-
PDF
- Split View
-
Views
-
Cite
Cite
Dolores D. Mruk, C. Yan Cheng, The Mammalian Blood-Testis Barrier: Its Biology and Regulation, Endocrine Reviews, Volume 36, Issue 5, 1 October 2015, Pages 564–591, https://doi.org/10.1210/er.2014-1101
Close - Share Icon Share
Spermatogenesis is the cellular process by which spermatogonia develop into mature spermatids within seminiferous tubules, the functional unit of the mammalian testis, under the structural and nutritional support of Sertoli cells and the precise regulation of endocrine factors. As germ cells develop, they traverse the seminiferous epithelium, a process that involves restructuring of Sertoli-germ cell junctions, as well as Sertoli-Sertoli cell junctions at the blood-testis barrier. The blood-testis barrier, one of the tightest tissue barriers in the mammalian body, divides the seminiferous epithelium into 2 compartments, basal and adluminal. The blood-testis barrier is different from most other tissue barriers in that it is not only comprised of tight junctions. Instead, tight junctions coexist and cofunction with ectoplasmic specializations, desmosomes, and gap junctions to create a unique microenvironment for the completion of meiosis and the subsequent development of spermatids into spermatozoa via spermiogenesis. Studies from the past decade or so have identified the key structural, scaffolding, and signaling proteins of the blood-testis barrier. More recent studies have defined the regulatory mechanisms that underlie blood-testis barrier function. We review here the biology and regulation of the mammalian blood-testis barrier and highlight research areas that should be expanded in future studies.
- I.
Introduction
- II.
Function and Structure of the Blood-Testis Barrier
- A.
Function
- B.
Structure
- A.
- III.
TJ Proteins of the Blood-Testis Barrier
- A.
Structural proteins
- B.
Scaffolding proteins
- C.
Signaling proteins
- A.
- IV.
Mechanisms of Blood-Testis Barrier Restructuring
- A.
Phosphorylation
- B.
Endocytosis
- A.
- V.
Future Directions in the Study of the Blood-Testis Barrier and Concluding Remarks on the Status of Male Contraceptive Research
I. Introduction
Spermatogenesis is comprised of a chronological series of cellular events that result in the production of mature spermatids. It initiates on postnatal day 5 in the rat, and it occurs within seminiferous tubules, the functional unit of the mammalian testis, under the regulation of several endocrine factors that include testosterone, FSH, LH, and estrogen. This cellular process takes approximately 48–53 days in the rat (for reviews, see Refs. 1–4). The seminiferous epithelium contains 2 types of cells, Sertoli and germ cells. Sertoli cells are polarized epithelial cells that extend from the base of the seminiferous tubule to its lumen. They send out extensive cytoplasmic processes that contact adjacent Sertoli cells and developing germ cells, which form the basis of the specialized cell junctions in the seminiferous epithelium. Spermatogenesis begins with type A spermatogonia that either self-renew by mitosis or differentiate into type B spermatogonia. Type B spermatogonia, which are connected by cytoplasmic bridges (for a review, see Ref. 5), subsequently detach from the basement membrane and give rise to preleptotene spermatocytes, followed by leptotene, zygotene, pachytene, and diplotene spermatocytes. Thereafter, spermatocytes undergo diakinesis, which completes meiosis I, giving rise to secondary spermatocytes. Secondary spermatocytes then undergo meiosis II to produce spermatids. Thereafter, spermatids undergo spermiogenesis, a 19-step process in the rat that involves acrosome formation, tail elongation and maturation, and nuclear changes to produce elongated spermatids. Spermatogenesis ends with spermiation, the release of mature spermatids as spermatozoa from the seminiferous epithelium. Furthermore, Sertoli and germ cells are not the only cells with roles in spermatogenesis. Peritubular myoid cells, contractile cells that encircle seminiferous tubules, function in the expulsion of spermatozoa out of seminiferous tubules and into the epididymis (6, 7). On the other hand, Leydig cells residing in the interstitium secrete testosterone in the presence of LH. Testosterone is needed for the maintenance of the blood-testis barrier, spermatogenesis, and fertility (for reviews, see Refs. 8, 9), and it promotes both Sertoli-germ cell junction assembly and disassembly (10, 11; for reviews, see Refs. 8, 12). For example, testosterone withdrawal results in the detachment of step 8–19 spermatids from the seminiferous epithelium (13, 14). Under normal physiological conditions, monocytes, macrophages, dendritic cells, T cells, natural killer cells, and mast cells are also present in the interstitium. Collectively, these cells maintain spermatogenesis in mammals.
A typical cross-section of the adult rat testis shows hundreds of seminiferous tubules, each at 1 of 14 stages of the seminiferous epithelial cycle (15, 16; for reviews, see Refs. 2, 17). These 14 stages repeat consecutively along the entire length of each seminiferous tubule in the testis, and 1 cycle is comprised of stages I–XIV. Each stage is defined by a unique arrangement of Sertoli and germ cells at different stages of development so that no 2 stages mirror each other. The 14 stages can be easily discerned by the shape of the acrosome and nucleus of spermatids, as well as by the position of elongating/elongated spermatids relative to the basement membrane. For example, spermiation involves enormous changes in Sertoli cell shape as step 19 spermatids line up along the luminal edge and then detach from the seminiferous epithelium at late stage VIII as spermatozoa. The release of spermatozoa is thought to occur in synchrony with the restructuring of the blood-testis barrier at stages VIII—XI, because the 2 events occur at opposite ends of the seminiferous epithelium. Sertoli cells create the blood-testis barrier that divides the seminiferous epithelium into 2 compartments, basal and adluminal. Spermatogonia and preleptotene spermatocytes reside in the basal compartment, whereas other primary and secondary spermatocytes, round spermatids, and elongating/elongated spermatids reside in the adluminal compartment. At late stage VIII/early stage IX, preleptotene/leptotene spermatocytes initiate passage across the blood-testis barrier, eventually entering the adluminal compartment as zygotene spermatocytes at stage XI. How preleptotene/leptotene spermatocytes cross the blood-testis barrier without disrupting spermatogenesis is one of the most intriguing questions in reproductive biology.
We review here the biology and regulation of the mammalian blood-testis barrier. We begin our review by discussing the structure and function of the blood-testis barrier and emphasizing its importance in spermatogenesis. This discussion is followed by several highly informative sections, which focus on specific proteins that contribute to the “fence” (ie, structural and scaffolding proteins) and “gate” (ie, signaling) functions of the tight junction (TJ), the most important component of the blood-testis barrier and the focus of this review. Although these sections are largely meant to serve as reference guides for future studies, we also provide insights on how the blood-testis barrier can be studied. Lastly, we discuss 2 mechanisms (ie, protein phosphorylation and endocytosis) in which proteins are rapidly regulated to bring about blood-testis barrier restructuring.
