Surgical aortic valve replacement (SAVR) has long been the standard of care for aortic valve replacement. More recently, transcatheter aortic valve replacement (TAVR) has been established as a safe, effective and less invasive method of valve replacement in patients with severe aortic stenosis who are at intermediate or high risk for complications related to SAVR.1–4 For patients who present with surgical bioprosthetic valve (BPV) degeneration, reoperation may be associated with increased risks.5,6 Valve-in-valve (VIV) TAVR has emerged as a safe and effective therapy for such patients.7,8 The US Food and Drug Administration has approved VIV TAVR with both self-expanding and balloon-expandable prostheses for patients with failed BPVs who are at high risk for complications related to reoperation.
Although VIV TAVR is a promising alternative to repeat SAVR, patient–prosthesis mismatch (PPM) is a concern. Broadly, PPM is defined as any situation in which the effective orifice area (EOA) of a prosthetic valve is smaller than the orifice of the patients’ native aortic valve; severe aortic PPM is defined as an indexed EOA ≤0.65 cm2/m2.9 Patients who undergo VIV TAVR are particularly at risk for PPM because the TAVR prosthesis is implanted within the frame of the previous BPV, thereby reducing the maximum EOA that can be achieved with the new valve. Importantly, severe PPM and small labelled BPV size (≤21 mm) have been associated with higher mortality following VIV TAVR.10–12 In the VIVID Registry, which is the largest series of VIV TAVR published to date, the incidence of severe PPM following VIV TAVR was 31.8 %.7 Furthermore, patients in the same series with a small labelled surgical valve size (≤21 mm) had a reduced survival at 1 year (74.8 %) compared with patients with an intermediate (21–25 mm) sized BPV (81.8 %) or a large (≥25 mm) BPV (93.3 %), suggesting that PPM has a negative impact on survival following VIV TAVR.
Several strategies have been developed to avoid PPM following VIV TAVR. Use of a transcatheter heart valve (THV) with supra-annular leaflet positioning (e.g. CoreValve Evolut, Medtronic, Minneapolis, MN, USA) may result in a larger EOA due to the supra-annular position of the prosthetic leaflets, and a higher THV implant depth may improve inflow dynamics and augment the effective EOA.13–17 Recently, several publications