Infections after transcatheter versus surgical aortic valve replacement: mid-term results of 200 consecutive patients
Introduction
Aortic stenosis (AS) is the most common heart valve disease in Europe and its incidence rises in elderly people (1). For decades, conventional surgical aortic valve replacement (SAVR) has been considered as the golden standard to treat patients with severe symptomatic AS (2). However, the increase in elderly patients along with multiple comorbidities increases the operative risk of AVR patients. Hence, at the end of the last century, great efforts have been made to decrease the operative trauma in surgical AVR, but in the same time Cribier and colleagues succeeded to implant the first percutaneous transcatheter aortic valve prosthesis in a high-risk patient (3).
In the subsequent years, transcatheter aortic valve implantation (TAVI) was widely adopted and became a popular option to treat high-risk AS patients (4-6). During the past 15 years, many studies and articles have been published comparing various outcomes of both procedures (7). Meanwhile, the valve academic research consortium (VARC) criteria have been published, defining some standard outcomes, which have to be reported (8).
Although, infectious complications after TAVI have not been widely and timely investigated, only few investigators reported such results (9-12), other investigators examined the inflammatory response after those procedures (13,14). Therefore, we aimed to examine infectious outcomes within the hospital stay, as well as the cumulative infections up to 2-year in a group of consecutive patients presenting with AS and undergoing either TAVI or conventional SAVR.
Methods
Study design
The present study was a prospective single-centre evaluation. The study was approved by the University-Hospital-Ethics-Committee (Ref# 15-6305-BO). Patients underwent either TAVI +/− percutaneous coronary intervention (PCI) or SAVR +/− coronary artery bypass grafting (CABG). The TAVI procedure was either performed by transapical, transfemoral or transaortic access. SAVR was usually performed though conventional full or partial sternotomy approach. Patients’ preoperative, operative and postoperative data were recorded in our institutional database. Infectious complications were reported postoperatively during hospital stay. Follow-up for infectious, as well as survival rates were recorded by active call protocol at 6, 12 and 24 months, and the 2-year results were 100% complete in April 2017.
Inclusion and exclusion criteria
In the period between June 2014 and April 2015, patients presenting with aortic valve pathology (severe stenosis or regurgitation or combined) with or without concomitant coronary heart diseases indicating surgical (i.e., CABG) or non-surgical (i.e., PCI and stenting) intervention were included in the present study. The cohort was divided in two groups SAVR (+/− CABG) or TAVI (+/− PCI), whereas each group consisted of 100 consecutive patients. Patients with severe mitral or tricuspid regurgitation necessitating intervention were excluded.
Definition of outcomes and endpoints
The study primary endpoint included all types of infectious complications, which were wound healing disorders, urinary tract as well as respiratory tract (e.g., pneumonia) infections, fever (>38.0 °C), endocarditis and sepsis. Secondary endpoints included, in addition to 30-day, 1 and 2-year mortality, in addition to the course of the main infective parameters [i.e., C-reactive protein (CRP), procalcitonin (PCT) and interleukin-6 (IL-6)]. Blood samples were collected preoperative, immediate postoperatively [on admission on the intensive care unit (ICU)], and every day morning during ICU and intermediate care unit (IMC) stay.