II. Function and Structure of the Blood-Testis Barrier
A. Function
The blood-testis barrier, which is essential for spermatogenesis, is located near the base of the seminiferous tubule, where it divides the epithelium into 2 distinct compartments, basal and adluminal. Spermatogonia and preleptotene spermatocytes reside in the basal compartment, whereas other primary and secondary spermatocytes, round spermatids, and elongating/elongated spermatids reside in the adluminal compartment. Thus, the function of the blood-testis barrier is to sequester germ cells residing in the adluminal compartment from the circulatory and lymphatic systems, and together with local immune suppression, to provide an immunoprivileged microenvironment for the completion of meiosis (for reviews, see Refs. 18–21). However, spermatogonia and preleptotene spermatocytes residing in the basal compartment are exposed to circulatory and lymphatic systems. Although a third transient compartment also exists at stages VIII–XI (22–25; for reviews, see Refs. 26–28), the blood-testis barrier creates 2, not 3, compartments. The intermediate compartment (Figure 1), which seals leptotene spermatocytes away from basal and adluminal compartments, is somewhat analogous to a hospital isolation room where there are 2 doors, both of which cannot be opened at the same time. As leptotene spermatocytes transit toward the tubule lumen, junction disassembly ahead of spermatocytes is coordinated with junction assembly behind these germ cells so that the 2 spermatogenic events are synchronized. In addition to Sertoli cells, peritubular myoid cells also contribute to barrier function. Interestingly, these cells prevent the entry of electron-opaque tracers (eg, lanthanum nitrate) into approximately 85% of rodent seminiferous tubules (for reviews, see Refs. 26, 29). Presently, it is not known why lanthanum nitrate penetrates some rodent seminiferous tubules and not others. Although TJs are present between peritubular myoid cells, they are not extensive and they remain poorly characterized (for a review, see Ref. 26). For example, TJ proteins that are expressed by Sertoli cells do not appear to be expressed by peritubular myoid cells, and it is not known how peritubular myoid cells contribute to barrier function. Thus, future studies should investigate whether peritubular myoid cells can enhance Sertoli cell barrier function in vitro. After the release of spermatozoa from the seminiferous epithelium, they remain isolated from the circulatory and lymphatic systems by blood-epididymis and blood-vas deferens barriers.
An electron micrograph of the intermediate compartment in the adult rat testis. This image shows 2 leptotene spermatocytes connected by intercellular bridges and enclosed within the intermediate compartment at stages IX–XI of the seminiferous epithelial cycle. The blood-testis barrier, which is created by Sertoli cells (SCs), is visible by the precipitation of lanthanum nitrate (arrowheads), which fails to advance beyond the TJs. Scale bar, 1 μm. (Reproduced from figure 1(e) of Mruk and Cheng [233] and used with permission.)
B. Structure
The blood-testis barrier is different from other tissue barriers in that it is composed of 4 different cell junctions. For example, TJs between capillary endothelial cells that line the cerebral vasculature form the blood-brain barrier, with astrocytes, pericytes, neurons, and the basal lamina indirectly regulating blood-brain barrier function. Capillary endothelial cells create a permeability barrier that is approximately 50–100 times tighter than the one present between peripheral endothelial cells, which is leaky, and they yield a maximal transendothelial electrical resistance measurement of 1500–2000 Ω/cm2 when cultured in vitro (for reviews, see Refs. 30–33). The blood-testis barrier is another example of “one of the tightest junctions” in the mammalian body (34). The concept of the blood-testis barrier is based on early biochemical studies that analyzed testicular fluid for specific proteins, amino acids, and ions, and on subsequent morphological studies that examined the passage of iv-injected dyes and large electron-opaque tracers into seminiferous tubules (22, 23; for reviews, see Refs. 26–28). Results from in vitro studies support the presence of a Sertoli cell barrier, except that its transepithelial electrical resistance measurement is 60–80 Ω/cm2 (35, 36), indicating the Sertoli cell barrier is leaky compared with the endothelial cell barrier. Although the reason(s) for this difference in Sertoli cell barrier function between in vitro and in vivo systems is not known, it may be due to the absence of peritubular myoid and germ cells in Sertoli cell cultures. As previously stated, peritubular myoid cells contribute to blood-testis barrier function in vivo, whereas germ cells regulate the Sertoli cell barrier in vitro (37), suggesting a better in vitro system is needed. Another reason for this difference between in vitro and in vivo systems may be due to the absence of a specific biological factor(s) that can enhance barrier function.
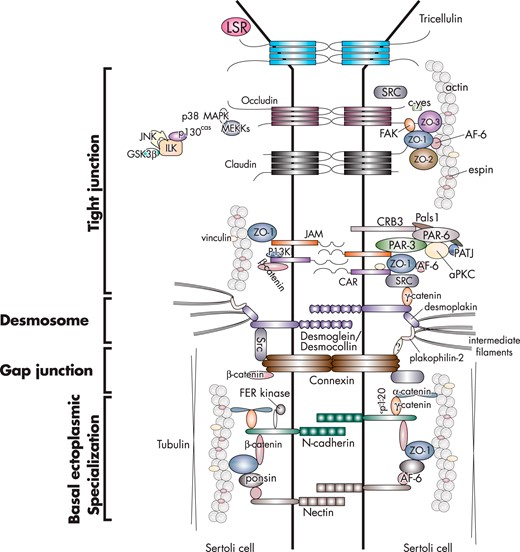
The blood-testis barrier is created by TJs, ectoplasmic specializations, desmosomes, and gap junctions that are present between Sertoli cells (for reviews, see Refs. 27, 38–44) (Figure 2). In vivo, there is no blood-testis barrier between Sertoli and germ cells, as well as between germ cells. The TJ has gate and fence functions, and it is the most important component of the blood-testis barrier. The gate function prevents the passage of water, solutes, and other large molecules between the paracellular space (ie, thereby creating a barrier), whereas the fence function restricts the movement of proteins and lipids between apical and basolateral domains (ie, thereby generating cell polarity). TJs in the testis coexist and cofunction with ectoplasmic specializations (Figure 3). Desmosomes and gap junctions are present between regions of the plasma membrane that contain TJs and basal ectoplasmic specializations (Figure 3). Desmosomes are cell-cell junctions that mediate robust adhesion, whereas gap junctions are cell-cell channels that allow diffusion of metabolites, second messengers, ions, and other molecules smaller than 1 kDa (for reviews, see Refs. 27, 38, 41, 44). TJs, ectoplasmic specializations, and gap junctions attach to actin microfilaments (for reviews, see Refs. 38, 45–47), whereas desmosomes attach to intermediate filaments (ie, vimentin) (48; for reviews, see Refs. 38, 41, 49) (Figure 4). Although recent studies in nontesticular cells show cross talk among actin microfilaments, intermediate filaments, and microtubules (for reviews, see Refs. 50–55), this is a largely unexplored area of research in the testis.