have reported on the concept of fracturing the surgical BPV ring with a high-pressure balloon inflation in order to dilate the BPV and permit further expansion of the THV,18–20 improving haemodynamic results in such patients.21–24 This review summarises our current knowledge of BPV fracture (BVF) to facilitate VIV TAVR, including information obtained from bench testing, procedural techniques, early clinical experience and future directions.
Bench Testing
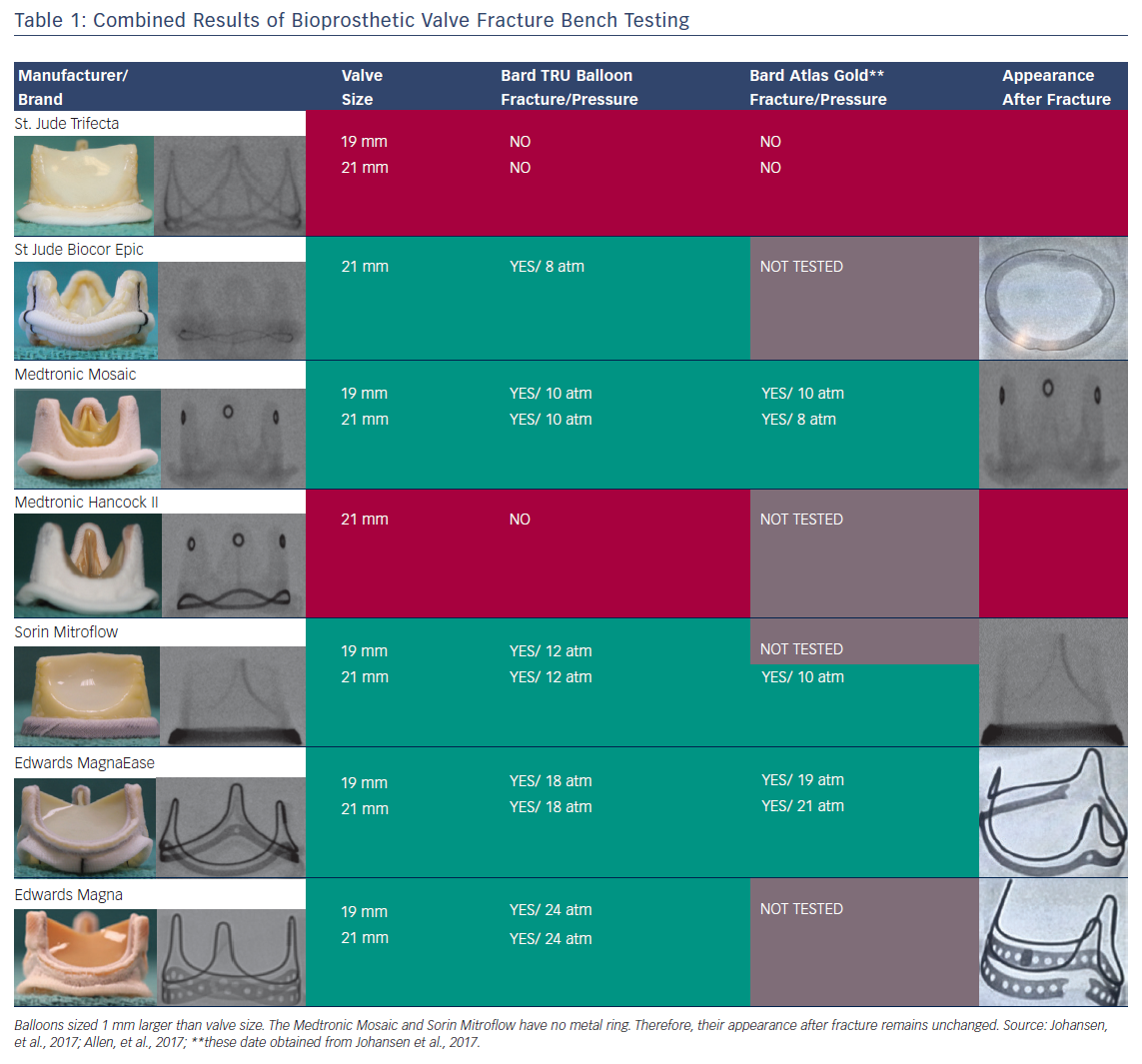
To date, there have been two published series regarding the tolerance of commercially available BPVs to high-pressure balloon inflation using non-compliant valvuloplasty balloons. Results of BVF in Trifecta (St Jude, Minneapolis, MN, USA), Mitroflow (Sorin, Milan, Italy), Magna Ease (Edwards Lifesciences, Irvine, CA, USA), Mosaic (Medtronic), Magna (Edwards Lifesciences), Hancock II (Medtronic) and Biocor Epic (St Jude) valves using Atlas Gold (Bard, Tempe, AZ, USA) and True Balloons (Bard) have been reported.21,24
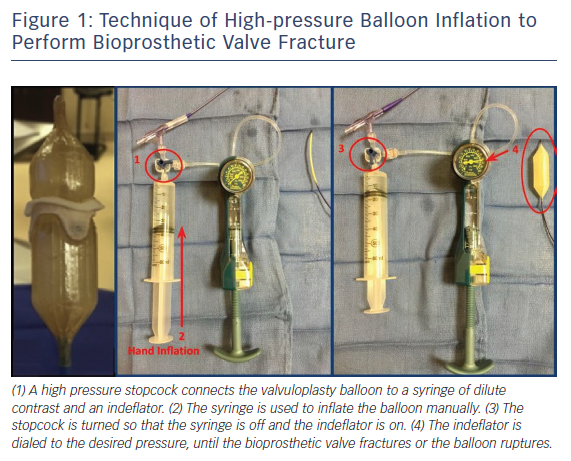
The procedural technique for BVF bench testing is displayed in Figure 1. Non-compliant balloons were positioned within small BPVs (i.e. labelled valve size 19 or 21 mm). Valvuloplasty balloons were sized 1 mm larger than the labelled surgical valve size. A high-pressure stopcock was used to separately attach a syringe and an indeflator to the balloon. With the stopcock open to the syringe, an initial hand inflation was performed to rapidly inflate the balloon, then the stopcock was opened to the indeflator, and the pressure was gradually increased in the balloon system until the BPV ring fractured. Fracture of the BPV ring was typically associated with a sudden decrease in inflation pressure, visible release of the balloon waist, and/or an audible “click”. BVF was considered unsuccessful if the balloon ruptured without evidence of valve fracture. The fracture threshold was reported as the lowest inflation pressure necessary to cause BPV ring fracture. Following BVF, the fractured valves were inspected for protruding elements or other potentially harmful results related to BPV disruption.21,24
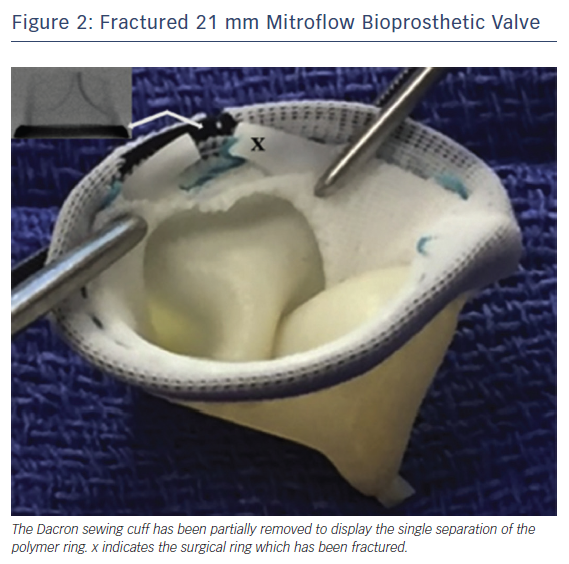
