-
PDF
- Split View
-
Views
-
Cite
Cite
Jessie Y.J. Liu, Remi Kowalski, Bryn Jones, Igor E. Konstantinov, Michael M.H. Cheung, Susan Donath, Christian P. Brizard, Yves d'Udekem, Moderately hypoplastic arches: do they reliably grow into adulthood after conventional coarctation repair?, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 4, April 2010, Pages 582–586, https://doi.org/10.1510/icvts.2009.223776
Close - Share Icon Share
Abstract
To determine whether conventional coarctation repair results in sustained growth of hypoplastic transverse arches, we examined the follow-up of 20 patients operated through a thoracotomy between 1990 and 1995 who had available serial echocardiographic examinations. Mean age at operation was 8.6±5.7 days. In the distal transverse arch, maximum change was observed in the early postoperative period and sustained growth was observed thereafter. At last follow-up, no patients had Z-scores of less than –2. In contrast, only minimal growth occurred in the proximal transverse arch (mean Z-score diameter before and after repair: –1.87±0.12 vs. –1.66±0.09; P=0.05) in the postoperative period. At last follow-up, seven patients (35%) kept a diameter Z-score of less than –2, and 5 of them had a gradient of 15 mmHg (P=0.01). No correlation was found between the size of the proximal arch at last follow-up and its size before repair or technique used. Conclusion: Patients with moderately hypoplastic arch treated by conventional coarctation repair have adequate growth of the distal arch demonstrated at long-term follow-up, but one-third of them keep a small proximal arch. This subset of patients is at risk of developing hypertension and may warrant further investigation.
1. Introduction
Up to 75% of the patients requiring a coarctation repair also have some degree of hypoplasia of the transverse arch [1–4]. While it is now agreed that the most severely hypoplastic arches should be repaired with a more extensive procedure performed on bypass via a sternotomy, the best approach for those presenting with moderate degree of transverse arch hypoplasia is still unclear. It has been advanced that a moderately hypoplastic segment of transverse arch will grow after repair of the narrow isthmic area, especially if the descending aorta is anastomosed to the arch as proximally as possible, but it is yet unknown if these patients will ultimately develop systemic hypertension [1, 3, 5–7]. We wanted to test the hypothesis that hypoplastic transverse aortic arches grow once normal forward flow is restored after conventional coarctation repair. We reviewed our experience with patients labelled to have a hypoplastic arch at the time of a conventional coarctation repair, and operated 15 years ago, to delineate the potential long-term growth of these segments of transverse arch.
2. Patients and methods
2.1. Patients
Between January 1990 and December 1995, 75 consecutive neonates with coarctation of the aorta were labelled to have a hypoplastic arch. All the patients who were labelled to have a hypoplastic arch were considered for the study irrespective of the actual size of the arch. Thirty-three of them underwent an end-to-side repair through a sternotomy and 42 underwent a conventional coarctation repair from a thoracotomy. Because abnormal blood flow across their arch was expected, 7 out of these 42 patients with single ventricles were excluded. Postoperative serial echocardiographic examination follow-up longer than 6 months were available in 20 patients. These 20 patients constituted the study group.
Patients' characteristics are shown in Table 1 . A subclavian flap repair was used in nine infants, an end-to-end anastomosis in seven and an extended end-to-end anastomosis in four. All operations were done via left posterolateral thoracotomy. Concomitant pulmonary banding was done in six patients. Mean age at operation was 8.6±5.7 days.
