What do urologists think patients need to know when starting on androgen deprivation therapy? The perspective from Canada versus countries with lower gross domestic product
Introduction
With increased use of prostate specific antigen (PSA) testing, men are being diagnosed with prostate cancer at increasingly younger ages and the incidence of the disease has been rising in recent decades (1-4). In the North American context, biochemical failure (i.e., a rising PSA after a potentially curative treatment, such as a radical prostatectomy) spurs many men to start on androgen deprivation therapy (ADT) even without overt signs of the disease. In the past, prostate cancer patients typically did not begin ADT until they showed signs of advanced disease, such as bone pain associated with metastasis. With men starting ADT earlier in the disease progression, they are on that treatment for a longer time. An extended duration on ADT increases the risk of adverse effects (e.g., cardiovascular disease, diabetes, bone loss, etc.) and men on ADT for longer periods of time must cope with side effects for a longer duration. Thus the need for educating patients about ways to manage the more debilitating side effects is more important than ever before. Here we explore what physicians believe patients need to be most educated about, when starting on ADT.
ADT is most commonly administered in the form of luteinizing hormone releasing hormone agonists (LHRHa) and is associated with many adverse effects that reduce the quality of life of prostate cancer patients (5-11). Our previous research has indicated a gap in patient knowledge about these adverse effects (12), which can influence patients’ ability to implement management strategies to mitigate these side effects. Tran et al. (12) examined the perspectives of Canadian uro-oncologists on patient education about ADT side effects. They found considerable diversity among treating physicians about which side effects they felt were essential to inform patients about. So, for example, less than 60% of physicians agreed that patients should be informed about increased risk for depression, diabetes, high cholesterol and anemia, the occurrence of genital shrinkage, and difficulties with orgasm. In contrast, there was strong agreement about informing patients about the following side effects: osteoporosis, erectile dysfunction, hot flashes, loss of libido, and loss of muscle mass. Respondents were also surveyed about side effect management strategies [reviewed in (13)] they recommended to patients (12) and a lack of concordance was also observed for management strategies.
Given the relative lack of agreement in the Canadian context, we sought to explore whether similar perspectives on educational practices with patients starting on ADT exist for urologists treating prostate cancer patients in other countries. It is likely that there are cultural and/or geographical differences in opinions about what knowledge is essential to disseminate in different clinical settings. In order to explore this question, our analysis focused on countries with lower Gross Domestic Product (GDP) than that of Canada. We hypothesized that variation in access to healthcare, the stage at which the cancer is diagnosed, agents used for ADT, and cultural differences in physician-patient relationships would be manifested in differences between uro-oncologists in Canada versus other countries. Here we identify such diversity and explore how these differences might influence what information physicians in the two settings feel their patients need to know.
Materials and methods
Urologists were anonymously surveyed about their perspectives on patient education regarding ADT adverse effects. Ethics approval was obtained from the Conjoint Health Research Ethics Board in Calgary, Alberta. Respondents represented a wide range of countries (n=28). Healthcare systems vary widely around the world and how medicine is practiced can be greatly influenced by economic factors. As such, countries were sorted into top, middle and bottom groups based on their per capita GDP (14); the top GDP countries (with number of responses in brackets) included: United States [4], Netherlands [1], Australia [3], Denmark [1], Ireland [1], Canada [4], Germany [4], United Kingdom [3] and France [1]. Countries classified as middle GDP included: Japan [4], Taiwan [1], Portugal [4], Poland [1], Latvia [1], Turkey [1], Malaysia [1], Argentina [1], Serbia [1] and Brazil [2]. The bottom GDP countries included: South Africa [3], China [1], Egypt [1], Indonesia [1], Philippines [1], Iraq [2], Nigeria [2], Ghana [2] and Kenya [1]. Not including the 4 Canadian responses, these tallied as 18, 17 and 14 respondents for high, middle, and lower GDP countries respectively.
Recruitment strategies included online invitations posted by professional associations to their members, and in-person recruitment at an international urology conference held in Vancouver, BC in the fall of 2013 (i.e., 33rd Congress of the Société Internationale d’Urologie). The majority of international respondents were recruited in person at the aforementioned urology congress. Of those approached, approximately 50% agreed to participate. The most common reason for declining was a lack of efficiency in English. Online invitations were successful for Canadian respondents and 4 international respondents only. The four additional Canadian responses collected at this conference, were added to the large sample of urologists who had previously completed the same survey in the Tran et al. (12)study. This yielded a tally of n=42 Canadian responses. The countries from the middle and low GDP groups were combined for a total of 31 responses (referred to here as “lower GDP countries”). Our subsequent data analysis compared these two samples.