The findings of systematic bench testing are summarised in Table 1. BPVs that could be consistently fractured in both series were Magna, Magna Ease, Mitroflow, Mosaic and Biocor Epic. Upon dissection of the sewing cuff, the fractured elements were directly visualised to confirm complete separation of the ring element (Figure 2).21,24 Hancock II valves could not be fractured in either series. BVF of 19 mm Trifecta valves was also unsuccessful in both series.21 A partial fracture of the 21 mm Trifecta valve (separation of the lower of two parallel rings of the frame) was noted by both groups. However, this partial fracture only occurred at very high inflation pressure (26 atm) or after the use of serial balloon inflations with increasing balloon diameter. Neither authors recommended BVF in Trifecta valves.
The minimum inflation pressures necessary to fracture similar valves were slightly different in the two series (Table 1). In general terms, BPVs with an alloyed metal ribbon ring (Magna and Magna Ease) demonstrated a higher fracture threshold (18–24 atm) than BPVs with a polymer ring (Biocor Epic, Mosaic, Mitroflow; 8–12 atm). Different techniques were used to measure inflation pressure at the moment of fracture in the two series, thus the small differences in observed fracture thresholds were not unexpected. In practical terms, precise knowledge of the fracture threshold may not be necessary. Rather, when a clinical case of BVF is planned, the most critical information is the knowledge that a particular type of valve can be fractured, and the approximate inflation pressure that will result in fracture. Therefore, the largely concordant findings between the two series serves as an excellent guide for operators when planning a BVF procedure.
Indications
At present, the indications to perform BVF to facilitate VIV TAVR are not fully defined. The majority of patients, in particular those with large surgical valves, are likely to achieve an adequate haemodynamic result with VIV TAVR, and patients without PPM following VIV TAVR have an excellent survival to 1 year.7,8 Therefore, patients who stand to benefit the most from BVF are those who are predisposed to PPM and high residual transvalvular gradients following VIV TAVR, including those with small BPVs (labelled valve size ≤21 mm) and/or stenosis as the mechanism of BPV failure.7,8 Whether patients with large BPVs (>21 mm labelled valve size) or intermediate transvalvular gradients (10–20 mmHg) after VIV TAVR stand to benefit from BVF is not known.