Patient characteristics
| Characteristic | |
| Demographic | |
| Male:Female | 15:5 |
| Mean age at operation (days)±S.D. | 8.6±5.7 |
| Mean age at last study (years)±S.D. | 9.0±5.6 |
| Associated cardiac and vascular anomalies, No. (%) | |
| VSD | 10 (50) |
| Mild LVOTO | 5 (25) |
| ASD | 1 (5) |
| Bicuspid aortic valve | 10 (50) |
| Dextraposition of the heart | 1 (5) |
| Complete AVSD | 1 (5) |
| Transposition of great arteries | 2 (10) |
| Mild LV hypoplasia | 4 (20) |
| Interrupted IVC | 1 (5) |
| Pulmonary artery stenosis | 1 (5) |
| Bovine arch | 1 (5) |
| Ebstein's anomaly | 1 (5) |
| Non-cardiac anomalies, No. (%) | |
| Scoliosis | 1 (5) |
| Noonan's syndrome | 1 (5) |
| Others | |
| Prematurity | 2 (10) |
| Characteristic | |
| Demographic | |
| Male:Female | 15:5 |
| Mean age at operation (days)±S.D. | 8.6±5.7 |
| Mean age at last study (years)±S.D. | 9.0±5.6 |
| Associated cardiac and vascular anomalies, No. (%) | |
| VSD | 10 (50) |
| Mild LVOTO | 5 (25) |
| ASD | 1 (5) |
| Bicuspid aortic valve | 10 (50) |
| Dextraposition of the heart | 1 (5) |
| Complete AVSD | 1 (5) |
| Transposition of great arteries | 2 (10) |
| Mild LV hypoplasia | 4 (20) |
| Interrupted IVC | 1 (5) |
| Pulmonary artery stenosis | 1 (5) |
| Bovine arch | 1 (5) |
| Ebstein's anomaly | 1 (5) |
| Non-cardiac anomalies, No. (%) | |
| Scoliosis | 1 (5) |
| Noonan's syndrome | 1 (5) |
| Others | |
| Prematurity | 2 (10) |
VSD, ventricular septal defect; LVOTO, left ventricular outflow tract obstruction; ASD, atrial septal defect; AVSD, atrioventricular septal defect; LV, left ventricle; IVC, inferior vena cava.
Patient characteristics
| Characteristic | |
| Demographic | |
| Male:Female | 15:5 |
| Mean age at operation (days)±S.D. | 8.6±5.7 |
| Mean age at last study (years)±S.D. | 9.0±5.6 |
| Associated cardiac and vascular anomalies, No. (%) | |
| VSD | 10 (50) |
| Mild LVOTO | 5 (25) |
| ASD | 1 (5) |
| Bicuspid aortic valve | 10 (50) |
| Dextraposition of the heart | 1 (5) |
| Complete AVSD | 1 (5) |
| Transposition of great arteries | 2 (10) |
| Mild LV hypoplasia | 4 (20) |
| Interrupted IVC | 1 (5) |
| Pulmonary artery stenosis | 1 (5) |
| Bovine arch | 1 (5) |
| Ebstein's anomaly | 1 (5) |
| Non-cardiac anomalies, No. (%) | |
| Scoliosis | 1 (5) |
| Noonan's syndrome | 1 (5) |
| Others | |
| Prematurity | 2 (10) |
| Characteristic | |
| Demographic | |
| Male:Female | 15:5 |
| Mean age at operation (days)±S.D. | 8.6±5.7 |
| Mean age at last study (years)±S.D. | 9.0±5.6 |
| Associated cardiac and vascular anomalies, No. (%) | |
| VSD | 10 (50) |
| Mild LVOTO | 5 (25) |
| ASD | 1 (5) |
| Bicuspid aortic valve | 10 (50) |
| Dextraposition of the heart | 1 (5) |
| Complete AVSD | 1 (5) |
| Transposition of great arteries | 2 (10) |
| Mild LV hypoplasia | 4 (20) |
| Interrupted IVC | 1 (5) |
| Pulmonary artery stenosis | 1 (5) |
| Bovine arch | 1 (5) |
| Ebstein's anomaly | 1 (5) |
| Non-cardiac anomalies, No. (%) | |
| Scoliosis | 1 (5) |
| Noonan's syndrome | 1 (5) |
| Others | |
| Prematurity | 2 (10) |
VSD, ventricular septal defect; LVOTO, left ventricular outflow tract obstruction; ASD, atrial septal defect; AVSD, atrioventricular septal defect; LV, left ventricle; IVC, inferior vena cava.
