Abstract
Background
The SINgapore GERiatric intervention study to reduce cognitive decline and physical frailty (SINGER) randomised controlled trial (RCT) uses a multidomain lifestyle interventions approach, shown to be effective by the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial, to delay cognitive decline.
Objective
To investigate the efficacy and safety of the SINGER multidomain lifestyle interventions in older adults at risk for dementia to delay cognitive decline.
Participants
1200 participants between 60–77 years old, with Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE) dementia risk score ≥6, fulfilling at least one of the following LIBRA index for diet, cognitive activity, physical activity and a Montreal Cognitive Assessment (MoCA) score ≥18, ≤27 points, will be recruited across Singapore.
Methods
SINGER is a 2-year multi-site RCT consisting of multidomain interventions: dietary advice, exercise, cognitive training, and vascular risk factors management. Participants will be randomised into either the Self-Guided Intervention (SGI; general lifestyle and health information and resources) or Structured Lifestyle Intervention (SLI) group. The SLI comprises diet training (6 group and 3 individual sessions over 12 months); exercise (supervised: 1-hour twice weekly for 6 months, unsupervised: 2–3/week for the rest of the study duration); cognitive sessions (15–30 minutes/session, 3/week for 6 months, together with 10 workshops in 24 months). Vascular management takes place every 3–6 months or otherwise as specified by study physicians. The primary outcome is global cognition measured using the modified Neuropsychological Battery assessing performance in various domains, such as episodic memory, executive function and processing speed. Secondary outcome measures include: domain-specific cognition and function, imaging evidence of brain and retinal changes, incidence and progression of chronic diseases, blood biomarkers, quality of life, mental health and cost-benefit analysis.
Conclusions
SINGER is part of the Worldwide-FINGERS international network, which is at the forefront of harmonizing approaches to effective non-pharmacological interventions in delaying cognitive decline in older adults at risk of dementia. By establishing the efficacy of multidomain interventions in preventing cognitive decline, SINGER aims to implement the findings into public health and clinical practices by informing policy makers, and guiding the design of community- and individual-level health promotion initiatives.
Similar content being viewed by others
Background
The prevalence of dementia is already high, particularly in Asia (1), and is projected to increase exponentially due to a rapidly ageing population, hence, dementia will strongly impact individuals, their families and healthcare systems (2–4).
Nearly one-third of dementia cases internationally are estimated to be attributable to modifiable lifestyle, vascular or metabolic risk factors (5). Of note, reducing the prevalence of each of these risk factors by 10% or 20% per decade may reduce Alzheimer’s Disease (AD) prevalence worldwide by 8 to 15% by 2050 (6). Studies using single-domain lifestyle interventions in reducing dementia risk, such as healthy diet, cognitive training, or blood-pressure management, appear less likely to have a sustainable impact (7).
By contrast, the approach of combining multiple intervention components may have synergistic effects. However, multi-domain intervention studies have demonstrated mixed outcomes (8–12). The 3-year French Multidomain Alzheimer Preventive Trial (MAPT) compared the effect of 3 interventions on cognitive decline (9). It was reported that the combined multidomain intervention groups showed a significant decrease in cognitive decline compared with the placebo group, in participants at higher risk of dementia. Additionally, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial produced promising results (10). This population-based 2-year randomised controlled trial (RCT) demonstrated the effectiveness of a multidomain lifestyle intervention comprising of cognitive training, physical activity, nutritional advice, social activities and vascular risk factors management on delaying cognitive decline in older adults at risk of dementia. Encouraged by these results, the Worldwide-FINGERS (WW-FINGERS) initiative was established in 2017 to adapt, test, and optimize the FINGER model in different geographical, cultural and economic settings (13, 14).
In Singapore, a 6-month pilot study demonstrated the cultural feasibility and practicality of the FINGER interventions and a set of locally adapted interventions in an Asian population (15). Hence, we propose to conduct a larger-scale trial to determine the efficacy of these interventions in an Asian population in Singapore, with the integration of novel digital intervention delivery and evaluation approaches.
In line with the WW-FINGERS international effort, this study will be investigating the efficacy and safety of the SINGER multidomain lifestyle interventions, incorporating more intensive blood pressure lowering, on cognition in older adults with increased risk of dementia. The SINGER interventions are based on standard healthcare recommendations and encompass a series of supervised and unsupervised sessions, to empower participants to continue sustaining these lifestyle modifications.