Clinical Application
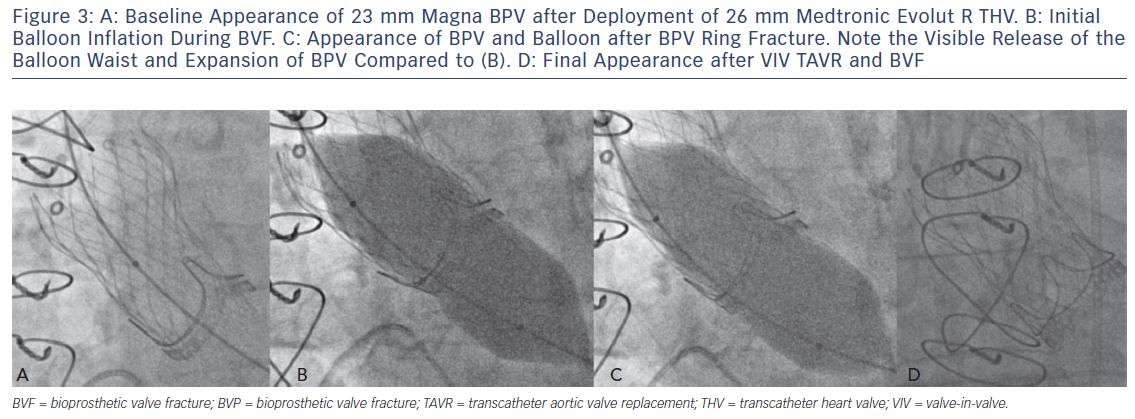
Translating the ex vivo BVF technique to a clinical setting is intuitive (Figure 2). During a case of VIV TAVR, a non-compliant valvuloplasty balloon, such as those used in bench testing, is positioned across the BPV ring (Figure 3a) over a stiff wire. At that point, the procedural technique is the same as depicted in Figure 1b,c. During rapid ventricular pacing, an initial hand inflation is used to fill the balloon with dilute contrast, then a coronary indeflator is used to increase the inflation pressure to the threshold for valve fracture. BPV fracture is accompanied by the same auditory, visual and haptic feedback as is observed during bench testing: a sudden drop in inflation pressure, a visible release of the balloon waist (Figure 3b,c) and/or an audible “click”. The valvuloplasty balloon is then deflated and removed. Figure 3d depicts the final result from the same clinical case, with a dramatic increase in expansion of both the TAVR prosthesis and BPV ring. Of note, given the prolonged nature of the pacing run that is often required, we prefer to perform all BVF procedures under general anesthesia to minimise any temporary neurologic sequelae.
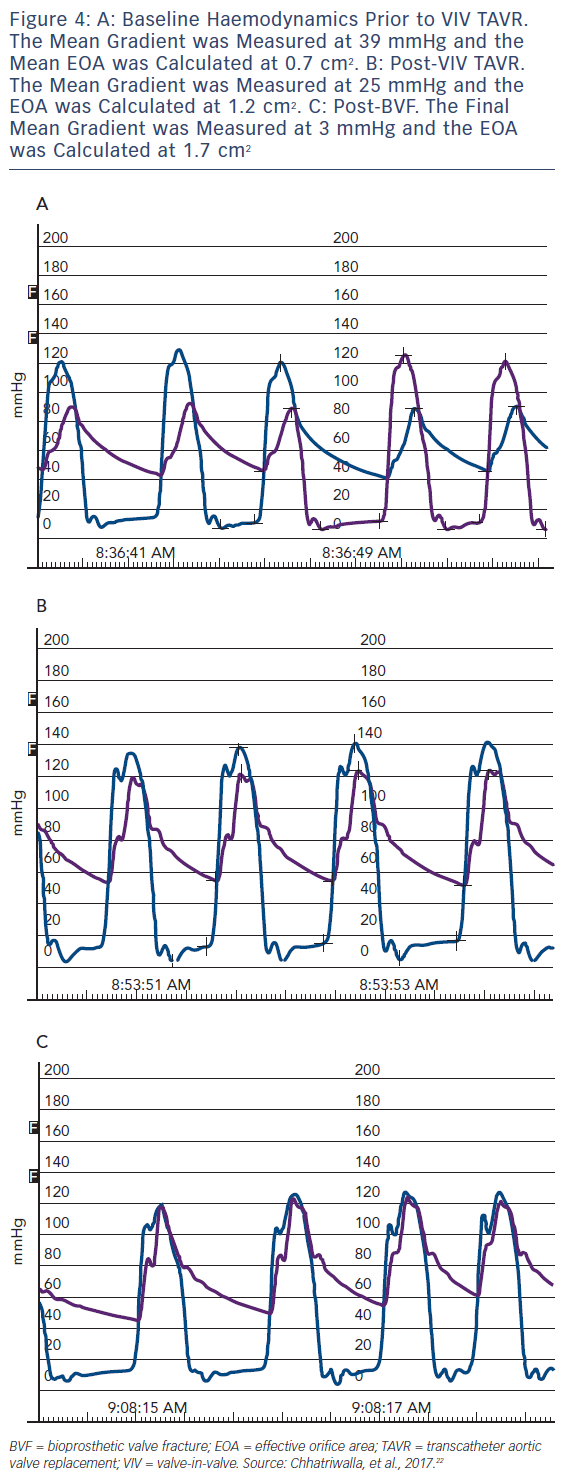
An example of the haemodynamic result of a clinical case of BVF with VIV TAVR is depicted in Figure 3. A 76-year-old man with a history of combined coronary artery bypass grafting (CABG) and SAVR, with a 23 mm Mosaic BPV 10 years prior, presented with symptoms of class III diastolic heart failure and severe BPV stenosis, with a mean transvalvular gradient of 49 mmHg, a peak Doppler velocity of 4.7 m/s and dimensionless index of 0.25. The Society of Thoracic Surgeons (STS) predicted risk of mortality was 8.2 %, and after a Heart Team evaluation, VIV TAVR was recommended. Baseline invasive haemodynamics (Figure 3a) demonstrated a mean gradient of 36 mmHg with a calculated valve EOA of 0.7 cm2. After deployment of a 26 mm CoreValve Evolut R self-expanding TAVR prosthesis, the mean gradient was reduced to 25 mmHg, with a corresponding EOA of 1.2 cm2 (Figure 3b). BVF was then performed with a 24 mm True Balloon, and the bioprosthetic ring fractured at 10 atm. Final haemodynamics