2.2. Methods
Hospital charts were reviewed. Data collected included patient demographics, associated cardiac and non-cardiac anomalies, operative data, and current clinical status. All previously performed echocardiographic examinations were reviewed. Measurements of aortic diameter on echocardiography were made at five different regions by one cardiologist blinded to patient history. The five regions were distal ascending aorta, proximal transverse arch, distal transverse arch, isthmus and descending aorta. The proximal transverse arch was defined as the segment of aorta between the innominate artery and the left carotid artery and the distal transverse arch, the segment between the left carotid and the left subclavian artery. Absolute values were converted to Z-score relative to body surface area (BSA) and to arch index ratios over ascending or descending aorta [8].
Recoarctation was defined as a blood pressure gradient of pressure between the upper and lower limb 20 mmHg or a peak instantaneous Doppler gradient 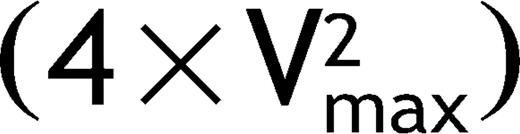 >25 mmHg.
>25 mmHg.
2.3. Statistical analysis
All data analysis was performed using Stata version 10 (Stata Corp, College Station, Texas). Fisher's exact test was used for discrete variables and paired Student's t-test for longitudinal growth analysis. Results were expressed as mean±standard deviation (S.D.) where appropriate.
3. Results
3.1. Clinical outcome
The mean follow-up time of the 20 patients who had a serial echocardiographic follow-up was 9.5±5.5 years. There was one late death following abdominal surgery which was unrelated to the coarctation repair. Recoarctation was identified in eight patients and six of these successfully underwent a balloon dilatation at the level of the isthmus. One patient was lost to follow-up after an echocardiographic gradient of 27 mmHg was detected, and another was scheduled for a balloon dilatation but on catheterization, only a minimal gradient of 5 mmHg was found and the patient received no further treatment. No patient needed reoperation. At the last follow-up, three patients were reported to be hypertensive by their cardiologists and nine were identified to have an arch gradient of 15 mmHg including all of those who had previously undergone a balloon dilatation.
The outcome of the 15 patients who did not have a serial echocardiographic follow-up was the following. One patient was lost to follow-up. After a mean of 14.3±4.6 years, three patients needed five reinterventions, one balloon dilatation and four redo surgery of the arch. Three patients were known hypertensive, two of them with an identified hypoplasia of the arch.
3.2. Echocardiographic follow-up
3.2.1. Proximal transverse arch
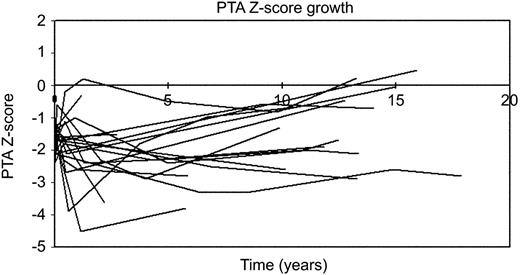
Whilst there was a slight increase in the size of the proximal transverse arch corrected for BSA, the dimension of this segment did not normalise (mean Z-score increased from –1.87±0.12 to –1.66±0.09; P=0.054). No significant growth was observed in subsequent periods. At last follow-up, seven patients (35%) still had a proximal transverse arch Z-score of less than –2 (Fig. 1 ).
There was a change in the relative size of the transverse aortic arch to the ascending during follow-up. This ratio gradually increased over time from preoperative values of 0.62±0.08 to 0.76±0.14 at 5 years (P=0.007) and to 0.81±0.10 at 15 years (P=0.01). Significant change also occurred between the 5 and 10 years period (P=0.02). When the transverse arch to descending aorta ratio was used, significant growth was observed at one year after the operation (P=0.03), no difference, however, was observed thereafter (Table 2 ).
