Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
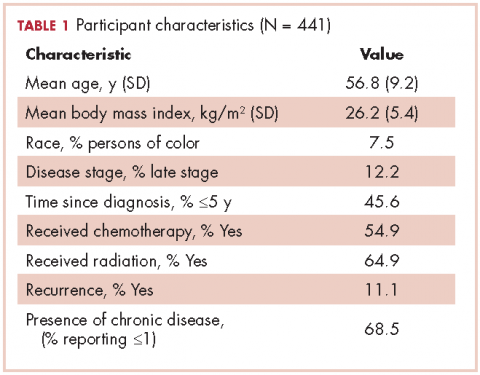
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
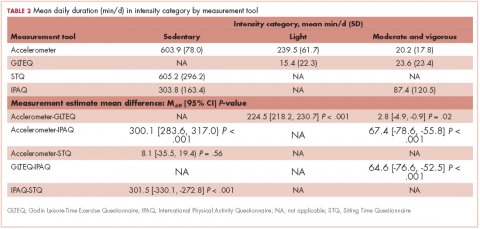
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
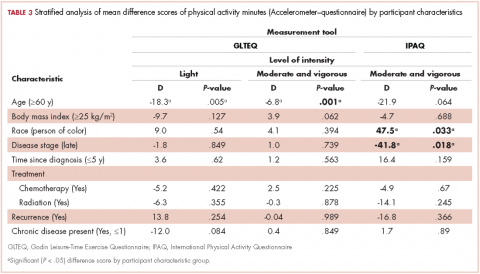
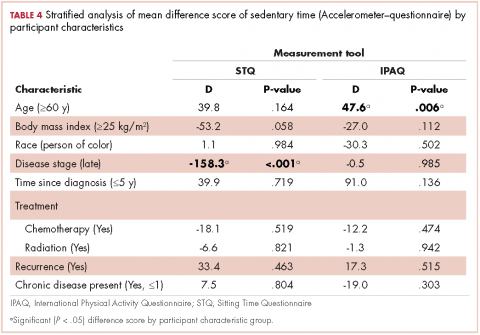
Also shown are the results of the stratified analyses to investigate whether congruence among the questionnaires and accelerometer measures were different based on participant characteristics for physical activity (Table 3) and ST (Table 4) estimates.Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are currently being used to estimate physical activity in breast cancer survivors and would provide useful information regarding how breast cancer survivors report their physical activity time.
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.