Methods
Study design
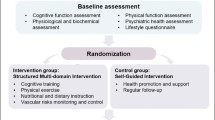
The multi-centre SINGER study is a 2-year randomized controlled trial (RCT) (Figure 1). 1200 older adults at risk of cognitive decline and dementia, determined using a Cardiovascular Risk Factors, Aging, and Incidence of Dementia (CAIDE) dementia risk score of ≥6, will be recruited from community dwelling and clinical cohorts of Singaporean older adults from August 2021.
Participants will be randomized to either a Self-Guided Lifestyle (SGL) or to a Structured Lifestyle Intervention (SLI) group, which focuses on regular aerobic and strength exercise, adherence to the FINGER diet, cognitive and social stimulation, and protocol-based vascular monitoring. Experienced neuropsychological raters evaluating cognitive outcome measures will be blinded to randomization group. Additionally, a digital administrative and patient-facing platform will be utilised. The SINGER study has been approved by the National Healthcare Group Domain-Specific Review Board and is registered under ClinicalTrials.gov (ID: NCT05007353). Written informed consent will be obtained from all participants before enrolment into the study. The study will be conducted in accordance with Good Clinical Practice guidelines.
Primary study objective
To determine whether randomization to a Self-Guided Intervention (SGI) versus Structured Lifestyle Intervention (SLI) group improves cognitive performance, measured by global cognitive z-scores.
Secondary study objectives
To examine (a) intervention effects on domain-specific cognition and function, (b) imaging evidence of brain and retinal changes, (c) blood biomarkers, (d) incidence and progression of chronic diseases, (e) quality of life, mental health and cost-benefit analysis.
Inclusion criteria
The SINGER study will recruit participants who are (1) aged 60–77 years, (2) at risk of dementia as determined by a CAIDE score of ≥6, (3) have modifiable lifestyle factors (fulfilling ≥1 of the LIBRA Questionnaires for diet, cognitive activity, physical activity), (4) Montreal Cognitive Assessment (MoCA) score between 18 and 27, both inclusive, (5) no plans to travel outside of Singapore for an extended period of time over the study period, (6) no physical disabilities that preclude study participation, (7) willing to complete all study-related activities for 24 months, (8) willing to be randomised to either lifestyle intervention group, (9) able to understand English or Mandarin Chinese.
Exclusion criteria
The SINGER study will exclude participants who have (1) malignant diseases, (2) dementia, (3) major depression, (4) symptomatic cardiovascular disease, (5) revascularisation within 1 year, (6) severe loss of vision, hearing or communicative ability, (7) other conditions that inhibit safe engagement in the prescribed intervention and other conditions preventing cooperation, as judged by the study physician.
Randomization
Participants will be randomized into either the SGI or SLI group, generated by a random allocation sequence by non-study related staff using STATA version 14 using a 1:1 schedule, stratified by site. Treatment allocation will only be revealed to the study team after receiving information demonstrating that the patient is eligible and has consented to the trial.
Intervention programme
Participants in the SGI arm will receive general lifestyle health education information, tools, resources, and support to encourage a healthier lifestyle (16). These resources exceed those provided to older adults in Singapore. They will also receive blood laboratory testing and blood pressure monitoring during their outcome visits. General written information about the clinical significance of measurements, and advice to seek treatment if needed will be provided.
Participants in the SLI arm will be assigned the following interventions (Figure 2).
Diet intervention
Participants will be allocated the FINGER diet, adapted from the Mediterranean diet, which has been recommended to be an effective diet by the World Health Organization (17, 18): 10–20% daily energy (E%) from proteins, 25–35% from fat, 45–55% from carbohydrates, 25–35g/day of dietary fibre, <5g/day salt and <5% from alcohol. They will be advised to have higher consumption of fruits and vegetables, wholegrain products, low-fat options in milk and milk products, limited added sugar intake, use of healthier oil (e.g. vegetable oil) instead of butter or saturated fat, and consumption of at least 2 portions of fish per week (10). Weight maintenance is expected.
Participants will receive 6 group-based nutrition advocacy workshops over 12 months. Each session is approximately 60–90 minutes and may be virtual if face-to-face meetings are disallowed. Discussions will include My Healthy Plate (19), food choice selection, and healthier food choices. They will also undergo 3 individual-based nutrition training sessions (30 minutes each), which are adapted to individual needs identified by the study team within the first year of the study. Participants will complete a 3-day food diary and questionnaires at each outcome visit.
