Clinical perspective of optical coherence tomography and intravascular ultrasound in STEMI patients
In the last years, the intravascular ultrasound study (IVUS) and further the optical coherence tomography (OCT) became two helpful tools to characterize of the atherosclerotic plaque.
These new technologies made possible to analyse in vivo the pathophysiologic mechanisms that previously were just speculated or observed post-mortem (1). Recently Dr. Higum and Prof. Jang published an interesting article named “A combined OCT and IVUS on plaque rupture, plaque erosion and calcified nodule in patients with STEMI”, useful to describe the different presentation of culprit lesions in ST-elevation myocardial infarction (2).
In this paper the authors describe the findings about 112 STEMI patients who underwent to OCT and IVUS. Incidence of plaque rupture (PR) was 64.3%, plaque erosion (PE) 26.8% and calcified nodule (CN) 8%. The highlight hallmarks of PR were a higher lipid content inside the plaque, major thin-cap fibroatheroma (TCFA) and more numerous microchannels, with a trend toward a positive remodelling of plaque. PE showed less “vulnerable” morphology of plaque because of lower degree of TCFA, lipid content of plaque and microchannels. The structure of lesion with PE was more eccentric than PR and this was observed better through IVUS rather than OCT. CN lesions demonstrated higher amount of calcium compared to the other lesions, arranged like a “calcium sheet” along with negative remodelling of plaque. After primary Percutaneous Coronary Intervention (PCI) PR was associated with higher rate of myocardial blush grade ≤1 and consequently with a larger incidence of no reflow because of elevated thrombogenic burden enhancing in situ-thrombosis and distal embolization, confirmed by the higher creatinine kinase (CK) peak in PR lesions respect to the others kinds.
The population was rather homogeneous, apart from difference in ages. Patients with CN were of older age with a larger significative incidence of diabetes mellitus, that was a factor causing increased degree of vessel calcification as already shown in different setting of patients (3). Unfortunately, the incidence of another factor of progressive and widespread calcification like chronic kidney disease (CKD) wasn’t reported.
Patients with culprit lesions characterized by PE were younger that those with PR, without relationship with gender. However, OCT and IVUS have showed some discrepancy due to its unclear definition and morphological criteria, so much that it in this study was just considered as a diagnosis of exclusion (4).
The results of this study confirmed the prevalence of PR in patients with STEMI and the elevated incidence of TCFA as risk factor of evolution toward myocardial infarction. A meta-analysis recently published by of our group (5) including 23 studies and 2,711 culprit lesions attested that at the observation through OCT the presence of PR and TCFA at 70.4% and 76.6% respectively, in STEMI patients (Figure 1). On the other side, in the others subsets of coronary artery disease the incidence of both these parameters resulted to be less important (NSTEMI 55.6% and 56.3%, UA 39.1% and 52.9%, and SAP 6.2% and 22.8%, respectively). Also in the evaluation of PR OCT and IVUS showed some discrepancy, a dated study with a lower number of patients (30 people) reported as cause of infarct in the 73% the disruption of fibrous cap evaluated by OCT, while 43% by IVUS.
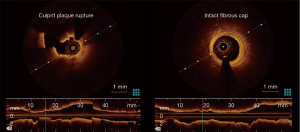
An interesting finding in the report by Dr. Higuma et al., and confirmed by our paper, is that clinical parameters seems not to be correlated with the presence of PR. This could be caused by limited sample size, limiting chances to reach statistical significance. Secondly classical cardiovascular risk factors such as hypertension, diabetes and age are surly correlated with atherosclerosis progression, so to the plaque burden, however plaque rupture itself mechanisms are not completely understood. It could be speculated that plaque rupture is a “stochastic” event determined by a “perfect storm” whom drops are the atherosclerotic burden, a vulnerable plaque, the sympathetic nervous system and the inflammation.
Surely this kind of study was very useful to describe pathological morphology of plaque, that just over a decade ago was a mirage. Despite both IVUS, and in particular OCT, raised interest in particular subsets of situations such as stent thrombosis (6), correct evaluation of stenosis diameter and stent’s struts apposition (7) or differential diagnosis in underestimated cause of acute coronary syndrome (such as coronary embolisms) (8), an important restriction of their use remains the costs and the unclear clinical impact of these technologies in common practice.