An illustration of the different types of cell junctions in the seminiferous epithelium of the adult rat testis. There are 4 types of cell junctions in the testis: TJs, ectoplasmic specializations, desmosomes, and gap junctions.
An electron micrograph of the blood-testis barrier in the adult rat testis. The blood-testis barrier is comprised of TJs, basal ectoplasmic specializations (ESs), desmosomes (DSs), and gap junctions (data not shown). TJs are typified by “kisses,” regions of contact between Sertoli cell plasma membranes (arrowheads). The basal ES is characterized by bundles of actin microfilaments (asterisks) positioned between the Sertoli cell plasma membrane and cisternae of endoplasmic reticulum (er). DSs are typified by electron dense material between Sertoli cells. Scale bar, 0.75 μm. (Reproduced from figure 4A of Sarkar et al [267] and used with permission).
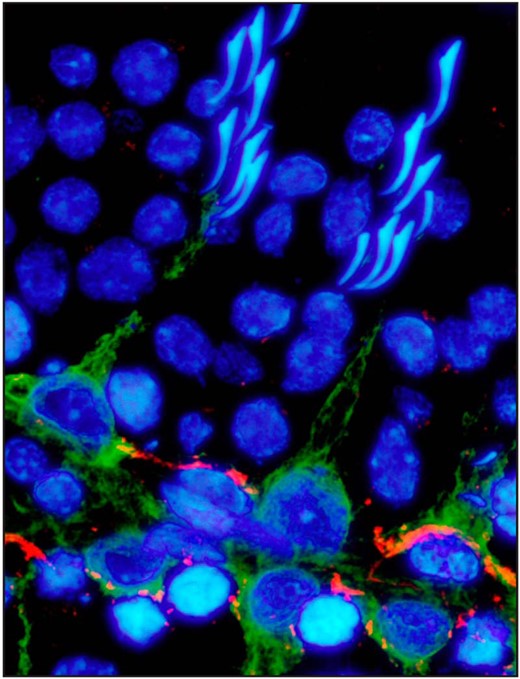
The localization of vimentin and γ-catenin in the adult rat testis. Testes were cryosectioned, fixed with methanol, and fluorescently immunostained for vimentin (green), a protein of the intermediate filament cytoskeleton, and γ-catenin (red), a protein of the desmosome and basal ectoplasmic specialization. This image shows a seminiferous tubule at stage IV of the seminiferous epithelial cycle. Vimentin localized to the Sertoli cell stalk, whereas plakoglobin localized at the blood-testis barrier. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole. Scale bar, 10 μm.
In addition to TJs, the blood-testis barrier is also created by ectoplasmic specializations, testis-specific adhesion junctions comprised of hexagonally arranged filamentous actin microfilaments positioned between the plasma membrane and a cistern of endoplasmic reticulum. Ectoplasmic specializations between Sertoli cells are defined as basal ectoplasmic specializations, whereas those between Sertoli cells and elongating/elongated spermatids are defined as apical ectoplasmic specializations (for reviews, see Refs. 38, 45). Ectoplasmic specialization-mediated adhesion is largely constituted by the cadherin-catenin multifunctional complex (for reviews, see Refs. 56, 57). Interestingly, the blood-testis barrier is compromised and fertility declines in mice with a heart-specific deletion of N-cadherin (Cdh2) (58), indicating cadherin is essential for barrier function. Furthermore, TJs and ectoplasmic specializations are very susceptible to damage caused by environmental toxicants or heat stress. For example, an increase in the scrotal temperature results in germ cell apoptosis, thereby inducing oligospermia or azoospermia in several species (59–62). When this occurs, there are reversible changes in the levels of TJ and ectoplasmic specialization proteins. Furthermore, the permeability of the blood-testis barrier is also affected, suggesting these changes are partly due to the unique organization of cell junctions within this structure.
III. TJ Proteins of the Blood-Testis Barrier
TJs constitute a specialized microdomain of continuous anastomosing fibrils that surround cells in a belt-like manner. Fibrils from adjacent cells periodically come together, which seals the paracellular space between cells and creates an impermeable barrier (Figure 3). Each fibril is comprised of an integral membrane protein that directly or indirectly associates with scaffolding and signaling proteins, as well as with cytoskeletal proteins (for a review, see Ref. 63).
A. Structural proteins
Tremendous progress has been made to identify, characterize, and study the proteins of the TJ, with the last integral membrane protein being identified in epithelial cells in 2005 (64). However, the proteins of the blood-testis barrier and their regulation remain understudied. In the next sections, we summarize results from studies on occludin, claudin, and tricellulin, the key structural proteins of TJ fibrils. We also discuss coxsackie and adenovirus receptor, because it is postulated to function in the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier.
Occludin
Occludin was identified after a chick liver junction fraction was used to produce a monoclonal antibody against an approximately 65-kDa protein (Figure 5) (65). Since this time, several splice variants of occludin have also been identified. However, these splice variants have not been studied in any organ thus far. Occludin is comprised of 4 membrane-spanning domains, 2 extracellular loops, and 2 intracellular segments, and it is expressed by several cell types and organs that include the brain and liver (for reviews, see Refs. 66, 67). Occludin is also expressed by the mouse and rat testis, but not by the human and guinea pig testis (68). The C terminus of occludin, which is rich in Ser, Thr, and Tyr residues, binds several proteins (for reviews, see Refs. 69–71) (Figure 5). Although occludin overexpression in epithelial cells increases transepithelial electrical resistance (72, 73), TJ fibrils are normal in occludin-deficient embryonic stem cells (74). Furthermore, zona occludens (ZO) proteins are still recruited to the TJ in occludin-deficient intestinal epithelial cells (75), revealing occludin is important, but not required, for barrier function. Occludin knockdown also affects the actin cytoskeleton, which is mediated by ras homolog family member A (76), a small GTPase (for a review, see Ref. 77). This is interesting because occludin can localize to the leading edge of migrating MDCK cells (78), and occludin overexpression induces spreading of AC2M2 cells (79). After occludin knockdown, atypical protein kinase C (PKC), par-3 family cell polarity regulator, and protein associated with Lin-7 homolog A (PALS)1-Pals-associated TJ protein (PATJ) fail to accumulate at the leading edge, and phosphoinositide-3-kinase (PI3K) fails to be activated, resulting in a reduced number of cell protrusions (78). These findings illustrate that occludin controls the localization of polarity proteins, the establishment of cell polarity, and the direction of cell movement. Occludin may have a similar role in the testis and facilitate the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier.
An illustration of the main proteins of the TJ, ectoplasmic specialization, desmosome, and gap junction at the blood-testis barrier in the adult rat testis.