Exercise intervention
Participants will be assigned to the modified FINGER exercise programme (20), which includes once to twice weekly supervised strength training sessions of up to 1 hour for 6 months. The sessions comprise of stretching, balance training, strengthening by targeting major muscle groups using free weights, TheraBands and/or weights machine, and exercise education. Additionally, a home exercise programme including aerobic exercise recommendations (30 minutes, up to 5 times/week) will be provided. Wearable activity monitors and home exercise weights will be recommended. After the initial 6-month training period, participants will be provided with a home exercise programme. An individual aerobic training program comprising activities such as walking, swimming, jogging and cycling will be planned with each participant. The progressive strength training program is based on 1 Repetition Maximum (RM) at baseline and re-measurements at month-12 and month-24 outcome visits. However, if gym access is restricted, Rating of Perceived Exertion (RPE) scale will be utilized. Participants will rate their level of difficulty for the exercises from 0 (extremely easy) to 10 (extremely hard) to assist with exercise titration, which can be done remotely. Strength training workload throughout the course of the study will be 60 to 70% 1RM/RPE 5–6, with a range of 12 to 15 repetitions for each set. 1RM/RPE will be assessed at baseline and at the end of month-6. Aerobic exercise intensity will be practised at moderate intensity levels at a recommended rating of 5–6 RPE. Participants will complete an exercise diary to allow review of the exercise prescription to achieve individualised targets: increasing from 1–2x/week for strength and 1–4x/week for aerobic in the first 6 months to 2–3x/week for strength and 3–5x/week for aerobic for the rest of the study duration.
Cognitive intervention
Ten cognitive training workshops, each lasting approximately 60–90 minutes, will take place throughout the study period. Discussions will include topics on age-related cognitive changes, memory strategies and everyday memory training. Participants will also undergo 2 periods of individualised computer-based cognitive training sessions of 6 months each, with a total of 144 sessions to be completed within 2 years. A tablet will be provided to all participants to complete the cognitive training sessions. The digital training program will take place thrice per week with each session taking 15–30 minutes (total 72 sessions in 6 months). The computerised cognitive training program will be used to conduct training; of note, a translated Mandarin version of the program will be used for Mandarin-speaking participants (10). The first 3-months will be supervised. Participants will then be advised to perform cognitive training at a recommended thrice a week unsupervised.
Vascular risk factors management
Participants will meet the study team every 3–6 months unless otherwise specified by the study physician to measure their blood pressure (BP), weight, body mass index (BMI), and hip and waist circumference. The study physician may request for additional glucose and cholesterol tests, if required. Participants will also meet the study team at screening, 12 and 24 months for detailed medical history taking and physical examination. At every visit, all participants will be provided with oral and written information and advice on healthy diets and physical, cognitive, and social activities beneficial for management of vascular risk factors and disability prevention. Blood samples will be collected at baseline and month-24. Relevant laboratory test results will be given to all participants, together with general written information about the clinical significance of measurements, and advice to seek treatment if needed. Management of metabolic and vascular risk factors will be based on Singapore Ministry of Health Clinical Practice Guidelines (21). BP management of participants with hypertension will be based on the SPRINT study protocol to target a systolic BP of <120mmHg (22). This target has shown both cardiovascular benefits and improved cognitive outcomes (15). BP management will include meetings with the study team at the outcome measure visits, and as requested by the study physician for measurements of BP, weight and BMI, and hip and waist circumference, physical examinations, and recommendations for lifestyle management. Study physicians will initiate medication if further management is needed and arrange for further clinical follow up. Adjustment of dose will be based on a mean of three BP measurements taken after 5 minutes of quiet rest while seated (22).
Primary outcome
The primary outcome is the composite z-score of global cognitive performance at month-12 and month-24, measured using the modified Neuropsychological Battery (mNTB) (23, 24), which includes Visual Paired Associates, Logical Memory Recall of the Wechsler Memory Scale-Revised, Rey Auditory Verbal Learning, Digit Span, Word and Category Fluency test, Trail Making Test, and Letter Digit Substitution test.