demonstrated a mean gradient of 3 mmHg and an EOA of 1.7 cm2 (Figure 3c). At 1 month follow up, the patient was doing well, with New York Heart Association class 1 functional status. An echocardiogram at that time demonstrated a mean transvalvular gradient of 8 mmHg, with a peak Doppler velocity of 2.0 m/s and a dimensionless index of 0.68.
Clinical Experience
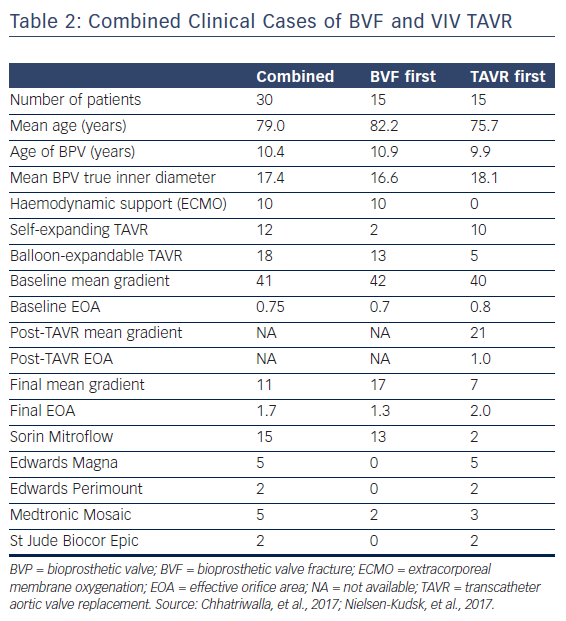
The procedural and haemodynamic results of patients who have been treated with VIV TAVR and BVF in two published case series are displayed in Table 2.22,23 A total of 30 patients with a mean age of 79.0 years were treated with VIV TAVR for failed BPVs. The majority of cases were performed to treat BPV stenosis, with a mean time from SAVR implant to VIV TAVR of 10.4 years. Fifteen patients were treated with TAVR prior to BVF, and 15 patients were treated with BVF followed by TAVR implant. There were no reports of perioperative death, coronary artery obstruction, annular rupture, aortic root injury, paravalvular leak or pericardial effusion. Two patients suffered small periprocedural strokes that were confirmed with MRI, and both patients later recovered full neurological function.22,23 Ten cases were performed with backup haemodynamic support with extracorporeal membrane oxygenation (ECMO), and 100 % long-term survival was noted in one case series (n=10), with a mean follow up of 438 days.23
The procedural results reported in these two case series highlight the haemodynamic benefit of BVF (Table 2). For the combined cohort, the mean gradient was reduced from 41 mmHg pre-procedure to 11 mmHg after BVF and VIV TAVR, which corresponds to an improvement in EOA from 0.75 to 1.7 cm2. In one series, most of the patients (15/20) were treated with BVF after VIV TAVR was performed. For this subset of patients, the mean pre-procedural gradient was reduced from 41.9 to 20.5 mmHg after VIV TAVR, and the mean gradient was further reduced from 20.5 to 6.7 mmHg following BVF. This corresponds to mean EOAs of 0.6, 1.0 and 1.7 cm2, respectively. The benefit of BVF to improve the procedural results of VIV TAVR is evident: with VIV TAVR alone, these patients would have been left with a suboptimal EOA of 1.0 cm2 and a final mean gradient of 20.5 mmHg.