Occludin-null mice present with several abnormalities, ranging from inflammation and hyperplasia of the gastric epithelium to calcification of the brain and atrophy of seminiferous tubules (80), as well as germ cell exfoliation and infertility in mice older than 10 weeks of age (234). However, these pathophysiological states are not life threatening, and occludin-null mice reach adulthood. In addition, synthetic peptides corresponding to part of the second extracellular loop sequence increase paracellular permeability in A6 and Sertoli cells by disrupting loop-loop interactions (81–83). The importance of this extracellular loop in TJ dynamics is further strengthened by the finding that it is required for the localization of occludin at the TJ (84). On polyacrylamide gels, occludin runs as a series of protein bands from approximately 62 to 82 kDa, which is due to protein phosphorylation, as well as other posttranslational modifications. In general, Ser/Thr-phosphorylated occludin concentrates at the TJ, whereas nonphosphorylated and nonjunctional occludin localizes within cytoplasmic vesicles (85). Taken collectively, these findings suggest that studies of TJ function, regardless of the cell type or organ in which they are performed, should include occludin as a target protein. However, other integral membrane proteins with a gate function at the TJ should be investigated as well.
Claudins
In mammals, the claudin family consists of at least 24 members (Figure 5) that are categorized into classical (claudins 1–10, 14, 15, 17, and 19) or nonclassical (claudins 11–13, 16, 18, and 20–24) types according to sequence similarities (86). Claudins form TJ fibrils based on a study that showed the overexpression of claudin 1 or 2 to induce TJ assembly in fibroblasts, which inherently lack TJs (87). Most cells express more than 2 claudins that together determine the overall paracellular electrical resistance and paracellular charge selectivity of TJs. In other words, a claudin molecule can associate with another claudin molecule of the same or different kind on the adjacent cell membrane (86). Similar to occludin, synthetic peptides corresponding to part of the claudin sequence disrupt claudin-claudin interactions, resulting in the mislocalization of claudin (88). In EpH4 cells, the mislocalization of claudin leads to the mislocalization of occludin (89). Claudins also mediate Ca2+-independent adhesion (90) and serve as receptors or coreceptors for the entry of different toxins and viruses such as Clostridium perfringens enterotoxin, HIV, and hepatitis C virus into cells (91–95).
In general, claudins 4, 5, 8, 11, 14, and 18 increase paracellular electrical resistance, whereas claudins 2, 7, 10, 15, and 16 increase paracellular cation permeability (86). Presently, it is not known whether claudin function is restricted to TJ assembly and maintenance because claudins also participate in other events such as the epithelial-mesenchymal transition, invasion, and cell migration. For example, matrix metalloproteases 3, 7, and 8 are up-regulated several-fold in the intestine of Cldn7−/− mice, causing epithelial cell sloughing, mucosal ulcerations, and inflammation (96). Moreover, the deletion of claudin 7 in vivo decreases the expression and affects the localization of integrin α2 (96), suggesting claudins indirectly regulate extracellular matrix degradation via matrix metalloproteases. These events likely trigger the production of cytokines to bring about extracellular matrix degradation and/or cell junction disassembly, because cytokines are known to regulate TJ function in several epithelial cells, including Sertoli cells (for reviews, see Refs. 97–99). Similar to occludin, there is a direct link between claudin function and cell motility. These events are mediated by neuronal Wiskott-Aldrich syndrome protein (N-WASP) (a ubiquitous protein that promotes actin nucleation) and ras homolog (RHO)-associated protein kinase (a Ser/Thr kinase that regulates the cytoskeleton), because claudin 5-deficient HECV cells cannot respond to wiskostatin (an N-WASP inhibitor) and Y-27632 (a RHO-associated protein kinase inhibitor) (100). The phosphorylation of claudins by protein kinases is also critical for TJ function (101), and nonphosphorylated claudin is rapidly internalized and degraded in lysosomes (102, 103).
In the testis, claudins 3 and 11 are highest at stages VI–VIII and V–VII, respectively, which precede the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier (104). Moreover, the expression of claudin 3, but not claudin 11, decreases in Arinvflox(exl-neo)Y;Tg(Amh-Cre) mice, which present for conditional androgen sensitivity and azoospermia (105), illustrating claudin 3 is regulated by androgens. The permeability of the blood-testis barrier is also affected in these mice based on a functional assay (105), revealing other claudin molecules cannot functionally substitute for its loss in the testis. These findings also illustrate that androgens promote barrier integrity (105–108). Equally important, claudin 3 is present at new TJs present behind migrating spermatocytes, but it is absent at old TJs present ahead of migrating spermatocytes (24, 105). Besides androgens, hormones (ie, FSH), cytokines (ie, TNFα, TGFβ), and germ cells also regulate claudin expression in Sertoli cells (109>–113). Furthermore, Sertoli cell polarity and TJ function are disrupted in Cldn11-null mice, resulting in Sertoli cell detachment and sloughing (114). These findings are in agreement with an earlier study that shows Cldn11−/− mice to be infertile due to the inability of germ cells to differentiate beyond the spermatocyte stage (115). Sertoli cells also proliferate in Cldn11−/− mice (114), suggesting claudin 11 inhibits postnatal Sertoli cell proliferation. Interestingly, this phenotype is similar to that observed in mice with a conditional deletion of connexin 43 (116), a protein of the gap junction that participates in blood-testis barrier function (117, 118). The detachment of Sertoli cells from the basement membrane also suggests that TJ proteins indirectly regulate hemidesmosome function. Besides claudins 3 and 11, the testis also expresses claudins 1, 4, 5, 7, and 8 (for a review, see Ref. 27). Of these, claudin 5 is expressed by Sertoli cells, preleptotene/leptotene spermatocytes, and spermatogonia (119). Presently, it is not known why germ cells express claudin 5, because they do not assemble TJs, but it is likely that claudin 5 facilitates spermatocyte movement across the blood-testis barrier (for a review, see Ref. 46).
Tricellulin and lipolysis-stimulated lipoprotein receptor (LSR)
Tricellulin is a TJ protein that localizes at both bicellular and tricellular contacts (Figure 5) (64, 120–123). It is expressed by several epithelial and endothelial cells and organs that include the kidney, liver, lung, brain, and testis (37, 64; for a review, see Ref. 124). Five tricellulin isoforms (TRIC-a–TRIC-e) have been identified (125, 126), and the largest isoform (TRIC-a) is the form that is referred to when the word “tricellulin” is used. At bicellular TJs, tricellulin reduces strand discontinuities, increases paracellular electrical resistance, and decreases the permeability to ions and larger solutes. At tricellular TJs, tricellulin seals epithelial sheets and excludes the passage of macromolecules (127). Interestingly, tricellulin activates cell division cycle 42 (CDC42) via TUBA (also known as dynamin binding protein), a guanine nucleotide exchange factor that promotes actin polymerization during cell junction assembly (128). Tricellulin is also critical for hearing, because a deletion of or a mutation in tricellulin results in deafness (125, 126, 129). Tricellulin contains 4 transmembrane domains and belongs to the TJ-associated family, which is also comprised of occludin (MARVELD1) and MARVELD3 (an alternatively spliced protein that colocalizes with occludin at TJs) (130). These proteins have in common a conserved MAL and related proteins for vesicle trafficking and membrane link (MARVEL) domain, and proteins having a MARVEL domain may function in events such as the biogenesis of transport vesicles (for a review, see Ref. 131).