Secondary outcomes
Cognitive domains
To examine intervention effects on specific cognitive domains, composite scores from the mNTB subtests (23, 24) including Episodic Memory (Visual Paired Associates tests, Logical Memory Immediate and Delayed Recall of the Wechsler Memory Scale-Revised, Rey Auditory Verbal Learning test), Executive Function (Digit Span, Word and Category Fluency test, Trail Making Test (TMT) Part B), and Processing Speed (Letter Digit Substitution test and Trail-Making Test (TMT) Part A) will be utilised. A digital cognitive battery will also be used alongside the mNTB to assess cognitive domains.
Functional abilities
To examine intervention effects on function, the Clinical Dementia Rating-Sum of Boxes (CDR-SB) (25), and functional abilities as measured on the ADCS-MCI Activities of Daily Living Inventory (ADCS-MCI-ADL) (26) will be performed.
Lifestyle factors
To examine intervention effects on lifestyle, a composite score based on self-reported physical activity, diet, and cognitive activity will be measured. Differences between intervention groups in physical function, mood, sleep quality, and subjective memory concerns will also be assessed. Scales used include the Geriatric Depression Scale (27), Pittsburgh Sleep Quality Index (28), Prospective-Retrospective Memory Questionnaire (29), Global Physical Activity Questionnaire (30), Leisure-Time Activities Questionnaire (31) and Physical Performance Test (32). See Supplementary Material.
Vascular risk factors
To examine differences between intervention groups in cardio-metabolic disease risk, changes in BP (in mmHg), lipid profile and glucose (both in mmol/L) will be measured, and incident events using serious adverse event reports assessed.
Neuroimaging
To examine intervention effects on changes in brain structural integrity, grey matter volume loss, white matter microstructure degradation, and increase of cerebrovascular disease markers (CeVD) seen on the magnetic resonance imaging (MRI) will be assessed. An evaluation of vascular polygenic risk scores (PRS) and its association with neurodegeneration, CeVD burden and cognitive decline will be performed.
Blood biomarkers
Novel and accessible blood markers to monitor AD- and CeVD-associated pathologies will be measured. Plasma concentrations of cardiac markers (High-sensitive cardiac troponin T (hs Troponin T), N-terminal pro b-type natriuretic peptide (NT-proBNP) and Growth/differentiation factor 15 (GDF 15)), and peripheral biomarkers of Aβ, tau and synaptic pathology, oxidative stress, endothelial/cardiovascular injury and degenerative protein modifications (DPMs) damaged proteins will be used.
Retinal imaging
Retinal imaging data will be used to predict cognitive decline. Retinal structural, vascular and neuronal changes will be assessed.
Cost-benefit Analysis
To develop macro (population level) and micro (individual level) systems models to simulate the causal relationship between health states, interventions/policies, and outcomes. Tests used include the Health-Related Quality of Life: 36-item Short Form Health Survey (SF-36 HRQoL) (33), Quality of Life Questionnaire (15D) (34) and 3) Resource Utilization Questionnaire: Resource Use Inventory (RUI) (35) (Supplementary Material).
Study flow
Potentially eligible participants will be pre-screened and assessed according to the inclusion and exclusion criteria. Medical history, brief clinical assessment and a brief cognitive assessment (5-min MoCA) will be conducted remotely. If participants pass these remote procedures, they will be invited on-site to undergo an electrocardiogram and further screening. Participants who pass all screening procedures will undergo full clinical, vascular, cognitive, physical and diet assessment at baseline, month-12 and month-24. Outcome assessments are divided into three sections - on-site, remote and self-administered (Table 1).
Digital Platform
In order to improve trial efficiency in a clinical trial environment altered by the COVID-19 pandemic, the SINGER trial will be using a digital platform to facilitate workflow and execution of interventions and outcome assessments. This platform will manage participants’ study activities through a dashboard to track their progress and to obtain an overview of the results and completion status of self-administered outcome measurements. Scheduling of one-to-one therapist-participant appointments, group training sessions, outcome visits and individual training sessions will also be made easier with the platform.
Participants will receive reminders to 1) attend training sessions and study visits, 2) complete their food, cognitive training, and exercise diaries, 3) log their vital signs for BP monitoring, and 4) complete their self-administered outcome measures. De-identified anthropometric measurements and laboratory results taken at the outcome visits will be available to participants. The platform will also facilitate communication between participants and the study team (e.g. discussions with other participants, video calls).