A rationale for using Canada as the contrasting group is that Canada is one of the top ranking GDP countries with universal healthcare, so options for managing ADT side effects in Canada are not typically limited by patients’ individual ability to pay for services. Furthermore, Canada’s universal healthcare practices are largely similar to that of the countries in our top GDP category (e.g., United Kingdom, Germany, with the exception of USA). In fact, the same analyses described below, were conducted on the Canadian vs. other high GDP countries, and no significant differences were found.
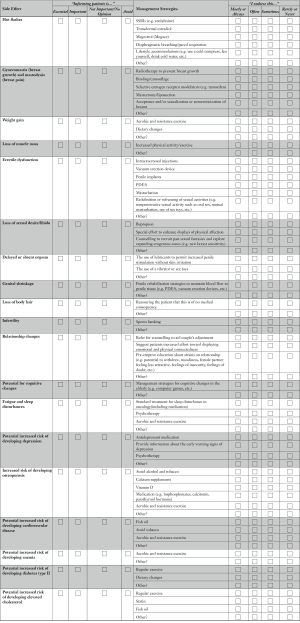
The same survey used by Tran et al. (12) was implemented. The survey included three parts: (I) a list of commonly reported side effects associated with ADT; (II) a list of management strategies for ADT side effects (15) (identified by the ADT Working Group); and (III) a brief case scenario that exemplified a typical ADT patient (i.e., a 65-year-old married man, commencing ADT after failing primary prostate cancer treatment, who was previously sexually active using a phosphodiesterase type 5 inhibitor to treat erectile dysfunction; see Online Supplement 1). Demographic variables of the respondents were also collected (e.g., gender, age, years in practice, rural vs. urban).
The first two sections of the survey contained two ranking exercises. The “categories of importance” scale was used to rate how essential physicians considered it to inform patients about specific side effects, and the “side effect management” scale was used to rate how frequently physicians endorsed a specific management strategy. Four categories were used to rate the importance of informing patients about specific side effect: “essential”, “important”, “not important/no opinion” and “avoid”. For statistical analysis the two categories of “essential” and “important” were collapsed into a single category: “inform”. similarly, “not important/no opinion” and “avoid” were combined into the single category: “not inform”.
To quantify the size of the difference of importance ranking for each individual side effect, an additional comparison was performed by applying a scoring system of 1 through 4 to each aforementioned ranking category, (i.e., “essential” =1, “important” =2, “not important” =3, and “avoid” =4), thus lower scores indicate higher importance.
Four categories were used to rate the endorsement of each management strategy and included: “always”, “often”, “sometimes/rarely” and “never”. These categories were also dichotomized, “always” and “often” categories were combined into the single category: “endorse”. Similarly “sometimes/rarely” and “never” were combined into the single category: “not endorse”.
Statistical analyses
A series of chi-square tests were performed (16) in order to identify statistically significant differences between the two groups of respondents using a conservative P value cut-off of 0.01 for significance in recognition of our multiple comparisons.
To statistically evaluate the difference between the two groups of urologists in the importance they gave to telling patients about specific side effects a series of Mann-Whitney U tests were performed, using a P value of 0.01.
A chi-square test was run to determine if there was a significant difference between the number of side effects ranked as important or essential (<2) by the two groups of urologists. Significance was determined at P value of 0.05.
Results
Surveyed urologists from Canada ranged in age from 33 to 86 years (mean ± SD =51.3±12.1). All 41 respondents were male. The average length of practice was 18.8 years (SD =11.3; range, 1–40). The average number of patients the physicians started on ADT per year was 6.9 (SD =4.9; range, 1–20).
The urologists from lower GDP countries ranged in age from 30 to 70 years (50.2±10.9); 28 were male and 3 were female. The average length of practice was 20.6 years (SD =11.1; range, 3–40). The average number of patients the physicians started on ADT per year was 7.8 (SD =10.5; range, 2–58).
Statistical differences between the two samples were not significant for age, length of practice, or average number of patients started on ADT per year, but were significant for gender, with 3 female urologists from lower GDP countries and none from Canada (P=0.042). Excluding these three individuals did not change the patterns of significance for any of the following analyses, so they were retained in the sample.