After tricellulin is silenced by RNA interference, bicellular and tricellular TJs are disorganized, and barrier function is compromised (64). Conversely, tricellulin overexpression excludes ions and larger solutes more effectively (127). Although tricellulin overexpression does not affect the molecular composition of TJs (127), tricellulin mislocalizes to bicellular TJs when occludin is silenced in MDCK II cells (132), suggesting occludin regulates tricellular TJ assembly by excluding tricellulin from bicellular TJs. Alternatively, tricellulin compensates for the loss of occludin (132). It is postulated that tricellulin and occludin are transported to the plasma membrane via a common pathway because both proteins localize to the same site during TJ assembly, except that tricellulin relocates to tricellular contacts after these ultrastructures are formed (133). At tricellular contacts, tricellulin links claudin-based TJ fibrils to LSR, resulting in the formation of vertical TJ fibrils. LSR, which was originally identified as a receptor for triglyceride-rich lipoproteins, functions at tricellular TJs in the liver, kidney, lung, intestine, ovaries, and testis (134). LSR also recruits tricellulin to tricellular TJs after occludin excludes tricellulin from bicellular TJs (132), illustrating LSR functions upstream of tricellulin in the formation of TJs. In addition, LSR is phosphorylated at Ser288 by c-Jun N-terminal kinase, which is necessary for LSR to localize at tricellular junctions (135). Although Lsr knockdown affects tricellular TJ assembly in vitro (136), Lsr−/− mice are embryonically lethal, except for 3 males that survive to reproductive age (137). Interestingly, these mice are infertile, with one mouse unable to develop testes altogether, revealing LSR has an important role in this organ. Interestingly, cytoplasmic bridges, which connect germ cells to form long chains (138; for a review see Ref. 139), pass through tricellular contacts. This conclusion is based on the observation that testis-expressed gene 14, a marker for cytoplasmic bridges, localizes at tricellular contacts (24). Taken collectively, these findings illustrate the importance of tricellulin and LSR, as well as occludin and claudin, in TJ dynamics in the testis. Future studies should investigate the roles of tricellulin and LSR in the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier.
Coxsackievirus and adenovirus receptor (CAR)
CAR (also known as CXADR), a single-span transmembrane protein with 2 extracellular Ig domains (Figure 5), is best studied in the heart because mice deficient for Cxadr or mice with a heart-specific deletion of Cxadr before E10 are embryonically lethal due to defects in this organ. However, mice with a heart-specific deletion of Cxadr after E11 evade major cardiac abnormalities and survive into adulthood (140–142), indicating CAR is essential for early embryonic heart development. Defects in several other organs that include the intestine, lung, pancreas, and thymus are also present in adult mice with a conditional deletion of Cxadr (143). At the cellular level, CAR has several functions. For example, the loss of CAR weakens cell adhesion and promotes cell migration via a mechanism that involves SRC-dependent endocytosis of E-cadherin (ie, an elevated CAR level promotes E-cadherin endocytosis) (144). CAR also interacts with occludin (145) and ZO-1 (146). However, CAR is not a component of TJ fibrils. Furthermore, barrier integrity is disrupted by soluble CAR, which is produced either by the de novo synthesis of an alternatively spliced mRNA (147) or the proteolytic cleavage of membrane-bound CAR (146). In human glioma cells, proteolytic cleavage of CAR by a disintegrin and metalloprotease 10 releases the ectodomain (∼32 kDa), whereas intramembrane proteolysis by presenilin/γ-secretase sends the intracellular domain (∼14 kDa) to the nucleus (148). Presently, it is not known whether a similar mechanism exists in the testis. Lastly, CAR functions as a receptor for type B coxsackieviruses and subgroup C adenoviruses. Although it is not completely clear how viruses gain access to CAR, which is sequestered within TJs, they likely disrupt TJ function by triggering the production of proinflammatory cytokines.
In the testis, CAR localizes to the blood-testis barrier, preleptotene/leptotene spermatocytes within the intermediate compartment, the convex side of the elongated spermatid head at stage VIII, and the acrosome (149–151). After Cxadr knockdown in Sertoli cells, occludin internalizes and interacts with early endosome antigen 1 (152), which localizes to the cytosolic face of early sorting endosomes, indicating CAR regulates the presence of occludin at the cell surface via endocytosis. During inflammation/infection, CAR is 1 of several proteins involved in the migration of immune cells across the endothelium (153). Although a similar mechanism has been proposed to explain the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier (for a review, see Ref. 154), cell junction dynamics, barrier integrity, germ cell movement, and fertility are not affected in Cxadr-deficient mice produced by the tamoxifen-inducible Cre/lox P system (ie, incomplete knockout) (155). Interestingly, CAR also binds and stabilizes tubulin, which is critical for cell migration (156). Likewise, it is postulated that Sertoli cell microtubules (Figure 6) facilitate the movement of elongated spermatids at stages IV–VI (157, 158). Thus, future studies should investigate whether CAR is involved. Lastly, it is not known why Cxadr knockdown affects the Sertoli cell barrier in vitro but not in vivo (152, 155).
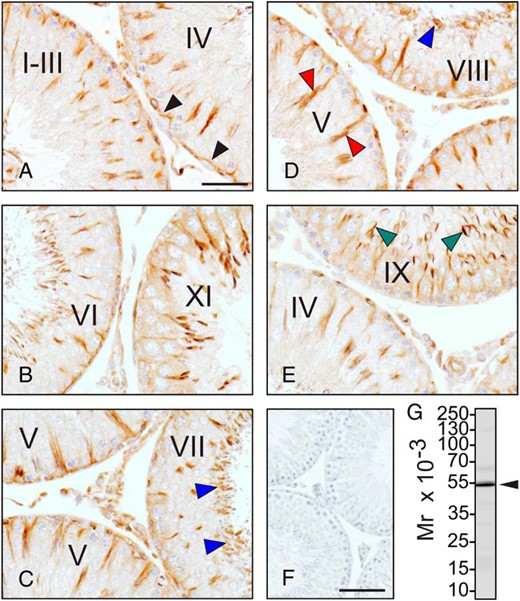
The localization of tubulin in the adult rat testis. Testes were fixed with Bouin's solution and embedded in paraffin wax. Tissue blocks were sectioned and immunostained for α-tubulin, a protein of the microtubule cytoskeleton. A–E, Seminiferous tubules at different stages of the seminiferous epithelial cycle. α-Tubulin localized at the blood-testis barrier (black arrowheads) (A), as well as to the luminal edge (blue arrowheads) (C and D), the Sertoli cell stalk (red arrowheads) (D), and the acrosome of late-step spermatids (green arrowheads) (E). There was no immunoreactive signal when the primary antibody was substituted with rabbit IgG (negative control) (F). The antibody was specific for α-tubulin when tested by immunoblotting (arrowhead) (G). The approximate positions of the molecular weight markers are shown to the left of the immunoblot. Scale bars, 50 μm (A–E) and 200 μm (F).