Training resources such as group workshop materials and pre-recorded exercise demonstration videos will be made available on the digital platform. Session notes will be uploaded and made visible to participants after each session. Additionally, the platform will aid in document management and data collection of clinical notes (e.g. clinical record forms), self-administered outcome questionnaires and intervention diaries.
Sample size
The targeted sample size for SINGER is 1200 participants followed over 2 years. According to projections from the SINGER-pilot study, approximately 5000 participants need to be pre-screened to reach the 1200 target. This will provide 80% power at a 5% significance level, assuming 15% drop-out over the follow-up, to detect a mean difference of 0.088 in the 2-year change in the mNTB global z-score composite between the two treatment groups, with a common standard deviation of 0.5 for the within group change. The assumed effect size for SINGER is close to the upper limit of the 95% confidence interval for the FINGER effect size (20), but lower than an earlier multi-domain lifestyle study in Singapore (36).
SINGER will be conducted in the context of several other similar trials in the Worldwide FINGER network, including the original FINGER. Thus, SINGER will not be viewed as a stand-alone assessment of a multidomain intervention, but as an important contributor to a broader assessment of efficacy. Hence the choice to target 80% power also conserves resources and provides a more efficient trial.
Statistical analysis
Primary analyses will be two-tailed and based on the intention-to-treat approach, where data from all participants will be analysed according to their original intervention assignment and full follow-up will be attempted regardless of intervention adherence. Univariate analyses will be performed to determine outliers and skewness of the data. The primary outcome for SINGER is the mNTB global composite cognitive z-score, measured by transforming the raw scores of all individual tests into standardized z-scores using the cohort-wide means and standard deviations (SD) at baseline. Additionally, individual cognitive domain z-scores will be obtained by averaging the cohort-wide composite mean and SD at baseline. Primary and secondary cognitive outcomes will be analysed using linear mixed models, with the dependent variable consisting of all composite outcomes measured from baseline through follow-up. Covariates include site (stratification factor) and clinic visit to control for potentially non-linear factors that may systematically affect both intervention groups. The fixed effects are intervention assignment and its interaction with follow-up time as a continuous variable - this interaction will be tested with one degree of freedom. Models will be fitted with restricted maximum likelihood to adjust for baseline differences among participants. Longitudinal correlations between measures collected over time within individual participants will be parameterized using an unstructured model. If this model for longitudinal covariance results is non-convergent, a first-order autocorrelation model will be used instead. The significance of the intervention will be determined based on a Wald test for the interaction between intervention assignment and time from randomization. Secondary outcomes of composite cognitive functions will be reported using 95% confidence intervals. Similarly, general linear models will evaluate if random assignment to the SGI or SLI group will affect CDR-SoB scores, functional status (ADCS-MCI-ADL), and a composite measure reflecting lifestyle practices involving diet, physical and cognitive/social activity. Furthermore, machine learning to predict future disease progression and intervention response based on multimodal biomarkers will be utilised. Logistic regression and survival analysis will be used for categorical variables.
Challenges and conclusion
Both the FINGER and SINGER interventions have been shown to be feasible in Singaporean older adults (15). However, due to the lack of sample size and short study duration, the pilot study could not determine the efficacy of the interventions. As a result, a large 2-year multidomain lifestyle intervention RCT will be conducted to evaluate the effects of a multiple lifestyle intervention on cognition in older adults at risk of dementia in Singapore. As a large portion of the study will be conducted over a digital platform, there may potentially be a slow uptake in participants utilising the resources and interventions on the digital platform. Furthermore, some subjects may have difficulty accessing and using the digital platform. To counter this challenge, the study team will be providing each participant a tablet with the digital platform already downloaded on the device. A group workshop will also be held to teach participants how to use the digital device and platforms so that they will be able to access and participate in the interventions. Encouraging learning and socialization, and providing access to devices, may potentially be a motivating factor for participants. The WW-FINGERS international network, which SINGER is a part of, will enable a more harmonious approach to effective non-pharmacological interventions in delaying cognitive decline in older adults at risk of dementia. SINGER will extend FINGER findings in a multi-ethnic Asian population. SINGER is a part of the WW-FINGERS global interdisciplinary network (wwfingers.com) which aims to share knowledge and experiences on trials for dementia prevention and risk reduction, harmonize data, and plan joint international initiatives for the prevention of cognitive impairment and dementia (37). SINGER will not only examine the efficacy of a large-scale lifestyle intervention, we will also explore the public health implications and translational pathway of incorporating the interventions into the Ministry of Health’s long-term vision and mission for the management of chronic disease burden nationally. The SINGER study aims to work closely with individuals, communities, public institutions and other partners to construct a framework for promoting healthy longevity.