Of those in the lower GDP category, LHRHa were listed as the most commonly used method of ADT by 54.8% of the sample (representing Portugal, Brazil, Poland, Taiwan, Malaysia, Iraq, Kenya, Turkey, China Argentina, Indonesia, South Africa and Ghana). An additional 29.0% indicated LHRHa was common, but so too were oral estrogens, anti-androgens, or surgical castration.
Part 1: what ADT side effects do urologists consider essential to inform the patients about?
The side effects that urologists in Canada considered most important to inform their patients about, in decreasing order, were: osteoporosis (100% ranked it essential or important), hot flashes (97.6%), erectile dysfunction (97.6%), loss of libido (94.9%) and gynecomastia (90.2%). Less than 50% of Canadian urologists thought it was important to inform their patients about 5 (out of 19) side effects: elevated cholesterol (46.2%), genital shrinkage (45.9%), delayed orgasm (39.5%), loss of body hair (23.7%) and infertility (10%). These and all other side effects examined are plotted in Figure 1.
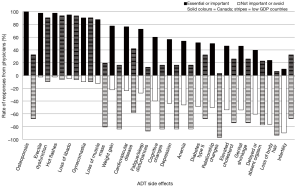
A gap was noticed between the side effects that were considered important and not important by urologists in the low GDP countries. Only 4 side effects were consistently considered important to inform the patients about, by both groups of urologists: hot flashes (93.5% ranked it essential or important), erectile dysfunction (90.3%), loss of libido (90.3%) and gynecomastia (90.3%). The remaining side effects were predominantly considered not important to discuss with patients by 58% or more of urologists. This ranking was a significantly different between the two groups of urologists (P=0.003). The perceived importance of informing patients about specific ADT side effects is summarized in Table 1.
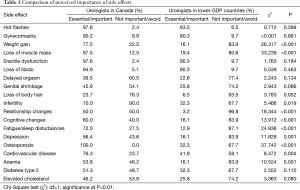
Full table
Differences in importance ranking for each individual side effect, are shown in Table 2. Lower scores indicate higher importance. The largest difference in importance was observed for osteoporosis, followed by loss of muscle mass, weight gain, fatigue/sleep disturbance, relationship changes, cognitive changes and loss of body hair (all P<0.001).

Full table
A comparison of the overall number of side effects determined important to tell patients about was also conducted. A total of 8 out of 19 side effects were given a summative ranking of <2 (essential or important) by Canadian urologists, but only 4 out of the 19 side effects where ranked similarly (<2) by urologists in lower GDP countries. The difference in rankings borders on significant (P=0.020).
Interestingly, only one side effect, infertility, was given an importance rating of larger than 3 by Canadian urologists, denoting that on average they categorize it as a topic on which to outright avoid discussion with patients. In the lower GDP countries however, 9 out of 19 ADT side effects were given an importance score of 3 or higher by the urologists. This ranking was also significantly different between the two groups of urologists (P=0.008) and indicated a strong propensity for urologists in our sample from lower GDP countries to avoid discussing almost half of the ADT side effects with their patients.
Part 2: what management strategies do urologists endorse for these side effects?
Table 3 and Figure 2 show ADT side effect management strategies in which significant differences in endorsement were found between urologists in Canada and urologists in lower GDP countries. Seven side effects had one or more management strategies that differed significantly between Canada and lower GDP countries (Figure 2).
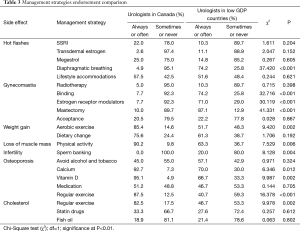
Full table
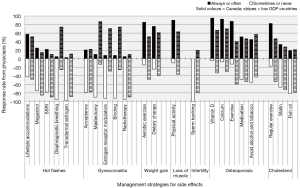
Although urologists in both groups rated hot flashes as an adverse effect that is essential to discuss with patients, they do not agree on what patients might do to reduce the burden of this side effect. For hot flashes, most pharmaceutical options are not strongly recommended in either group. But, in the lower GDP countries, the behavioral intervention, which has no cost associated with it, i.e., diaphragmatic breathing, receives much more promotion than in Canada (74.2% vs. 4.9%). Canadian urologists do appear to endorse pharmaceuticals more than those in lower GDP countries, in the management of hot flashes (25.0% vs. 14.4% for Megestrol; 22.0% vs. 10.3% for selective serotonin reuptake inhibitors). This specific finding may reflect economic disparities. Though recommended strategies differ, recall that there is no difference between the two groups in how important it is to talk to patients about this side effect.