B. Scaffolding proteins
The cytoplasmic plaque of the TJ is the area adjacent to the TJ. It is enriched in several scaffolding proteins that bring together structural proteins and link them to the cytoskeleton (Figure 5) (for a review, see Ref. 63). These scaffolding proteins also invite signaling proteins that regulate TJ function. Furthermore, many scaffolding proteins contain putative nuclear localization sequences, indicating they transit to the nucleus to turn on or off the expression of target genes (for a review, see Ref. 159). In the next section, we discuss the ZO protein family and highlight relevant findings in the testis.
Zona occludens
ZO-1 (tight junction protein 1, TJP1), ZO-2 (TJP2), and ZO-3 (TJP3) are well-studied adaptor proteins primarily used by integral membrane TJ proteins such as occludin and claudin for attachment to the actin cytoskeleton (Figure 6). ZO-1, ZO-2, and ZO-3 belong to the membrane-associated guanylate kinase (MAGUK) family, which is comprised of 10 distinct subfamilies; ZO proteins comprise 1 of these subfamilies. Several domains define this family, but only 3 of these domains (ie, postsynaptic density 95, discs large, and ZO-1 proteins [PDZ]; Src homology [SH]3; and GUK) are found within most MAGUK family members. Specifically, ZO proteins have 3 PDZ domains, 1 SH3 domain, and 1 GUK domain (for a review, see Ref. 160). These domains allow ZO proteins to interact with several proteins that support TJ function. ZO proteins also function outside of the TJ, because they are present within the nucleus and at other cell junctions (for reviews, see Refs. 161, 162). One of these functions is to regulate cell growth and proliferation (163; for reviews, see Refs. 161, 164, 165). For example, ZO-1 inhibits cell proliferation by binding ZO-1-associated nucleic acid binding protein (ZONAB), a Y-box transcription factor, which prevents the transit of ZONAB into the nucleus, thereby inhibiting its transcriptional activity (166; for a review, see Ref. 159). APG-2, a heat shock protein that controls ZONAB transcriptional activity, competes with this transcription factor for binding to ZO-1 (167, 168). However, Apg-2 knockdown does not prevent TJ assembly. Instead, a deceleration in the rate at which ZO-2, ZO-3, and occludin are recruited to the TJ is observed without any apparent effects on the actin cytoskeleton (167). These results agree with ZO-1 knockdown/knockout studies in other cells (169, 170).
In the testis, ZO-1 also coimmunoprecipitates and colocalizes with connexin 43, and it regulates gap junctional communication (Figure 6) (171). Interestingly, phosphorylation of connexin 43 at Ser373 by protein kinase B (PKB) disrupts ZO-1-connexin 43 interactions, increases gap junction size, and promotes gap junctional communication (172), indicating ZO-1 is a negative regulator of gap junctional communication. Furthermore, ZO-1 mislocalizes from Sertoli cell TJs after the endocytosis of gap junctions is triggered by γ-hexachlorocyclohexane (171). Although the mislocalization of ZO-1 may be due to its interaction with connexin 43, ZO-1 binds other integral membrane proteins such as occludin and claudin (75, 173). Interestingly, these proteins could not retain ZO-1 at the plasma membrane. Not surprisingly, both Tjp1- and Tjp2-deficient mice are embryonically lethal; Tjp1-deficient mice die midgestation (174), whereas Tjp2-deficient mice die postimplantation (175). When the lethality of Tjp2 knockout mice is rescued by injecting Tjp2−/− stem cells into wild type blastocysts to produce ZO-2 chimera, subfertility ensues (176). Although the expression of ZO-1 and ZO-3 is unaffected, blood-testis barrier function is compromised in these mice (176). By contrast, Tjp3-deficient mice show no apparent phenotype (176).
ZO-1 and ZO-2 also promote claudin polymerization, which is essential for TJ assembly (177). For example, epithelial cells polarize but fail to assemble TJs in the absence of claudin polymerization caused by the complete or partial loss of ZO-1 and ZO-2 (177). Claudins also fail to polymerize after the N terminus of ZO-1, which contains PDZ domains 1–3, is introduced into Tjp1 knockout/Tjp2 knockdown cells (177). Instead, PDZ domains 1–3, the SH3 domain, and the GUK domain are all required, indicating SH3 and GUK domains are indispensable for claudin polymerization. Other studies demonstrate that afadin, an actin-binding protein that regulates cell adhesion, is critical for TJ assembly because its knockdown prevents the recruitment of TJ proteins to sites of cell adhesion (178). In the testis, ZO-1 localizes to the blood-testis barrier, but also to the apical ectoplasmic specialization, which surrounds elongated spermatids (179; for a review, see Ref. 27). Furthermore, germ cells do not express ZO-1 (37). ZO-1 localizes to the convex side of the head of elongated spermatids at stages IV–VI of the seminiferous epithelial cycle when spermatids move toward the basement membrane and embed within Sertoli cell crypts. Thus, future studies should investigate whether ZO-1 directly or indirectly interacts with tubulin (Figure 6), tubulin-binding proteins (eg, end-binding protein, a plus-end tracking protein), and/or microtubule-dependent motor proteins (eg, dynein, a minus-end directed motor protein). The involvement of connexin 43 in spermatid movement during these stages should also be investigated because it binds ZO-1. For example, a disruption of ZO-1-connexin 43 interactions may affect spermatid movement at these stages. The use of staged seminiferous tubules provides a good starting point for future experiments.
C. Signaling proteins
Polarized epithelial cells are divided into distinct apical and basal domains that are created by the asymmetric distribution of proteins, lipids, RNA and/or cytoskeletal components (for reviews, see Refs. 180–183). Cell polarity is mediated by 3 well-conserved protein modules: 1) the apical Crumbs (CRB)-PALS-PATJ complex; 2) the apicolateral partitioning-defective (PAR)3-PAR6-atypical protein kinase C (aPKC) complex; and 3) the basolateral Scribble (SCRIB)-discs-large (DLG)-lethal giant larvae (LGL) complex (for reviews, see Refs. 184–187). These 3 modules function together in the formation of cell polarity, and in the assembly and maintenance of TJs. A fascinating feature of these protein modules is that they antagonize each other, and manipulating different proteins within them can alter the relative surface area of the apical and basal domains, resulting in the formation of cuboidal, columnar, or squamous cell shapes (for a review, see Ref. 188). Although these 3 modules localize to the apical/apicolateral domain in most cells, they are mostly confined to the basal/basolateral domain in Sertoli cells. In the next sections, we discuss these 3 protein modules. We will not discuss planar cell polarity, which refers to the polarity of cells within the horizontal plane.