References
Alzheimer’s Disease International. Dementia in the Asia Pacific Region. 2014. https://www.alzint.org/resource/dementia-in-the-asia-pacific-region/.
Chen C, Xu X, Chew E, Henry CJ, Koo EH, Singapore Intervention Study To Prevent Cognitive Impairment And Disability (Singer) Initiative. Alzheimers Dement 2017; Featured Research Session: F4-09: Global Dementia Prevention Initiative: Applicability of the Multi-Domain Interventions. https://doi.org/10.1016/j.jalz.2017.07.408
United Nations General Assembly. Resolution adopted by the General Assembly on 14 December 2020: United Nations Decade of Healthy Ageing (2021–2030). 2020.
Ministry of Health Singapore. Speech By Dr Amy Khor, Senior Minister Of State For Health, At The 13th International Congress Of The Asian Society Against Dementia, Held On Thursday 29 August 2019, At The Shangri-La Hotel Singapore. In 13th International Congress Of The Asian Society Against Dementia. 2019. Singapore.
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. The Lancet Commissions 2017;390:2673–734. https://doi.org/10.1016/S0140-6736(17)31363-6
Norton S, Matthews FE, Barnes D, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurology 2014;13:788–794. https://doi.org/10.1016/S14744422(14)70136-X
Lisko I, Kulmala J, Annetorp M, et al. How can dementia and disability be prevented in older adults: where are we today and where are we going? Journal of Internal Medicine 2020;289(6):807–830. https://doi.org/10.1111/joim.13227
Moll van Charante E, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016;388:797–805. https://doi.org/10.1016/S0140-6736(16)30950-3
Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurology 2017;16:377–89. https://doi.org/10.1016/S1474-4422(17)30040-6
Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet 2015;385(9984):2255–2263. https://doi.org/10.1016/s0140-6736(15)60461-5
de Souto Barreto P, Pothier K, et al. A Web-Based Multidomain Lifestyle Intervention for Older Adults: The eMIND Randomized Controlled Trial. The Journal of Prevention of Alzheimer’s Disease ((PAD) 2020;8:142–150. https://doi.org/10.14283/jpad.2020.70
Srisuwan P, Nakawiro D, Chansirikarnjana S, et al. Effects of a Group-Based 8-Week Multicomponent Cognitive Training on Cognition, Mood and Activities of Daily Living among Healthy Older Adults: A One-Year Follow-Up of a Randomized Controlled Trial. The Journal of Prevention of Alzheimer’s Disease (JPAD) 2019;7(2):112–121. https://doi.org/10.14283/jpad.2019.42
Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 2020;16(7):1078–1094. https://doi.org/10.1002/alz.12123
Rosenberg A, Mangialasche F, Ngandu T, Solomon A & Kivipelto M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. The Journal of Prevention of Alzheimer’s Disease (JPAD) 2019;7(1):29–36. https://doi.org/10.14283/jpad.2019.41
Chew KA, Xu X, Siongco P, et al. SINgapore GERiatric intervention study to reduce physical frailty and cognitive decline (SINGER)-pilot: A feasibility study. Alzheimers Dement TRCI 2021;7(1):e12141. https://doi.org/10.1002/trc2.12141
World Health Organization. A healthy lifestyle. 2021. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle.
National Nutrition Council. Finnish nutrition recommendations - Diet and physical activity in balance. 2005, Edita: Oy, Finland.
Renzella J, Townsend N, Jewell J, et al. What national and subnational interventions and policies based on Mediterranean and Nordic diets are recommended or implemented in the WHO European Region, and is there evidence of effectiveness in reducing noncommunicable diseases? 2018. WHO Regional Office for Europe: Copenhagen.
Health Promotion Board. Health Promotion Board Introduces My Healthy Plate to Inculcate Healthy Eating Habits amongst Singaporeans. 2014. https://www.hpb.gov.sg/article/health-promotion-board-introduces-my-healthy-plate-to-inculcate-healthy-eating-habits-amongst-singaporeans
Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement 2013;9(6):657–65. https://doi.org/10.1016/j.jalz.2012.09.012
Ministry of Health Singapore. Clinical Practice Guidelines (Medical). 2017. https://www.moh.gov.sg/hpp/all-healthcare-professionals/guidelines/GuidelineDetails/clinical-practice-guidelines-medical.