Definitions: management of infectious complication was mainly based on the clinical state of the patient and our institutional guidelines, i.e., in patient who develops recurrent fever (>38.5 °C) with elevation of infect parameters; a blood, urine, stool, sputum or broncho-alveolar lavage if patient was intubated would be sampled for culture and a chest X-ray and echocardiography were performed in addition to change of the current catheters (central venous line, arterial and urinary catheters) to define the source of infection. Urinary tract infections were diagnosed by positive urine culture in addition to clinical symptoms (e.g., dysuria), respiratory infection was defined as incidence of pneumonia within hospital stay and was diagnosed with clinical symptoms associated with elevation of the infect parameters and confirmed by infectious signs in the chest X-ray, post-discharge respiratory infection was included when diagnosed by a physician (e.g., family doctor) and confirmed with laboratory and chest X-ray examination, it may or may not indicate rehospitalisation, postoperative endocarditis was diagnosed according to the Duke’s criteria (15) and was confirmed by echocardiography (16), finally sepsis was diagnosed as incidence of systemic inflammatory response syndrome (SIRS) due to severe infectious status associated with positive evidence of bacteria in the bloodstream confirmed by blood culture, wound disorders was diagnosed when primary healing failed with or without incidence of wound infection (i.e., it was confirmed only if wound swap culture was positive). Finally, fever was involved if patient had recurrent hyperthermia >38.0 °C, but negative blood culture and exclusion of the above mentioned infectious endpoints, hence it reflects a state of non-infectious SIRS (17). Infectious related rehospitalisation was recorded for patients only if due to infectious cause (endocarditis, wound or respiratory infections).
Statistics
Statistical analyses were performed with the SPSS software (version 22.0, Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation (SD) or medians with interquartile ranges (IQRs), and categorical data were expressed as percentages or frequencies. The Kolmogorov-Smirnov test was used to check for the normality of distribution of the data in the two groups prior to final analysis. Differences between the two groups were compared with the use of a χ2 test or Fisher’s exact test (if the expected cell frequencies were <5) for categorical variables and the t-test or Wilcoxon rank-sum test for continuous variables. Kaplan-Meier survival curves were generated to estimate the survival function for patients in both groups for 2-year follow-up and differences were evaluated by the use of the log-rank test. All reported P values are two-sided and a value of P<0.05 was considered statistically significant. Finally, Excel 2016 software (version 16.0, Microsoft, Albuquerque, NM, USA) was used to create the boxplot diagrams showing the course each infect parameter.
Results
Patient population
A total of 200 consecutive patients presenting with AS between June 2014 and April 2015 were included. Patients underwent either TAVI (n=100) [either isolated (n=94) or combined with PCI (n=6)] or SAVR (n=100) [either isolated (n=52) or combined with CABG (n=48)]. TAVI procedure was transapical (n=50), transaortic (n=3), or transfemoral (n=47). TAVI patients were significantly older (mean age, 81±6 versus 69±11 years, P<0.001) and presented more comorbidity and therefore a higher EuroSCORE (23.1%±13.8% versus 8.7%±9.5%, P<0.001) than SAVR patients, respectively. Mild to moderate mitral regurgitation was present in (63% versus 58%, P=0.47) of the patients and mild to moderate tricuspid regurgitation was present in (50% versus 34%, P=0.02), respectively, which didn’t require intervention at the time of aortic valve procedure. Coronary heart disease was present in (62% versus 57%, P=0.47) of the patients, who indicated earlier PCI (35% versus 13%, P<0.001) or CABG (21% versus 1%, P<0.001), meanwhile, rest of the patients required either PCI or CABG simultaneous to the aortic valve procedure. Detailed baseline characteristics of all patients are summarized in Table 1.
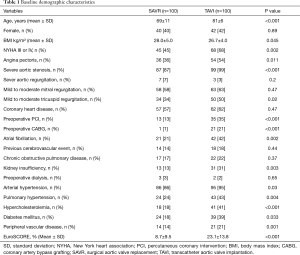
Full table
Postoperative outcomes
Table 2 summarizes early postoperative outcomes. SAVR patients had a longer operation time (236±71 versus 85±38 min, P<0.001), as well as intensive and IMC stay [4.4 (3–7.4) versus 3 (2–5.2) days, P=0.036]. Early infectious results showed no difference in TAVI versus SAVR patients in regard to the incidence of fever (5% versus 5%, P=1.0), pneumonia (14% versus 19%, P=0.45, most common pathogens Klebsiella pneumonia versus Staphylococcus aureus), urinary tract infections (4% versus 7%, P=0.54, most common pathogen: Escherichia coli in both groups), sepsis (5% versus 6%, P=1.0, most common pathogens Staphylococcus epidermidis versus Enterobacter cloacae) or endocarditis (0% versus 1%, P=1.0, pathogen caused endocarditis was Staphylococcus aureus), except for wound infections, which were significantly lower in the TAVI-treated patients (1% versus 8%, P=0.035, most common pathogen: Staphylococcus epidermidis in both groups), respectively. In addition, incidence of postoperative dialysis (8% versus 3%, P=0.12), re-intubation (6% versus 11%, P=0.09) and 30-day mortality (10% versus 4%, P=0.09) showed no difference between both groups, respectively.