The CRB-PALS-PATJ cell polarity complex
CRBs are single-span transmembrane proteins that regulate cell polarity, and the CRB-PALS-PATJ cell polarity complex is the only polarity protein module to contain a transmembrane protein (189; for reviews see Refs. 183, 186, 187, 190, 191) (Figure 5). CRBs also function outside of cell polarity; they are tumor suppressors (for reviews, see Refs. 192–194), as well as regulators of cell division, survival, and growth (195–199). Three CRBs exist in mammals: CRB1, CRB2, and CRB3 (200; for a review, see Ref. 186), and several organs that include the brain and testis express Crb3, the most widely expressed Crb (201, 202). The short cytoplasmic domain of CRB, which consists of a membrane proximal juxtamembrane domain and a C-terminal PDZ-binding motif, is critical for apical domain formation. In addition, the juxtamembrane domain binds proteins (eg, talin, ezrin, radixin, moesin, focal adhesion kinase [FAK]) (for a review, see Ref. 203) that contain a 4.1 protein, ezrin, radixin, and moesin binding domain (189; for reviews, see Refs. 204, 205). These results illustrate that cell polarity involves cross talk among the plasma membrane (ie, TJ), cytosol (ie, cytoskeleton), and extracellular matrix (ie, focal contact and/or hemidesmosome). Interestingly, crb overexpression in Drosophila ectodermal epithelial cells results in the expansion of the apical domain at the expense of the basal domain (206), whereas Crb3 overexpression in MDCK cells delays the assembly of TJs (207). Likewise, the loss of Crb3 disrupts TJ function (208, 209), illustrating CRB manipulation affects TJ function. In Drosophila, Crb regulates the Hippo signaling pathway (196, 210; for a review, see Ref. 211), which controls organ growth, and the Notch signaling cascade, which controls development, differentiation, and survival (for reviews, see Refs. 212, 213). Notch receptors, single-span transmembrane proteins that are activated by cell-cell contact, are cleaved by a disintegrin and metalloprotease or γ-secretase upon ligand binding. This process generates an intracellular fragment, which transits to the nucleus to turn on the expression of target genes that control growth and development (for reviews, see Refs. 214, 215). Crb inhibits the γ-secretase complex (199, 216), illustrating it is a negative regulator of the Notch signaling pathway. In the testis, aberrant Notch signaling results in defects in germ cell fate, an increase in germ cell apoptosis, and infertility (217–219). However, the role of CRB in blood-testis barrier dynamics is not known.
PALS, a scaffolding protein of the MAGUK family, binds PATJ (220) and mammalian homolog of Lin-7 (also known as lin-7 homolog A) (221, 222) (Figure 5). In MDCK cells, Pals knockdown results in a decrease in Patj expression and an increase in barrier permeability. It also affects the targeting of aPKC to TJs, thereby disrupting cell polarity and TJ function (223). Interestingly, the loss of Pals1, but not Patj, disrupts the trafficking of newly synthesized E-cadherin to the plasma membrane and the assembly of adherens junctions in MDCK cells (224–226), suggesting the retromer complex is involved. The retromer complex sorts and transports proteins from 1) the endosome to the trans-Golgi network, 2) the endosome to the plasma membrane, and 3) the endosome to the trans-Golgi network, and then to the plasma membrane (for a review, see Ref. 227). CRB passes through the trans-Golgi network and associates with the retromer complex (228, 229). Although PALS1 sequesters E-cadherin to the adherens junction, it is not known whether the trafficking of other integral membrane proteins is also affected. Thus, additional studies are needed to determine the roles of CRB and PALS1 in protein trafficking.
PATJ is the third member of this cell polarity complex (Figure 5) (230, 231). PATJ interacts with several proteins that include claudin 1 and ZO-3, with the latter protein sequestering PATJ to the TJ (232). Similar to the knockdown of Crb and Pals, Patj knockdown disrupts TJ function (225, 226). PATJ also binds junction enriched and associated protein, which is expressed by several organs such as the brain and testis (235). Originally identified as a protein that functions in cell movement (236–238), junction enriched and associated protein removes PATJ from the plasma membrane and delivers it to the recycling endosome (239), thereby disrupting TJs. Presently, there is no detailed study on the CRB-PALS-PATJ cell polarity complex in the testis.
The PAR3-PAR6-aPKC cell polarity complex
PAR3 and PAR6 are scaffolding proteins that recruit different proteins to the TJ via their PDZ domains (Figure 5) (240, 241). They are required for the establishment and maintenance of cell polarity. PAR also participates in directional cell movement by regulating actin and microtubule cytoskeletons (242–244; for reviews, see Refs. 180, 245). Both PAR3A and PAR3B partially colocalize with ZO-1 (246–250), and they are also present at TJs that lack highly developed fibrils (251), suggesting they also function outside of the TJ. PAR3 is recruited to the plasma membrane by binding junctional adhesion molecule-A (JAM-A), which is present at newly assembled TJs before PAR3 (252–255). This is followed by the drafting of PAR6 and aPKC (256). At the apical domain, the PAR complex facilitates the apical localization of the CRB complex and the basolateral localization of the SCRIB complex, thereby promoting cell polarity. Specifically, PAR3 interacts with CRB via PAR6 and aPKC. PAR3 also interacts with 14–3-3 (also known as PAR5) (257; for a review, see Ref. 258), but this is largely dependent on the phosphorylation state of PAR3, which is regulated by aPKC (259, 260). Interestingly, there is a delay in the delivery of proteins (ie, ezrin) to the apical domain of MDCK cells after Par3 knockdown (261), illustrating PAR3 is important for apical domain development, TJ maturation, and cytoskeletal remodeling. Likewise, Par3 knockdown in Sertoli cells disrupts the delivery of JAM-A and ZO-1 to sites of cell-cell contact after calcium switch (201). However, the significance of these findings is not clear because JAM-A is recruited to the plasma membrane independent of PAR3. Furthermore, only 1 out of 3 PAR isoforms was silenced by approximately 50%, indicating Sertoli cells still expressed Par3 after knockdown. Furthermore, the splice variant of Par3 (Par3c, it lacks residues 827–856 within the aPKC binding domain) can functionally substitute for the loss of canonical Par3 during the morphogenesis of dendritic spines (262). Thus, Par3c likely compensates for the loss of Par3 expression in Sertoli cells. Presently, additional studies are needed to determine the role of the retromer complex in PAR-mediated protein trafficking.