Growth of the proximal aortic arch
| Preoperative | Postoperative | 6 month | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.91±0.43 | –1.66±0.33 | –1.91±0.97 | –1.99±1.52 | –2.37±0.89 | –1.69±0.92 | –1.01±1.32 |
| PTA/AA ratio | 0.62±0.079 | 0.69±0.090 | 0.69±0.14 | 0.73±0.21 | 0.76±0.14* | 0.73±0.12† | 0.81±0.10* |
| PTA/DTA ratio | 0.70±0.10 | 0.72±0.10 | 0.80±0.20 | 0.82±0.17* | 0.76±0.24 | 0.79±0.17 | 0.86±0.21 |
| Preoperative | Postoperative | 6 month | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.91±0.43 | –1.66±0.33 | –1.91±0.97 | –1.99±1.52 | –2.37±0.89 | –1.69±0.92 | –1.01±1.32 |
| PTA/AA ratio | 0.62±0.079 | 0.69±0.090 | 0.69±0.14 | 0.73±0.21 | 0.76±0.14* | 0.73±0.12† | 0.81±0.10* |
| PTA/DTA ratio | 0.70±0.10 | 0.72±0.10 | 0.80±0.20 | 0.82±0.17* | 0.76±0.24 | 0.79±0.17 | 0.86±0.21 |
*P-value<0.05 compared with preoperative values. †P-value<0.05 compared with preceding age group values.
Growth of the proximal aortic arch
| Preoperative | Postoperative | 6 month | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.91±0.43 | –1.66±0.33 | –1.91±0.97 | –1.99±1.52 | –2.37±0.89 | –1.69±0.92 | –1.01±1.32 |
| PTA/AA ratio | 0.62±0.079 | 0.69±0.090 | 0.69±0.14 | 0.73±0.21 | 0.76±0.14* | 0.73±0.12† | 0.81±0.10* |
| PTA/DTA ratio | 0.70±0.10 | 0.72±0.10 | 0.80±0.20 | 0.82±0.17* | 0.76±0.24 | 0.79±0.17 | 0.86±0.21 |
| Preoperative | Postoperative | 6 month | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.91±0.43 | –1.66±0.33 | –1.91±0.97 | –1.99±1.52 | –2.37±0.89 | –1.69±0.92 | –1.01±1.32 |
| PTA/AA ratio | 0.62±0.079 | 0.69±0.090 | 0.69±0.14 | 0.73±0.21 | 0.76±0.14* | 0.73±0.12† | 0.81±0.10* |
| PTA/DTA ratio | 0.70±0.10 | 0.72±0.10 | 0.80±0.20 | 0.82±0.17* | 0.76±0.24 | 0.79±0.17 | 0.86±0.21 |
*P-value<0.05 compared with preoperative values. †P-value<0.05 compared with preceding age group values.
3.2.2. Distal transverse arch
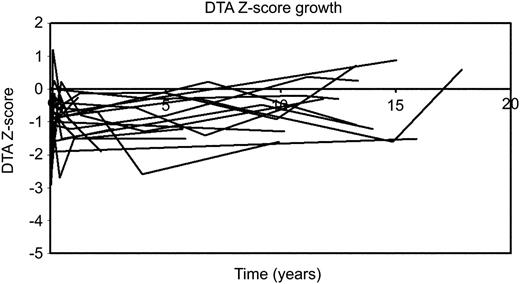
In the distal transverse arch, maximum change was observed in the early postoperative period (mean diameter Z-score –1.67±0.19 vs. –0.86±0.21; P=0.01). Sustained growth was observed thereafter (mean Z-score at 15 years follow-up: –0.49±0.35; P=0.02). At last follow-up, no patients had Z-score of less than –2 (Fig. 2 ). When distal transverse arch to ascending or descending aorta ratio were calculated, similar pattern of growth as observed for the proximal transverse arch were obtained, with relative growth of the distal transverse arch relative to the ascending and descending aortic segments over time (Table 3 ).