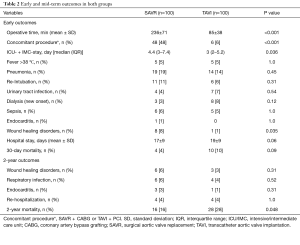
Full table
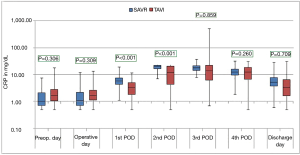
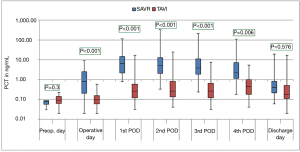
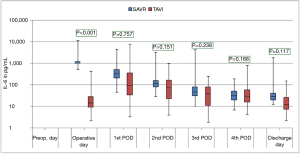
The courses of the laboratory infective parameters (CRP, PCT and IL-6 values) are illustrated as boxplot diagrams (Figures 1-3). Concerning the CRP-course, significant higher mean-values were observed on the first (P<0.001) and second (P<0.001) postoperative day in SAVR-patients, as well as the PCT values were significantly higher (P<0.001), however this significant difference was only present in the operative day between both groups regarding the mean value of IL-6 (P<0.001). Finally, a constant decrease in the mean values of all the infective parameters could be observed until discharge. To be noted that most common pathogens causing different infections in both group are summarized in Table 3.

Full table
Mid-term outcomes
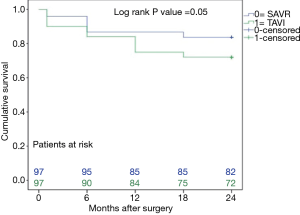
The mid-term results are represented by the collective 2-year follow-up, whereas no difference was found between the overall TAVI and SAVR patients during follow-up regarding infectious related rehospitalisation (4% versus 4%, P=1.0), as well as the incidence of wound healing disorders (3% versus 6%, P=0.31), respiratory infections (4% versus 6%, P=0.52) or endocarditis (1% versus 3%, P=0.31) as shown in Table 2. Finally, TAVI-group shows significant higher 2-year mortality (28% versus 16%, P=0.048) than SAVR group as illustrated with Kaplan-Meier curves in Figure 4. Additionally, further analysis has been applied dividing the cohort into four subgroups involving isolated SAVR (n=52), SAVR and CABG (n=48), transfemoral-TAVI (n=47) and transapical-TAVI in addition to transaortic-TAVI (n=53) subgroups whereas each result is reported in each corresponding subgroup as summarized in Table 4. Here, the transfemoral access appeared favourable regarding the incidence of pneumonia and wound healing disorders.
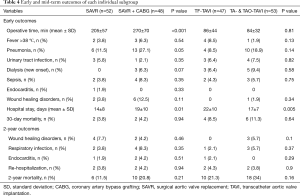
Full table
Discussion
The present study is evaluating the infectious complications of TAVI-procedure. The main findings of this study are: (I) postoperative pneumonia is the most common infectious complication in both groups; (II) logically, SAVR-group showed more postoperative wound healing disorders; (III) postoperative infect parameters (CRP, PCT & IL-6) showed significant higher levels in the SAVR-group early postoperatively, but decreased to normal levels within time; (IV) finally, TAVI-group reported significant higher mid-term mortality as expected.