Complications
Although the haemodynamic benefit of BVF is clear, the incremental risk posed by BVF in addition to VIV TAVR is not fully known. In the two clinical series published to date (total n=30), complications were few. Two patients suffered a small periprocedural stroke, with complete resolution of neurological deficits. One patient suffered complete atrioventricular block requiring a permanent pacemaker.22,23 However, stroke and heart block are potential complications of TAVR even in the absence of BVF. Despite the relatively low complication rate in the published series and unpublished clinical experience, there are some theoretical risks specific to the BVF procedure that must be considered. Although there were no incidents of aortic root disruption, paravalvular leak, aortic insufficiency or coronary occlusion in the published case series, it is important to acknowledge that the clinical experience with BVF is still early. There is still much to learn about the specific clinical and anatomic features that predispose patients to complications.
Coronary Artery Obstruction
As is true of VIV TAVR, one of the major concerns with BVF is the potential for coronary artery obstruction. A recent registry of VIV TAVR reported an incidence of coronary artery obstruction of 3.5 % with VIV TAVR,25 which appears to be decreasing as experience with VIV TAVR grows.26 There are several risk factors for coronary obstruction during VIV TAVR: narrow coronary sinuses, low coronary artery ostia, bulky bioprosthetic leaflets, reimplanted coronary arteries and type of BPV, i.e. those with leaflets mounted external to the stent frame (Mitroflow, Trifecta).26 Whereas the typical concern with native valve TAVR is coronary ostial height in relation to the native aortic annulus, during VIV TAVR the most important factor is the anticipated distance between the coronary ostia and the final position of the BPV leaflets.26 This relationship can be assessed during pre-procedural coronary angiography as well as by computed tomography, wherein a “virtual THV” can be superimposed on the CT images to determine the relationship between the BPV leaflets and the coronary arteries.26 A virtual THV to coronary distance of less than 3 mm is considered to place a patient at high risk for coronary occlusion.26
With BVF, the architecture of the BPV is altered such that the final position of the BPV leaflets is less certain. In bench testing, measurement of the BPV following BVF demonstrated a maximum gain in BPV diameter of 3–4 mm. Further expansion of the BPV is restricted by the Dacron sewing cuff, which remains intact after valve fracture.21 The additional space in the coronary sinuses necessary to accommodate BVF is not fully understood. Extrapolating from the recommended safety margins of VIV TAVR, it is reasonable to estimate that a BPV to coronary distance of less than 5 mm could be considered to place a patient at high risk for coronary occlusion when BVF is performed. To date, there are no published cases of coronary occlusion attributable to BVF. If there is pre-procedural concern for coronary occlusion, standard precautions are recommended, including wire protection of the coronary artery as deemed appropriate by the operators.
THV selection
Careful selection of the THV is an important part of the evaluation prior to BVF. Both self-expanding and balloon-expandable THVs are currently approved for use in VIV TAVR in the US. There are some data to suggest that self-expanding THVs result in superior procedural haemodynamics and increased EOA when used for VIV TAVR, compared with balloon-expandable valves,14,17 largely due to the supra-annular position of the prosthetic leaflets on the self-expanding frame. However, BVF can be successfully performed in the setting of VIV with both self-expanding and balloon-expandable THVs. There is some concern that the high-pressure balloon inflation used to perform BVF may cause structural damage to the self-expanding valve frame or leaflets, resulting in severe acute valvular regurgitation. This can largely be avoided by using a balloon smaller than the constrained segment of the self-expanding THV, and by positioning the BVF balloon such that the balloon shoulder is lower (i.e. more ventricular) than where the leaflets are anchored to the frame.21 However, care must be employed to ensure the valvuloplasty balloon and delivery wire are well-positioned in the ventricle as well.