Growth of the distal aortic arch
| Preoperative | Postoperative | 6 months | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.62±0.75 | –0.86±0.81*† | –0.78±0.84* | –0.99±0.59 | –0.88±0.80* | –0.61±0.60* | –0.51±1.08* |
| DTA/AA ratio | 0.53±0.077 | 0.66±0.17*† | 0.67±0.15* | 0.64±0.13 | 0.73±0.14* | 0.70±0.10* | 0.72±0.12* |
| DTA/DA ratio | 0.59±0.10 | 0.68±0.15*† | 0.77±0.18* | 0.71±0.10* | 0.75±0.21* | 0.73±0.081* | 0.75±0.19* |
| Preoperative | Postoperative | 6 months | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.62±0.75 | –0.86±0.81*† | –0.78±0.84* | –0.99±0.59 | –0.88±0.80* | –0.61±0.60* | –0.51±1.08* |
| DTA/AA ratio | 0.53±0.077 | 0.66±0.17*† | 0.67±0.15* | 0.64±0.13 | 0.73±0.14* | 0.70±0.10* | 0.72±0.12* |
| DTA/DA ratio | 0.59±0.10 | 0.68±0.15*† | 0.77±0.18* | 0.71±0.10* | 0.75±0.21* | 0.73±0.081* | 0.75±0.19* |
*P-value<0.05 compared with preoperative values. †P-value<0.05 compared with preceding age group values.
Growth of the distal aortic arch
| Preoperative | Postoperative | 6 months | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.62±0.75 | –0.86±0.81*† | –0.78±0.84* | –0.99±0.59 | –0.88±0.80* | –0.61±0.60* | –0.51±1.08* |
| DTA/AA ratio | 0.53±0.077 | 0.66±0.17*† | 0.67±0.15* | 0.64±0.13 | 0.73±0.14* | 0.70±0.10* | 0.72±0.12* |
| DTA/DA ratio | 0.59±0.10 | 0.68±0.15*† | 0.77±0.18* | 0.71±0.10* | 0.75±0.21* | 0.73±0.081* | 0.75±0.19* |
| Preoperative | Postoperative | 6 months | 1 year | 5 years | 10 years | 15 years | |
| Z-score | –1.62±0.75 | –0.86±0.81*† | –0.78±0.84* | –0.99±0.59 | –0.88±0.80* | –0.61±0.60* | –0.51±1.08* |
| DTA/AA ratio | 0.53±0.077 | 0.66±0.17*† | 0.67±0.15* | 0.64±0.13 | 0.73±0.14* | 0.70±0.10* | 0.72±0.12* |
| DTA/DA ratio | 0.59±0.10 | 0.68±0.15*† | 0.77±0.18* | 0.71±0.10* | 0.75±0.21* | 0.73±0.081* | 0.75±0.19* |
*P-value<0.05 compared with preoperative values. †P-value<0.05 compared with preceding age group values.
The sizes of both proximal and distal arch at last follow-up were not related to preoperative size or by the type of repair.
3.2.3. Arch gradient and transverse arch size
When comparing with patients with minimal residual gradient, patients with residual gradient ≥15 mmHg at last follow-up had significantly smaller preoperative proximal transverse arch (Z-score –2.36±1.39 vs. –1.02±0.94; P=0.03). In five of the seven patients with a PTA Z-score ≤–2, a gradient ≥15 mmHg was found; in comparison, only one patient (14%) with a PTA Z-score less than –2 was found to have a gradient ≥15 mmHg (P=0.01).