Generally, patients undergoing cardiac surgery are usually at higher risk for postoperative infections, due to various factors, which involves the surgical incision, the stress situation of the patient, the presence of predisposing factors, especially advanced age, high body mass index (BMI) or diabetes mellitus (18,19), in addition to perioperative blood transfusion, prolonged anaesthesia time with endotracheal intubation and mechanical ventilation, as well as other factors such as central venous or urinary catheter placement, parenteral nutrition, and nosocomial infections during hospital stay. In the current study, the incidence of various infections in both groups was similar in regard to the incidence of urinary tract infections (4% versus 7%, P=0.54), endocarditis (1% versus 0%, P=1.0) or sepsis (6% versus 5%, P=1.0), apart from wound healing disorders, which was significant higher in the SAVR group (8% versus 1%, P=0.035). This could be attributed in addition to the more invasiveness of the surgical AVR to the higher BMI (28.0±5.0 versus 26.7±4.0, P=0.045) in this group. In earlier reports of Rodes-Cabau et al. (20), and Godino and colleagues (21), sepsis occurred in 2.9% and 8.4% of a TAVI treated cohort. This variety, however, might be attributed to the number of patients included in each study, and not to the procedure itself, taken in consideration that the TAVI population are usually older in age and has more risk factors i.e., higher EuroSCORE.
In the present analysis, pneumonia represented the incidence of postoperative respiratory infections within hospital stay, which were the most common cause for infections, with a slight non-significant higher incidence in the SAVR than the TAVI group (19% versus 14%, P=0.45). The main explanation of this result could be attributed to the endotracheal intubation and mechanical ventilation (22), with the risk of nosocomial respiratory infections, in addition to the instability of the thoracic cage after surgery this proves the highest incidence of pneumonia in those patients undergoing concomitant SAVR and CABG as reported in Table 4. Patients in that group were intubated for longer time and have a complete sternotomy for surgery whereas pain and improper coughing advocates respiratory infections, in comparison to those who undergo isolated SAVR where they were shorter intubated and have a mini-sternotomy allowing stability of thoracic cage, resulting in less respiratory infections as reported in earlier studies (23,24). On the other hand, patients undergoing TF-TAVI develop the least incidence of pneumonia (4 patients), in comparison with the other TAVI accesses. In our institute TF-TAVI is routinely performed without intubation i.e., under sedition with local anaesthesia (only 6 out of the 47 TF-TAVI patients were intubated during procedure), even though only one out of the four patients who develop pneumonia was intubated during procedure. Based on that, one could speculate that the less invasiveness in isolated SAVR or TF-TAVI reduces the risk of postoperative pneumonia. Furthermore, it is expected that not only the incidence of pneumonia but also the incidence of urinary tract infections will continuously decrease, especially in the transfemoral TAVI cohort, due to recent implementation of a so-called minimalistic TF-TAVI approach, which not only avoids general anaesthesia and intubation but also insertion of a urinary catheter for the procedure. Even with the higher renal insufficiency and dialysis (8% versus 3%, P=0.12) in the overall TAVI group in comparison to the SAVR group, which most related to contrast media injection during procedure (25).
In the current study, three infect markers (CRP, PCT & IL-6) have been analysed to describe the peri-procedural inflammatory response for patients undergoing SAVR and TAVI. Those parameters are basically the main infect parameters, which are considered in our institutional guidelines as a reflection of various infections. Blood samples were collected preoperatively, immediate postoperatively (on admission to the ICU), every day morning during ICU stay and at discharge. Comparing the different procedures, the peri-procedural inflammatory response differed significantly depending on the type of procedure, which showed significant higher levels in the SAVR-group early postoperatively, but the values decreased over time to normal levels until discharge. The main explanation of this finding is the presence or absence of cardiopulmonary bypass activating the inflammatory pathway (26), which is a known fact that cardiac surgery with cardiopulmonary bypass activates a systemic inflammatory response that is associated with adverse outcomes (27). These results are similar to the results of Erdoes et al. who reported a significant activation of various inflammatory pathways in invasive procedures (e.g., SAVR) in comparison to less invasive procedures (e.g., TAVI) (14). However, in addition to the amount of inflammation, a persistent elevated inflammatory marker seems to have a negative association with outcomes including mortality (17). In agreement with earlier reports, we also observed a peak level of CRP after TAVI on the third postoperative day (14,28). In comparison to CRP and PCT, IL-6 showed a significant difference on the immediate postoperative course with significant higher values in the SAVR-group. This significance disappeared, however, directly after the first postoperative day. IL-6 is another marker of inflammatory response, and its release is closely associated with tissue trauma (29). However, cardiac myocytes have been shown to be the major source of IL-6 synthesis (30).