Gynecomastia is the one side effect where urologists in lower GDP countries endorsed more management strategies and offer more advice to patients, than do urologists in Canada. Specifically, binding the chest, prescribing selective estrogen receptor modulators, and mastectomy, are all endorsed as management strategies for gynecomastia by at least 71% of urologists in lower GDP countries and by only 10% or less of urologists in Canada. This suggests that gynecomastia is perceived as a more stigmatizing or burdensome condition for men in those countries than in Canada. However, it is also possible that the greater focus on gynecomastia in the lower GDP countries reflects a racial or regional difference in the prevalence of gynecomastia (and concomitant mastalgia) for men on ADT in those countries. There have been documented racial differences in the prevalence of hot flashes in menopausal women (17,18), though this has not yet been studied in an ADT population.
For side effects such as weight gain, loss of muscle mass, osteoporosis, and elevated cholesterol one management strategy—increased physical exercise—is consistently endorsed by Canadian urologists, significantly more so than by urologists in lower GDP countries. Canadian urologists are more likely to endorse vitamin D (95.1%) and exercise (85.4%), for weight gain, for example, than are urologists in the sample of lower GDP countries (66.7%, 51.7%). Also, none of the Canadian urologists endorsed sperm banking as a management strategy for infertility, while 20% of urologists in lower GDP countries did. Gender biases (i.e., male vs. female urologists) in the recommendation for sperm banking were not found, possibly due to the small sample of females (n=3). With regards to sarcopenic obesity, Canadian urologists are far more likely to recommend exercise as a management strategy for the side effects of muscle loss and weight gain.
Part 3: do urologists modify their discussion with patients according to patients’ characteristics?
The majority of urologists in both groups reported they would not change their responses based on patient’s age, relationship status, previous sexual history, and if the patient were prescribed ADT as short term therapy in combination with external beam radiotherapy, or as an intermittent hormonal therapy for systemic disease. Differences were not significant between the two analyzed groups of urologists.
Do urologists routinely provide educational materials to patients on ADT?
In Canada, 73.2% of urologists reported they routinely provide educational material (such as booklets, books, websites, DVDs) to their patients on ADT compared to only 19.4% of urologists in lower GDP countries. Differences between the two groups were highly significant (P<0.001).
What additional screenings and referrals do urologists make?
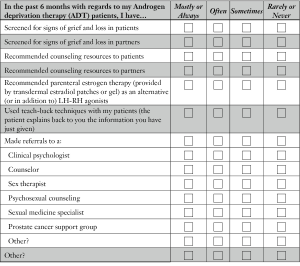
Most urologists rarely screen their ADT patients or their partners for signs of grief and loss. Canadian urologists reported that they rarely refer their patients to counseling and, if they do, most (41.5%) refer their patients to a prostate cancer support group. Surprisingly, the data indicate that significantly more referrals are made by urologists in lower GDP countries to “clinical psychologists and sexual medicine specialists”, than are referrals made by urologists in Canada. An overview of these findings is given in Table 4.
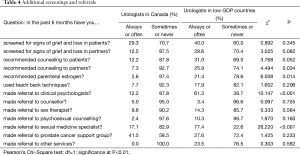
Full table
Conclusions
Throughout the world countries establish national guidelines for when to prescribe specific medications and there is a global consensus that some form of ADT is appropriate for treating systemic prostate cancer. To the best of our knowledge there are in contrast no “standards of care” concerning patient education about ADT adverse effects or ways to mitigate these adverse effects. The data show profound differences between the side effects that Canadian urologists consider important to discuss with patients compared to urologists in lower GDP countries.
Several factors may account for this overall difference, starting with the agents used to suppress testosterone. Though the most common pharmacological agents used for ADT among international respondents are LHRHa (85%), they are not used exclusively and different ADT agents have different side effect profiles. Across Canada, LHRH agonists are the most common form of ADT, with rising use of LHRH antagonists in recent years. These drugs induce both testosterone and estrogen deprivation and have different side effect profiles than drugs that do not cause estrogen deprivation. LHRH agonists and antagonists are among the most expensive drugs licensed in Canada and are out of economic reach for many patients in lower GDP countries. Cheaper alternative agents include the synthetic steroidal antiandrogen, cyproterone acetate, the synthetic non steroidal estrogen diethylstilbestrol, and even conjugated equine estrogen (commercially sold as Premarin; pers. comm., 2015). These cheaper agents all have estrogenic effects. Where physicians prescribe estrogenic agents for androgen suppression, gynecomastia would be expected to be a more common concern and therefore, more emphasized by the urologists. Similarly hot flashes and osteoporosis would be less frequent in their patients and thus less heavily emphasized in their discussions with those patients.
