org/10.2165/00002018-200427140-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200427140-00004