4. Discussion
After a conventional coarctation repair through a thoracotomy, the patients are at risk of developing hypertension in adult life, and its associated cardiovascular comorbidities, and decreased survival [9, 10]. Leaving a segment of arch hypoplasia has been identified as one of the risk factors for early mortality, recoarctation, hypertension and late mortality (Jahangiri et al., 2000: 4 [1]; Lacour-Gayet et al., 1990: 6 [2]; Trinquet et al., 1988: 5 [4]; Weber et al., 1993: 7 [11]; Karl et al., 1992: 8 [12]; Kappetein et al., 1994: 10 [13]).
The alternative approach to conventional repair is the end-to-side anastomosis performed between the detached descending aorta and a proximal segment of the aortic arch. It is usually reserved to the most severely hypoplastic arches, because it is performed via a sternotomy and requires a short period of circulatory arrest [12].
The evidence that transverse arch grows after conventional repair of aortic coarctation relies on studies reporting relatively short-term outcomes, with poor definition of the studied arch anatomy, and an obvious lack of criteria of optimal growth to achieve [1, 3, 5–7]. The study of Myers in 1992 seems to be one of the rare longitudinal angiographic studies examining specifically the proximal and distal transverse arch size (Myers et al., 1992: 15 [3]). It was performed in 17 patients followed for a mean of 4 years. It was, however, not focused on patients with hypoplastic arches but examined their available data on all-comers undergoing a coarctation repair. The remaining studies reported follow-up ranging from 5 days to 8 years, but none of these reported the final size of the proximal arch, only a few reported clinical gradients, and none documented systemic blood pressure at last follow-up [1, 5–7].
The main difficulty encountered when studying hypoplasia of the aortic arch is the definition of hypoplasia. The most common definition has been introduced by Moulaert following a study performed on autopsy specimens [1, 4, 7, 14]. It defines the proximal and distal arches to be normal, if the ratio between their diameters and the diameter of the ascending aorta was 0.6 and 0.5, respectively. In our study, we found a great variability in the sizes of the ascending and the descending aorta of our patients. We believe that the growth of the aorta could be affected by a residual obstruction. Therefore, the more recent interpretation of the normality of the aortic size by Z-score estimates seemed to us a more realistic approach. Ideally, the optimal growth of the transverse arch should result in normotension in adults. In two retrospective studies, hypertension late after surgery has been linked to a lack of growth of the transverse arch [11, 15]. Because we lack longitudinal studies of patients with moderate degree of hypoplasia of the arch at the time of the repair, it is difficult to estimate the prevalence of persisting hypoplasia of the arch, and even more so the late prevalence of hypertension in these patients.
Our study is one of only a few analysing separately the proximal and the distal arch, and demonstrates that the growth of these two segments may not be uniform [3, 11]. It could be argued that, based on the Moulaert criteria, the hypoplastic segments of proximal transverse arch ultimately grew to normal diameters. This interpretation, however, would mask the fact that more than a third of the patients still had proximal arches with Z-scores less than –2. More importantly, five out of the seven patients who ended with small proximal arches had developed residual gradients 15 mmHg, and it is possible that these patients will be more prone to develop hypertension in the future.
The discrepancy in growth of the proximal and the distal arch can probably be explained by technical considerations. It is likely that the more contemporary techniques of coarctation repair will better address the issue of distal arch hypoplasia than the original subclavian flap repair technique. There might be differences between surgical techniques and between surgeons in the capacity to enlarge the distal arch at the time of a coarctation repair performed by thoracotomy, and these differences may explain the differences observed in the growth of the distal transverse arch described in previous studies with similar techniques [1–7, 11]. Our study seems to demonstrate that in some patients, despite our best attempts, the proximal arch remained small. None of the current techniques are likely to address a narrowing between the innominate artery and the left common carotid artery. While the present study highlights the lack of growth of the proximal arch, it did not have the power to identify potential risk factors for this. Techniques used, initial size of the aorta, and presence of associated lesions could all potentially have an impact on this growth.