A second possibility is that there may, in fact, be substantial variation in the incidence or severity of specific side effects along both racial and regional (climatic) lines. Our data suggests that this could be relevant for the side effect of gynecomastia. These differences may also be present for bone density loss strategies, given the high incidence of vitamin D deficiency in Canada (associated with its high latitude global position). In comparison with lower GDP countries (which are largely more equatorial) citizens in those countries are more likely to have adequate sunlight exposure for the natural production of endogenous vitamin D. However at this time we are unaware of any studies documenting ADT side effect incidence and burden across racial or geographic lines.
Another variable may be that patients are more likely to present with more advanced disease in lower GDP countries (19). This, in turn, may reflect the health status of patients when they are first offered ADT as a treatment. The patient’s life expectancy and morbidity would alter the perceived balancing between the cost and benefit of managing ADT side effects. In Canada, a large number of patients are regularly screened for prostate cancer and as a result are diagnosed at a relatively early stage in the disease trajectory (20). Patients diagnosed with prostate cancer are likely to be followed with repeated PSA tests and are more likely to start on ADT in the context of biochemical failure, before presenting with metastatic signs of systemic disease.
In contrast, in lower GDP countries, where screening is far less common (19,21) patients are more likely to be diagnosed upon metastatic presentation. Mortality rates from prostate cancer in developing countries are twice that of mortality rates in developed countries (21). This difference may account for many of the noted differences in perspectives on management of ADT side effects. For example, osteoporosis is of far more concern to urologists in Canada than to urologists in the lower GDP countries. It is possible that for patients starting ADT in Canada, where the majority have a good life expectancy and minimal evidence of skeletal-related events, managing the risk of osteoporosis is a valid concern. In contrast, in low GDP countries, where patients first present with metastatic bone lesions and reduced life expectancy, the long term management of osteoporosis would appear to be less relevant as life expectancy is shorter.
Several other side effects, which Canadian urologists endorse as essential for discussion with patients, but urologists from lower GDP countries do not, similarly appear to reflect the typical disease status of the patients that they start on ADT. Results indicate that Canadian urologists are far more likely to recommend exercise as a management strategy for muscle loss and weight gain. This difference is statistically significant, but should be interpreted cautiously given the finding that there is less concern in general about these side effects in lower GDP countries. We suggest that the greater concern about loss of muscle mass and weight gain by Canadian urologists reflects the concern in more affluent countries about obesity in general. In lower GDP countries, where obesity is less common [although on the rise; see (22)] managing sarcopenic obesity would also be less common and thus would be seen as comparably less important by urologists prescribing ADT.
Furthermore, for patients with more advanced disease at first presentation, when overall life expectancy may be at best, only a few years, concerns about cardiovascular risk, cholesterol, type 2 diabetes, cognitive changes, depression, anemia, or the impact on relationships, all would seem to be of less relevance. That would appear to explain why management strategies to deal with those side effects are not rated as important in lower GDP countries as they are in Canada.
In contrast, patients showing no overt signs of systemic disease, and with good life expectancy, are more likely to be negatively impacted by the same adverse effects, and for a longer duration. Such patients may be more commonly found in Canada. As such, we would expect that Canadian urologists would rate those side effects as important to discuss with their patients. This is consistent with our findings. The management strategy most recommended by Canadian urologists, is physical exercise, which is in fact a well-documented intervention to collectively reduce the majority of ADT adverse effects (3,15,23,24).
Cultural factors may also play a role in education practices. There is cultural variation in the degree to which the patient versus the physician is responsible for the decision to treat with ADT. In general, Canadian urologists consider it important to discuss with patients far more side effects than do urologists in lower GDP countries (Table 1). A focus on shared-decision making and patient-empowerment in Western medicine has largely come to replace more traditional or paternalistic model of the doctor-physician relationship (25). This shift may reflect a fundamental difference between the extent to which the patient is informed about side effects, so that he can be part of the decision-making process.