The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–2116. https://doi.org/10.1056/NEJMoa1511939
Shen JHQ, Shen Q, Yu H, et al. Validation of an Alzheimer’s disease assessment battery in Asian participants with mild to moderate Alzheimer’s disease. Am J Neurodegener Dis 2014;3(3): p. 158.
Harrison J, Minassian SL, Jenkins L, et al. A neuropsychological test battery for use in Alzheimer disease clinical trials. Arch Neurol. 2007;64(9):1323–9.
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–4.
Pedrosa H, De Sa A, Guerreiro M, et al. Functional evaluation distinguishes MCI patients from healthy elderly people—the ADCS/MCI/ADL scale. J Nutr Health Aging 2010;14(8):703–9.
Snowdon J. Validity of the geriatric depression scale. Am Geriatr Soc. 1990;38(6):722–3.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh sleep quality index (PSQI). Psychiatry Res. 1989;28(2):193–213.
Smith G, Del Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory 2000;8(5):311–21.
Bull FC, Maslin TS, Armstrong T. Global Physical Activity Questionnaire (GPAQ): Nine Country Reliability and Validity Study. Journal of Physical Activity and Health 2009;6(6):790–804.
Karp A, Paillard-Borg S, Hui-Xin W, et al. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders 2006;21(2):65–73.
Delbaere K, Van den Noortgate N, Bourgois J, Vanderstraeten G, Tine W, Cambier D. The Physical Performance Test as a predictor of frequent fallers: a prospective community-based cohort study. Clin Rehabil. 2006;20(1):83–90.
McHorney CA, Ware Jr JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3): p. 247–63.
Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5): p. 328–36.
Sano M, Zhu CW, Whitehouse PJ, et al. ADCS Prevention Instrument Project: pharmacoeconomics: assessing health-related resource use among healthy elderly. Alzheimer Dis Assoc Disord 2006;20:S191.
Ng TP, Ling LHA, Feng L, et al. Cognitive Effects of Multi-Domain Interventions Among Pre-Frail and Frail Community-Living Older Persons: Randomized Controlled Trial. Journals of Gerontology: Medical Sciences 2018;73:806–812. https://doi.org/10.1093/gerona/glx207
World-Wide FINGERS. A global network of clinical trials for prevention and risk reduction of dementia. 2021. https://wwfingers.com/.
Acknowledgements
The authors thank Dr. Miia Kivipelto, Dr. Tiia Ngandu, Dr. Alina Solomon and the FINGER team for their support and guidance throughout the planning of the project. The authors would also like to acknowledge Dr. Francesca Mangialasche, Dr. Emily Meyers, Dr. Heather Snyder, Dr. Laura Baker, Dr. Susan Landau and the US-POINTER team for their help in the development and harmonisation of the study protocol design.
Funding
Funding: This study is funded by a National Medical Research Council of Singapore Open Fund Large Collaborative Grant (OF-LCG). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: X. Xu has nothing to disclose. K. Chew has nothing to disclose. ZX. Wong has nothing to disclose. A. Phua has nothing to disclose. E. Chong has nothing to disclose. C. Teo has nothing to disclose. N. Sathe has nothing to disclose. YC. Chooi has nothing to disclose. W. Chia has nothing to disclose. C. Henry has nothing to disclose. E. Chew has nothing to disclose. MC. Wang has nothing to disclose. AB. Maier has nothing to disclose. N. Kandiah has nothing to disclose. C. Chen reports grants from the National University of Singapore and National Medical Research Council.
Ethical standards: The SINGER study has been approved by the National Healthcare Group Domain-Specific Review Board and is registered under ClinicalTrials.gov (ID: NCT05007353). Written informed consent will be obtained from all participants before enrolment into the study. The study will be conducted in accordance with Good Clinical Practice guidelines.
Supplementary Material
Rights and permissions
About this article
Cite this article
Xu, X., Chew, K.A., Wong, Z.X. et al. The SINgapore GERiatric Intervention Study to Reduce Cognitive Decline and Physical Frailty (SINGER): Study Design and Protocol. J Prev Alzheimers Dis 9, 40–48 (2022). https://doi.org/10.14283/jpad.2022.5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2022.5