Finally, our study shows a non-significant difference of all-cause 30-day mortality between TAVI and SAVR groups (10% versus 4%, P=0.09), but the difference between survivals reached statistical significance at 2-year follow-up (28% versus 16%, P=0.048) as shown in the Kaplan-Meier survival curves. These results are in accordance with others from Triado-Conte et al., who reported a significantly higher 2-year mortality in a TAVI-treated group with in-hospital infections in comparison to those without infections (44% versus 21% P=0.001) (11). Moreover, significant higher 1-year mortality in TAVI-treated patients was reported in a recent study (9), whereas they speculated that infectious complications might impact the outcomes of such critically ill patients. Opposite to that, Smith et al. reported a non-significant slightly higher 1 year mortality in high-risk SAVR patients (26.8% versus 24.2%, P=0.44) (6). Our findings could be expected, as patients in the TAVI group were older and presented with higher risk factors and EuroSCORE, in addition to that, mortality was defined due to all cause, and not only the cardiac ones.
Study limitations
Our study was performed at a single centre with a relatively small cohort; however, it represents one of the first that investigates infectious results in TAVI patient group in a comprehensive manner. As expected, there was difference in the baseline characters between both groups, for instance the SAVR group included 48% of patients having concomitant CABG, whereas the TAVI group included 6% of patients having concomitant PCI and stenting, this in turn increased the operative time hence it might affect the outcomes. Based on the nature of the study, being prospective, the long-term results of both groups of patients are still under investigation.
Conclusions
Infectious complications after TAVI are still enquiry. As expected, extracorporeal circulation is associated with elevation of infect markers though marked activation of the inflammatory pathway. Although, patients in the TAVI-group were at high-risk (older and presented higher EuroSCORE), no difference between TAVI and SAVR groups regarding infectious complications were reported. However, both groups are at risk for postoperative pneumonia, yet the transfemoral TAVI access appeared favourable regarding the incidence of pneumonia. Finally, SAVR group show more wound healing disorders but less mortality than the overall TAVI group.
Acknowledgements
The authors are sincerely grateful to Andreas Sander and Wolfgang Ristau (Institute of Quality Controlling, West German Heart and Vascular Centre Essen) for their generous effort and support in data review to finish this work.
Footnote
Conflicts of Interest: This work was presented at the 65th annual meeting of the European Society of Cardiovascular Surgery, April 2016, Belgrade, Serbia.
Ethical Statement: The study was approved by the University-Hospital-Ethics-Committee (Ref# 15-6305-BO).