In Canada, and the affluent world in general, there is a strong emphasis on independence and autonomy, such that it is largely accepted that patients must be well-informed about side effects in order to make informed decisions about their treatment. In westernized, English speaking countries, patients who are younger and have higher education, tend to prefer a more active role in treatment decision-making (26). Among other types of cancer patients (e.g., gynecological, lung and colorectal), American breast and prostate cancer patients tend to report the highest percentage of shared and active role preferences (27). Furthermore, consistent with this notion is the increased emphasis placed on individuals to take responsibility for their health; therefore, self-management strategies that focus on lifestyle (i.e., diet and exercise) are more likely to be promoted.
In non-western cultures, there is an understanding that the responsibility for treatment decisions rests to a higher degree with the physician than with the patient (27-31). This may be even more so when the patient has advanced disease. As such, a need to discuss side effects at length with patients may be seen as less essential in these countries. In fact extensive discussion of ADT side effects may even be perceived of as unnecessarily distressing to patients. In this context, shared decision-making may be less commonly expected by both physician and patient, and therefore less emphasis may be placed on educating patients about ADT side effects. Trill and Holland (32) contrast the assumption in North American culture, that individuals are expected to gain mastery over their environment, whereas in Eastern cultures the value is for individuals to strive to live in harmony with their environment. They further argue that individuals in Eastern cultures have increased acceptance of illness. By extension, patients in lower GDP countries may be more accepting of adverse treatment effects, whereas Canadian patients may feel a greater need for managerial control over those same side effects.
In non-western countries, where the average patient starting on ADT may be more likely to have advanced disease, and physicians hold more power to make treatment decisions on behalf of their patients, information disclosure may be even more restricted. When patients have a terminal diagnosis, there is large cultural variability in the degree to which such information is shared with the patient, such that in many countries (e.g., Saudi Arabia, Egypt, Singapore, Japan, and China) there is a high degree of concealment of information from the patient [reviewed in (30-33)] and varied disclosure to their family. Even in some European countries (e.g., Italy, Spain, and Greece) a cancer diagnosis may not be disclosed directly to the patient [reviewed in (33)]. This is in stark contrast to Anglo-Saxon and Northern European countries where a more forthcoming communication style is preferred [reviewed in (34)].
Another influencing variable is likely to be divergent cultural, social, and religious beliefs around perceived masculinity. Such differences appear to be most relevant to a cluster of side effects that are considered more important for physicians within lower GDP countries to discuss with patients. These side effects include loss of libido, gynecomastia, genital shrinkage, and infertility. What is intriguing about this group of side effects is that they all relate to masculinity, male identity, and sexual performance.
It is possible that these side effects are more stigmatizing in the lower GDP countries than they are in Canada. We speculate that gender role distinction, and in particular markers of masculinity may be more important for males in lower GDP countries, than for males in Canada. The only side effect that was ranked more often by lower GDP country respondents, than Canadian respondents as important, was infertility (see Table 2). With the mean age of prostate cancer diagnosis in the Canadian population now in the mid-60s, it makes intuitive sense that the effect of infertility may be seen as less important. This does not appear to hold though for lower GDP countries. It is possible that masculinity, fertility, genital size, and libido, may collectively be seen as more important for male self-esteem and well-being in lower GDP counties than in Canada, though this has not been documented in the literature.
Lastly, the finding that respondents from lower GDP countries were more likely than Canadian respondents to refer their patients to clinical psychologists and sex therapists was surprising. We speculate that the definition that is used for clinical psychologists is different in these countries as compared to Canada, as psychosocial oncology in general is a far newer field in less economically advanced countries. We would also assume that availability of these health care professionals would be scarcer. It is possible that the phrasing of the question lead to misleading findings, “In that past 6 months have you made a referral to…” reflects only an occasional referral, rather than a routine practice.
There are several obvious limitations to this study; the most outstanding is the small sample size. Because of that, it is not possible to correlate in any meaningful way the specific agents recommended by the physicians for androgen deprivation with their particular answers to questions about side effects and their recommended management strategies. Also, generalizations cannot be made about responses from any particular country. Recruitment of international survey respondents was challenging and online invitations were largely unsuccessful. Future efforts in this area are likely to be most successful from face-to-face recruitment at international urology conferences.