Another interesting study about OCT in STEMI was OCTAVIA trial (9), which performed OCT on the culprit lesion during acute events and after follow-up of 9 months the coronary angiography was remade with the addition of evaluation through OCT. The population was divided according the presence of ruptured fibrous cap (RFC) and those with intact fibrous cap (IFC). They report similar ruptured cap rate compared with the study by Dr. Higuma.
Autoptical studies endorsed the plaque rupture incidence approximately at 75% (10,11). A higher prevalence of plaque rupture is comprehensible, as demonstrated by Dr. Higuma plaque rupture is correlated with an impaired myocardial blush and slow flow leading to a worse acute prognosis of these patients and probably more often to death.
The introduction of OCT made a remarkable contribution, because it improved the quality of plaque evaluation, performing the display in vivo intracoronary thrombus, plaque ruptures and erosion, mostly TCFA minor than 65 µm (12).
IVUS and mostly OCT demonstrated to be very close to the classic anatomicopathological description, certainly they corroborated the theories on autoptical field about the morphology of plaque; however the clinical impact of these data on treatment is unclear. A spot of controversy was concentred to differentiate the type of therapy in patients with STEMI on the basis of plaque’s features, attempting a more custom intervention. Observing the pathophysiology knowledge’s the presence of ruptured plaque should prompt for an aspiration procedure, followed by implantation of drug eluting stents and an aggressive medical therapy (in particular statins and antiplatelet therapy). While performing thrombus aspiration in case of plaque with IFC could be useless or even dangerous, while performing antiplatelet therapy beyond the time necessary for the stent endothelialization could be not necessary.
Nevertheless, OCTAVIA trial showed similar healing response to follow-up of nine months for plaque with RFC or IFC, probably for local effect of aggressive antiplatelet therapy and also of stenting, shortening the hopes for a custom therapeutic address.
Indeed, the time to pass from the pure scientific speculation through the clinical application has come, further clinical trial in this sense are needed to reach a per-patients tailored therapy in order, as told by the Ulysses of Lord Tennyson “to strive, to seek, to find”.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Yue Liu (Associate professor, Department of Cardiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- D'Ascenzo F, Agostoni P, Abbate A, et al. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: a meta-regression of randomized clinical trials. Atherosclerosis 2013;226:178-85. [Crossref] [PubMed]
- Higuma T, Soeda T, Abe N, et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc Interv 2015;8:1166-76. [Crossref] [PubMed]
- D'Ascenzo F, Cerrato E, Calcagno A, et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis 2015;240:197-204. [Crossref] [PubMed]
- Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol 2007;50:933-9. [Crossref] [PubMed]
- Iannaccone M, Quadri G, Taha S, et al. Prevalence and predictors of culprit plaque rupture at OCT in patients with coronary artery disease: a meta-analysis. Eur Heart J Cardiovasc Imaging 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Iannaccone M, D'Ascenzo F, Montefusco A, et al. The butler did it! A very late stent thrombosis of TAXUS evaluated with Optical Coherence Tomography. Int J Cardiol 2015;187:141-3. [Crossref] [PubMed]
- Iannaccone M, D’Ascenzo F, Templin C, et al. Optical Coherence Tomography Evaluation of Intermediate-Term Healing of Different Stent Types: Systemic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2016. [Epub ahead of print].
- Iannaccone M, Montefusco A, Omede' P, et al. All that glitters ain't gold! A case of embolic STEMI demonstrated by OCT. Int J Cardiol 2015;196:14-5. [Crossref] [PubMed]
- Saia F, Komukai K, Capodanno D, et al. Eroded Versus Ruptured Plaques at the Culprit Site of STEMI: In Vivo Pathophysiological Features and Response to Primary PCI. JACC Cardiovasc Imaging 2015;8:566-75. [Crossref] [PubMed]
- Cheruvu PK, Finn AV, Gardner C, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol 2007;50:940-9. [Crossref] [PubMed]
- Falk E, Nakano M, Bentzon JF, et al. Update on acute coronary syndromes: the pathologists' view. Eur Heart J 2013;34:719-28. [Crossref] [PubMed]
- Prati F, Uemura S, Souteyrand G, et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovasc Imaging 2013;6:283-7. [Crossref] [PubMed]