In terms of TAVR prosthesis selection, BVF adds an extra element of pre-procedural consideration. Appropriate THV selection for VIV TAVR is guided by the true inner diameter of the BPV, rather than the labelled surgical valve size, as there can be considerable difference in these measurements between different valve models.27 BVF results in structural expansion of the BPV, changing the true inner diameter of the BPV considerably. Based on measurements made during bench testing, it appears that the maximum gain in diameter that can be achieved with BVF is between 3 and 4 mm.21 Thus, there are some situations in which a larger TAVR prosthesis may be appropriate for a BVF procedure than would be selected for a stand-alone VIV TAVR. For example, in a patient with a 21 mm Mitroflow, which has an inner diameter of 17 mm, BVF might result in expansion of the inner diameter to 20–21 mm. In this situation, it is not known whether a partially constrained 23 mm transcatheter valve would result in better haemodynamics than a fully expanded 20 mm transcatheter valve. In vitro testing has suggested that a larger prosthesis, even if expanded to a less than nominal diameter, may result in a more favourable transvalvular gradient.17 However, this concept has not been rigorously tested in clinical practice, and the interaction between TAVR prosthesis expansion and optimal haemodynamics after BVF is not fully understood.
Selection of Valvuloplasty Balloon
Both Atlas Gold and True Dilatation balloons are consistently able to fracture small surgical valves, both in bench testing (Table 1) and clinical experience.21–24 In bench testing and the majority of clinical cases, balloons sized 1 mm larger than the labelled valve size were utilised. However, during some clinical cases, smaller balloons (i.e. sized 1 mm larger than the true inner diameter of the BPV) were used successfully. It stands to reason that balloons need only be sized larger than the internal diameter of the BPV to fracture the valve, especially if a THV is already implanted in the BPV. Whether smaller balloons consistently fracture BPVs remains to be seen. Furthermore, it is not known if larger BPVs (i.e. >21 mm) will fracture consistently with valvuloplasty balloons sized 1 mm larger than the labelled BPV size, as the force exerted by larger balloons in larger prostheses may be somewhat different than in small valves, and this has not been systematically tested clinically or on the bench.
Timing of BVF
Whether BVF is optimally performed before or after implantation of the TAVR prosthesis is not known. There are theoretical advantages to both strategies. If BVF is performed first followed by TAVR implantation, this may allow for confirmation that the BPV can be fractured prior to TAVR implantation, which in theory may allow for selection of a larger TAVR prosthesis. However, bench testing and clinical experience have demonstrated consistent success in fracturing most BPVs, thus it does not appear to be necessary to ensure BVF is successful prior to THV implantation.
Some operators have preferred to perform BVF prior to VIV TAVR to avoid the theoretical concern of subclinical damage to the prosthetic leaflets during high-pressure balloon inflation, which might impact long-term THV durability. Performing BVF before VIV TAVR has the potential benefit of sparing the THV the high-pressure balloon inflation. However, it should also be noted that balloon-expandable THV leaflets are subjected to pressure at the time of crimping, and at the time of implantation regardless of whether BVF is performed. Furthermore, as noted above, in self-expanding THV valves, BVF should be performed by placing the balloon shoulder below the level of the supra-annular valve leaflets to avoid any possibility of leaflet injury. At this time there are no robust long-term data regarding THV durability after BVF. The 100 % long-term survival in one case series (n=10) is a promising sign that patients do well after BVF. However, haemodynamic and quality of life data are not available in this cohort.