All told, there are major differences about what urologists around the world think patients need to know about ADT side effects and how to manage them. Across different regions, variations in cultural ideologies (e.g., value of shared decision-making, patriarchal socialization) and health care systems (e.g., prostate cancer screening, privatized vs. universal health care) may strongly influence patient education practices. Recognizing these differences may help physicians treating patients from different cultures and countries attend to side effects of ADT that are likely to be most bothersome to their individual patients.
Recently the American Cancer Society published Prostate Cancer Survivorship Care Guidelines (35), which have now been formally endorsed by the American Society of Clinical Oncology (36). These guidelines include recommendations for managing many ADT side effects. Notably the guidelines focus on the physiological side effects of osteoporosis, cardiovascular risk, and sarcopenic obesity. Conversely they provide little attention to ADT side effects related to sexual performance or masculine identity. Although neither the ACS nor ASCO qualify their recommendations with a statement that they are specific to the USA or other high GDP countries, our data suggest that such a caveat is appropriate. Urologists from lower GDP countries are likely to find that the ACS guidelines do not reflect what they perceive of as the particular needs and concerns of their patients starting on ADT.
Acknowledgements
We thank Phil Pollock, Susan Tran, Robyn Jackowich, Kendra Lawrence and Naomi Liu for help in collecting data. Alison Ludgate helped with data entry. John Robinson, Imhokhai Ogah, Erik Wibowo and Tia Higano provided critical comments on draft manuscripts.
Funding: This research was variously funded by the Natural Sciences and Engineering Research Council of Canada, the Urology Fund of the University of British Columbia, the Vancouver Prostate Centre, the Alberta Cancer Foundation for post-doctoral funding and a Prostate Cancer Canada Rising Star Grant (#RS-2015-03) supporting LM Walker, and the Program for Undergraduate Research, University of Calgary, for S. Tran.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by The University of Calgary Conjoint Health Research Ethics Board of NO. E23369-2011G. Written informed consent was obtained from the participants for publication of this article. A copy of the written consent is available for review by the editor-in-chief of this journal.
Online supplement: ADT educational practices survey
Please read these instructions carefully:
The following is a list of potential side effects of androgen deprivation therapy (ADT) (left half) and potential management strategies for each side effect (right half). Please read the clinical scenario, and answer the following questions based on the prostate cancer patient presented in the scenario at the bottom of this page. Please fill out the survey in a left-to-right fashion, beginning with the side effect, and then the corresponding management strategies.
For the side effects, rate how important it is to inform patients of each of these potential side effects by checking it off as either “essential”, “important”, “not important/no opinion”, or “avoid”.
For the management strategies, rate the degree to which you would endorse each strategy by checking it off as either “mostly or always”, “often”, “sometimes”, “rarely or never”.
*Note:
- - “essential” = it is absolutely necessary to inform patients of this side effect in almost all circumstances
- - “important” = it is desirable, but if, for example, time is limited, informing patients of this side effect could be dropped
- - “not important/no opinion” = it is not necessary to inform patients of this side effect, or either you don’t have a strong opinion or you don’t have any opinion at all about this
- - “avoid” = it should definitely be avoided informing patients of this side effect in almost all circumstances
(Adapted from Feldman-Stewart et al., 1997)
Clinical scenario: A 65-year-old married man who has failed primary treatment for prostate cancer now has a rising prostate-specific antigen (PSA). He is previously sexually active using oral medications (PDE5i). You, the man, and his wife agree that commencing androgen deprivation therapy is the best course of action.
Would you change any of your replies above if the patient was…
For each question, please rate the degree to which each applies to you in the past 6 months, by checking off one of the boxes on the scale.
For each question in this last section, please rate the importance of each to you by checking one of the boxes on the scale.
Do you routinely provide educational material(s) on androgen deprivation therapy to your patients?
Demographic Data
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Weir HK, Thompson TD, Soman A, et al. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 2015;121:1827-37. [Crossref] [PubMed]
- Ahmad AS, Ormiston-Smith N, Sasieni PD. Trends in the lifetime risk of developing cancer in Great Britain: comparison of risk for those born from 1930 to 1960. Br J Cancer 2015;112:943-7. [Crossref] [PubMed]
- Allan CA, Collins VR, Frydenberg M, et al. Androgen deprivation therapy complications. Endocr Relat Cancer 2014;21:T119-29. [Crossref] [PubMed]
- Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl 2012;14:226-31. [Crossref] [PubMed]
- Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825-36. [Crossref] [PubMed]
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA 2005;294:238-44. [Crossref] [PubMed]
- Sountoulides P, Rountos T. Adverse effects of androgen deprivation therapy for prostate cancer: prevention and management. ISRN Urol 2013;2013:240108.