References
- Vahanian A, Alfieri OR, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2008;34:1-8. [Crossref] [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1-148. [Crossref] [PubMed]
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755-63. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Castrodeza J, Amat-Santos IJ, Blanco M, et al. Propensity score matched comparison of transcatheter aortic valve implantation versus conventional surgery in intermediate and low risk aortic stenosis patients: A hint of real-world. Cardiol J 2016;23:541-51. [PubMed]
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. EuroIntervention 2012;8:782-95. [Crossref] [PubMed]
- Falcone M, Russo A, Mancone M, et al. Early, intermediate and late infectious complications after transcatheter or surgical aortic-valve replacement: a prospective cohort study. Clin Microbiol Infect 2014;20:758-63. [Crossref] [PubMed]
- van der Boon RM, Nuis RJ, Benitez LM, et al. Frequency, determinants and prognostic implications of infectious complications after transcatheter aortic valve implantation. Am J Cardiol 2013;112:104-10. [Crossref] [PubMed]
- Tirado-Conte G, Freitas-Ferraz AB, Nombela-Franco L, et al. Incidence, Causes, and Impact of In-Hospital Infections After Transcatheter Aortic Valve Implantation. Am J Cardiol 2016;118:403-9. [Crossref] [PubMed]
- Onsea K, Agostoni P, Voskuil M, et al. Infective complications after transcatheter aortic valve implantation: results from a single centre. Neth Heart J 2012;20:360-4. [Crossref] [PubMed]
- Ruparelia N, Panoulas VF, Frame A, et al. Impact of clinical and procedural factors upon C reactive protein dynamics following transcatheter aortic valve implantation. World J Cardiol 2016;8:425-31. [Crossref] [PubMed]
- Erdoes G, Lippuner C, Kocsis I, et al. Technical Approach Determines Inflammatory Response after Surgical and Transcatheter Aortic Valve Replacement. PLoS One 2015;10. [Crossref] [PubMed]
- Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994;96:200-9. [Crossref] [PubMed]
- Heinle S, Wilderman N, Harrison JK, et al. Value of transthoracic echocardiography in predicting embolic events in active infective endocarditis. Duke Endocarditis Service. Am J Cardiol 1994;74:799-801. [Crossref] [PubMed]
- Sinning JM, Scheer AC, Adenauer V, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J 2012;33:1459-68. [Crossref] [PubMed]
- De Marinis L, Folli G, D'Amico C, et al. Differential effects of feeding on the ultradian variation of the growth hormone (GH) response to GH-releasing hormone in normal subjects and patients with obesity and anorexia nervosa. J Clin Endocrinol Metab 1988;66:598-604. [Crossref] [PubMed]
- Gadaleta D, Risucci DA, Nelson RL, et al. Effects of morbid obesity and diabetes mellitus on risk of coronary artery bypass grafting. Am J Cardiol 1992;70:1613-4. [Crossref] [PubMed]
- Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90. [Crossref] [PubMed]
- Godino C, Maisano F, Montorfano M, et al. Outcomes after transcatheter aortic valve implantation with both Edwards-SAPIEN and CoreValve devices in a single center: the Milan experience. JACC Cardiovasc Interv 2010;3:1110-21. [Crossref] [PubMed]
- Hortal J, Munoz P, Cuerpo G, et al. Ventilator-associated pneumonia in patients undergoing major heart surgery: an incidence study in Europe. Crit Care 2009;13:R80. [Crossref] [PubMed]
- Shehada SE, Öztürk Ö, Wottke M, et al. Propensity score analysis of outcomes following minimal access versus conventional aortic valve replacement. Eur J Cardiothorac Surg 2016;49:464-9; discussion 469-70. [Crossref] [PubMed]
- Shehada SE, Elhmidi Y, Mourad F, et al. Minimal access versus conventional aortic valve replacement: a meta-analysis of propensity-matched studies. Interact Cardiovasc Thorac Surg 2017;25:624-32. [Crossref] [PubMed]
- Elhmidi Y, Bleiziffer S, Piazza N, et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J 2011;161:735-9. [Crossref] [PubMed]
- Wan IY, Arifi AA, Wan S, et al. Beating heart revascularization with or without cardiopulmonary bypass: evaluation of inflammatory response in a prospective randomized study. J Thorac Cardiovasc Surg 2004;127:1624-31. [Crossref] [PubMed]
- Cremer J, Martin M, Redl H, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 1996;61:1714-20. [Crossref] [PubMed]
- Krumsdorf U, Chorianopoulos E, Pleger ST, et al. C-reactive protein kinetics and its prognostic value after transfemoral aortic valve implantation. J Invasive Cardiol 2012;24:282-6. [PubMed]
- Cruickshank AM, Fraser WD, Burns HJ, et al. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990;79:161-5. [Crossref] [PubMed]
- Kukielka GL, Smith CW, Manning AM, et al. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation 1995;92:1866-75. [Crossref] [PubMed]