It is also not known whether there are any differences between procedural outcomes of BVF performed before or after VIV TAVR. However, some data suggest that there may be some disadvantage to performing BVF first, especially when a balloon-expandable THV is used. In bench testing, if a balloon-expandable THV was implanted nominally within a fractured BPV using standard inflation, the compliant delivery balloon was not sufficient to fully expand the TAVR prosthesis, and a notable constraint remained after the TAVR valve was implanted.21 A final, high-pressure balloon inflation was necessary to fully expand the balloon-expandable TAVR prosthesis. Interestingly, when self-expanding TAVR valves were implanted within fractured BPVs, there appeared to be sufficient radial strength to re-expand the fractured BPV and achieve nominal deployment diameter, without a final high-pressure balloon inflation.21
Haemodynamic data may also support the strategy to perform VIV TAVR prior to BVF. In the currently published series (n=30), the final haemodynamics when VIV TAVR was performed first, followed by BVF, appear to be superior to the results when BVF was performed first (Table 2). Although patients treated with BVF first (n=15) had a similar pre-procedural gradient and EOA to those who underwent TAVR first (n=15), the final mean gradient and EOA in the BVF-first group were less favourable compared with the haemodynamics in the TAVR-first group (14 mmHg and 1.4 cm2 vs 7 mmHg and 1.7 cm2, respectively). However, this comparison might be confounded by differences in the valves that were fractured or the THV selected for VIV TAVR. In the BVF-first group, 13 of 15 patients (87 %) were treated with a balloon-expandable THV, with a nominal pressure implantation, and no high-pressure balloon inflation following the THV. These data suggest that a final high-pressure inflation following BVF with a balloon-expandable THV may be necessary to optimise THV expansion and procedural results. It is important to interpret these findings with caution, as the total number of patients in each group is small and the results are subject to confounding.
Longer-term Outcomes
There are some available data regarding haemodynamic durability at 1 month following BVF. In a series of 18 patients who underwent BVF prior to VIV TAVR, the baseline mean transvalvular gradient and EOA were 42.8±17.0 mmHg and 0.8±0.3 cm2, respectively, which improved to 8.1±3.6 mmHg and 1.96±0.58 cm2, respectively, after VIV TAVR and BVF. At 1 month, mean gradient and EOA by echocardiography were 12.7±5.0 mmHg and 1.64±0.3 cm2, respectively, which were not statistically different to the final procedural haemodynamic measurements. Long-term follow-up of these patients is ongoing.
In contrast to concerns that BVF may result in impaired TAVR durability, it is also possible that BVF actually improves durability, considering that a high residual gradient after VIV TAVR is associated with worse outcomes. Poor expansion of prosthetic leaflets has been associated with early BPV failure, as leaflet folding results in stress and strain points on the leaflets, which leads to leaflet calcification and fibrosis, and can accelerate BPV degeneration.28 In theory, by optimising THV expansion and reducing the transvalvular gradient, BVF might decrease leaflet stress and degeneration and improve long-term THV durability. If this is indeed the case, then all patients undergoing VIV TAVR might benefit from BVF, irrespective of the size of their BPV or the residual gradient following THV implantation. Ultimately, comparisons of long-term outcomes between patients who undergo BVF before or after VIV TAVR first will be important to understand the optimal BVF strategy.
Haemodynamic Support
Due to concern that BVF may result in aortic root injury or coronary artery obstruction, some operators have preferred to perform BVF only in the setting of full haemodynamic backup with ECMO (Table 2). To date, no published reports of aortic root injury or haemodynamic collapse attributable to BVF exist. As clinical experience with BVF has accumulated, it appears that full haemodynamic backup is not routinely necessary. We do not recommend routine use of ECMO during these cases, especially as ECMO requires additional further large-bore vascular access, further exposing the patient to potential vascular complications. However, in certain situations ECMO may be beneficial, such as a patient with very high risk for coronary occlusion with VIV TAVR and BVF.
Future Directions
The initial bench testing and clinical experience with BVF and VIV TAVR establishes a firm foundation for this procedure in patients with failed aortic BPVs. However, there are many unanswered questions relating to this novel technique. Whether BVF has an impact on the survival of patients who are at risk of PPM following VIV TAVR remains to be seen. Further data are needed as to the quality of life benefit that is gained from VIV TAVR with BVF compared with VIV TAVR alone. The feasibility of BVF in patients with larger BPVs (>21 mm labelled BPV size) has not been well studied, and whether this will improve the procedural results or outcomes for these patients is equally unknown. The safety margins for performing BVF in patients at risk of coronary obstruction and aortic root injury are not fully understood and warrant further study. Finally, it remains to be seen whether BVF can be performed safely and successfully, and with benefit, in conjunction with VIV procedures in the mitral, pulmonary or tricuspid positions. As our experience grows, the indications for and technique of BVF will undoubtedly continue to be refined.