- Walker LM, Robinson JW. A description of heterosexual couples' sexual adjustment to androgen deprivation therapy for prostate cancer. Psychooncology 2011;20:880-8. [Crossref] [PubMed]
- Walker LM, Robinson JW. Sexual adjustment to androgen deprivation therapy: struggles and strategies. Qual Health Res 2012;22:452-65. [Crossref] [PubMed]
- Tran S, Walker LM, Wassersug RJ, et al. What do Canadian uro-oncologists believe patients should know about androgen deprivation therapy? J Oncol Pharm Pract 2014;20:199-209. [Crossref] [PubMed]
- Elliott S, Latini DM, Walker LM, et al. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med 2010;7:2996-3010. [Crossref] [PubMed]
- Kaiser Family Foundation’s Global Health Facts. Data Source: World Bank, World Development Indicators 2015. The World Bank, International Bank for Reconstruction and Development. Available online: http://kff.org/global-indicator/gdp-per-capita
- Walker LM, Robinson JW. Androgen Deprivation Therapy: An Essential Guide for Prostate Cancer Patients and Their Loved Ones. New York: Demos Health, 2014.
- IBM SPSS Statistics for Windows, Version 21.0. IBM Corp. Released 2012. Armonk, NY: IBM Corp.
- Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40-55 years of age. Am J Epidemiol 2000;152:463-73. [Crossref] [PubMed]
- Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric 2007;10:197-214. [Crossref] [PubMed]
- Ajape AA, Babata A, Abiola OO. Knowledge of prostate cancer screening among native African urban population in Nigeria. Nig Q J Hosp Med 2010;20:94-6. [Crossref] [PubMed]
- Canadian Task Force on Preventive Health Care, Bell N, Connor Gorber S, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ 2014;186:1225-34. [Crossref] [PubMed]
- Kanavos P. The rising burden of cancer in the developing world. Ann Oncol 2006;17 Suppl 8:viii15-viii23.
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. [Crossref] [PubMed]
- Lee CE, Leslie WD, Lau YK. A pilot study of exercise in men with prostate cancer receiving androgen deprivation therapy. BMC Cancer 2012;12:103. [Crossref] [PubMed]
- Cormie P, Newton RU, Taaffe DR, et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis 2013;16:170-5. [PubMed]
- Gafni A, Charles C, Whelan T. The physician-patient encounter: the physician as a perfect agent for the patient versus the informed treatment decision-making model. Soc Sci Med 1998;47:347-54. [Crossref] [PubMed]
- Tariman JD, Berry DL, Cochrane B, et al. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol 2010;21:1145-51. [Crossref] [PubMed]
- Nilchaikovit T, Hill JM, Holland JC. The effects of culture on illness behavior and medical care. Asian and American differences. Gen Hosp Psychiatry 1993;15:41-50. [Crossref] [PubMed]
- Berger JT. Culture and ethnicity in clinical care. Arch Intern Med 1998;158:2085-90. [Crossref] [PubMed]
- Butow PN, Tattersall MH, Goldstein D. Communication with cancer patients in culturally diverse societies. Ann N Y Acad Sci 1997;809:317-29. [Crossref] [PubMed]
- Mitchell JL. Cross-cultural issues in the disclosure of cancer. Cancer Pract 1998;6:153-60. [Crossref] [PubMed]
- Ruhnke GW, Wilson SR, Akamatsu T, et al. Ethical decision making and patient autonomy: a comparison of physicians and patients in Japan and the United States. Chest 2000;118:1172-82. [Crossref] [PubMed]
- Trill MD, Holland J. Cross-cultural differences in the care of patients with cancer. A review. Gen Hosp Psychiatry 1993;15:21-30. [Crossref] [PubMed]
- Surbone A. Cultural aspects of communication in cancer care. Support Care Cancer 2008;16:235-40. [Crossref] [PubMed]
- Mystakidou K, Parpa E, Tsilila E, et al. Cancer information disclosure in different cultural contexts. Support Care Cancer 2004;12:147-54. [Crossref] [PubMed]
- Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin 2014;64:225-49. [Crossref] [PubMed]
- Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol 2015;33:1078-85. [Crossref] [PubMed]