This study has two practical implications. Firstly, for both clinical and scientific purposes, the size of the proximal and the distal arch should be analysed separately. Secondly, one should not be too confident that remodelling of the proximal arch will occur after conventional coarctation repair in a patient with proximal arch hypoplasia. Whether these patients should be offered end-to-side repair through sternotomy and at what stage they should be offered this technique will remain debatable as long as no larger studies are performed. The benefits of not risking late hypertension may ultimately justify what is a much more invasive procedure with its associated risks.
In conclusion, patients with a moderately hypoplastic arch treated by conventional coarctation repair have adequate growth of the distal arch demonstrated at long-term follow-up, but one-third of them still have proximal arch hypoplasia. This subset of patients is at risk of developing hypertension and may warrant further investigation.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
References
Conference discussion
Dr. M. Pozzi (Ancona, Italy): Actually it is one of the issues where we'll always be looking for an answer. In a way, you've given part of the answer in the sense that certainly a repair of coarctation by itself I think does not reliably assure a growth of the arch.
And the other thing which I think you already pointed out is the fact it is important to distinguish between the proximal part and the distal part of the arch. The distal part of the arch can be dealt with by a surgical technique, so I think that's probably not a major issue.
The proximal part, I would agree with you, is a very difficult issue because we're talking about newborns where 1 mm in diameter would make the difference between belonging to a good arch or a hypoplastic arch. And with also the variability of echo assessment, individual operator variation, it's really a tough game.
From that point of view, I would agree with you that a much larger series, maybe even a multi-institutional series, is necessary.
With regard to the proximal part, I think at the end of the day, the bottom line question is what are we going to do? I'd like to know what your approach is now, but as far as I'm concerned, considering that repair of the aortic arch from the midline is becoming a very frequent and safe operation with antegrade perfusion (I think there is no more need for circulatory arrest), I would be inclined, when in doubt about moderate hypoplasia or hypoplasia of the arch, to go through the midline and do a complete repair from the front. But I would like to know what your practical approach is tomorrow when you go back.
Dr. d'Udekem: As I mentioned, we don't have a cutoff. We don't have a cutoff for a size of the transverse arch under which we are doing the operation from the front; we are investigating that.
For the time being, we do the same as you. We trust our surgical feelings, and we don't push ourselves. If we don't want to do it from the side because it looked a bit small, we do not hesitate to go from the front, and that's as scientific as it gets.
Dr. H. Lindberg (Oslo, Norway): I totally agree with you that it's almost impossible to say which arch grows and does not. However, I have a certain feeling that if there is an increased distance between the left carotid and the left subclavian artery, if that distance is increased, the arches do not grow. Have you had the possibility to look if that special configuration of the arch is looking like this and not like that, if that has any impact on the growth or not?
Dr. d'Udekem: We did not. We thought about measuring the length of the arch, but it was very difficult. Some of the studies were 20 years old. It was really very difficult to acquire all the segmented analysis of the transverse arch.
We could not analyze the length between the emergence of the innominate artery, for example, and the emergence of the left carotid artery. So I have no answers for that.
Dr. Lindberg: No, I was just thinking about the nonparallelity about the take-off of the vessels. Because when they have their trapezoid configuration, it's my experience those arches do not grow, but we'll come into that later.
Dr. J. Fragata (Lisbon, Portugal): Before you go, I would like to ask if you could precisely tell us what your definition for “hypoplastic” is, because this was lacking in your presentation.
Dr. d'Udekem: The definition of hypoplasia, and that's why we took all the measurements, is any patients where we found written hypoplasia either in the description of the surgical report or in the description of the echo by the cardiologists, and these are the patients we investigated.
Dr. Fragata: So no measurements at all?
Dr. d'Udekem: That's how we selected the patients. And then we reported all the measurements in Z-score, and obviously all the severe hypoplasia of the arch were operated from the front in Melbourne during that time period already.
So the only ones left were those with the moderate hypoplasia, which sometimes is very minimal and sometimes a little bit more severe.