On the other hand, PAR6 is encoded by 3 different genes, Par6A/C, Par6B, and Par6D/G. Besides binding PAR3 and aPKC (263–266), PAR6 also interacts with active CDC42 and ras-related C3 botulinum toxin (RAC) (263, 267–269). This interaction allows CDC42 to activate PAR6, PAR3, and aPKC, thereby assembling a CDC42-PAR3-aPKC-PAR6 module. This protein module then associates with T-cell lymphoma invasion and metastasis 1 (a RAC-specific guanine nucleotide exchange factor that is highly expressed by the brain and testis) (270), resulting in RAC1 activation, cytoskeletal remodeling (ie, actin polymerization and microtubule stabilization) (243, 271), lamellipodia formation, and cell migration (272). In addition, PAR6C regulates cell polarity by functioning downstream of TGFβ in NMuMG cells (273). Specifically, TGFβ receptor II phosphorylates PAR6C, resulting in the recruitment of Smad ubiquitination-related factor 1 (an E3 ubiquitin ligase) (for a review, see Ref. 274), degradation of ras homolog family member A, disruption of TJs, and induction of the epithelial-mesenchymal transition (273, 275, 276). In the literature, few studies address PAR6 function. Par6 knockdown affects the morphogenesis of actin-rich dendritic spines (262) and the development of blastocysts (277). In another study, PAR6 interacts with MAPK organizer 1 (278), a scaffolding protein that mediates the binding of the PAR6-aPKC complex to CRB3 (278). Presently, it is not known whether these protein-protein interactions exist in the testis. In the testis, PAR6 interacts with fascin1, an actin cross-linking protein (for reviews, see Refs. 279, 280). However, immunoprecipitated PAR6 electrophoreses at a higher molecular weight than nonimmunoprecipitated PAR6, indicating additional biochemical experiments are needed to substantiate these findings. Furthermore, PAR6 does not colocalize with fascin1 (281).
The third component of this cell polarity complex is aPKC, a Ser/Thr kinase that is crucial for TJ assembly and maintenance (Figure 5). aPKCs are different from conventional PKCs, because they possess an N-terminal Phox and Bem1 domain, which allows them to interact with PAR6 (249; for a review, see Ref. 282). During junction assembly, aPKC forms a complex with PAR6 and LGL, followed by the phosphorylation and dissociation of LGL from the aPKC-PAR6 complex (283) and by the association of this complex with PAR3. It also allows aPKC to phosphorylate PAR3 at Ser827 (284, 285) and JAM-A at Ser285 (286), thereby facilitating TJ maturation. aPKC also regulates microtubule dynamics and vesicle trafficking (287–289).
The SCRIB-DLG-LGL cell polarity complex
The third cell polarity complex, SCRIB-DLG-LGL localizes to the basolateral domain of epithelial cells (for reviews, see Refs. 183, 290–292). SCRIB is a large multidomain protein that functions in cell polarity, migration, cell cycle progression, apoptosis, and protein sorting and trafficking (293–299). For example, SCRIB regulates the passage of CRB through the retromer complex via aPKC-dependent and -independent mechanisms, even though the 2 cell polarity complexes antagonize each other (300). SCRIB also functions as a tumor suppressor and as a target for viral oncoproteins (301–304; for reviews, see Refs. 194, 305). Although Scrib knockdown disrupts tight and adherens junction function in different cells (295, 306), it does not affect TJ function in Sertoli cells (307). However, the concurrent knockdown of Scrib, Dlg, and Lgl decreases its permeability, revealing the increase in barrier function is due to the loss of Dlg and/or Lgl (307). Furthermore, concurrent knockdown of Scrib, Dlg, and Lgl affects the orientation of step 19 spermatids within the seminiferous epithelium, but it is not known why a tighter blood-testis barrier would adversely affect spermatid orientation at the luminal edge. Nevertheless, Scrib knockout mice are embryonically lethal due to a defect in neural tube closure (308, 309), illustrating SCRIB is essential for normal morphogenesis. Besides binding and partially colocalizing with ZO-2 (310), SCRIB also binds pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP) (311, 312), a membrane-associated Ser/Thr protein phosphatase that inactivates PKB and PKC (for reviews, see Refs. 313, 314). After Scrib knockdown, PHLPP1 transits to the cytosol, resulting in an increase in PKB phosphorylation and cell proliferation. SCRIB is unable to bind PKB in the absence of PHLPP1, and PHLPP1 is unable to bind PKB in the absence of SCRIB, indicating these 3 proteins form a heterotrimeric complex (311). Furthermore, SCRIB colocalizes with E-cadherin (315) and stabilizes E-cadherin-p120 catenin interactions (316), which maintain cell adhesion. Taken collectively, these results illustrate that SCRIB is an important regulator of tight and adherens junctions.
DLG and LGL are the other key proteins of this cell polarity complex. DLG, a member of the MAGUK family, functions in cell polarity, vesicle trafficking, cell junction dynamics, and tumor suppression (for reviews, see Refs. 290, 317, 318). Four DLGs have been identified in mammals, with DLG1 being the best studied member of this protein family. DLG1 also localizes to the adherens junction, where it binds E-cadherin and links it to PI3K (319, 320). A disruption of E-cadherin-PI3K interactions results in the mislocalization of E-cadherin, indicating DLG1 stabilizes E-cadherin. On the other hand, 2 LGLs have been identified in mammals (for reviews, see Refs. 321, 322). Interestingly, LGL phosphorylation by aPKC is critical for its function because nonphosphorylated LGL associates with the apical domain in MDCK cells (323–325). Although Dlg1 and Lgl2 are expressed by the testis (307), their role in blood-testis barrier dynamics requires further study.
IV. Mechanisms of Blood-Testis Barrier Restructuring
The blood-testis barrier is a dynamic ultrastructure that restructures during the movement of preleptotene/leptotene spermatocytes at stages IX–XI of the seminiferous epithelial cycle. Sertoli cell junction disassembly is mediated by several mechanisms that generally involve changes in the levels, localizations, and interactions of structural, scaffolding, and signaling proteins. Many of these proteins were discussed in the preceding sections. For example, the opposing actions of kinases and phosphatases rapidly regulate the phosphorylation state of cell junction proteins, thereby altering their localization and interaction with other proteins. In this section, we discuss 2 key mechanisms, phosphorylation and endocytosis, that regulate blood-testis barrier dynamics.
A. Phosphorylation
The SRC kinase family is comprised of at least 8 members in the human: SRC, YES, FYN, and FGR, which constitute the SRC A subfamily; and LYN, HCK, LCK, and BLK, which constitute the SRC B subfamily. SRC, YES, and FYN are expressed by several organs, whereas the others are primarily expressed by lymphoid and myeloid tissues (for reviews, see Refs. 326, 327). Most cells and organs express more than one member of the SRC kinase family. In addition to these SRC kinases, at least 2 other members exist. FRK is expressed by the brain, breast, colon, and bladder, whereas BRK is expressed by the colon, small intestine, and prostate. SRC, YES, and FYN are the best studied kinases in the mammalian testis (328–332). In the next section, we summarize key results from studies on SRC.
In general, SRC kinases consist of an N-terminal 14-carbon myristoyl group attached to an SH4 domain, followed by a unique region, an SH3 domain, an SH2 domain, an SH2 kinase linker, a kinase domain (also known as the SH1 domain), and a C-terminal domain containing a negative regulatory Tyr residue (for a review, see Ref. 327). Myristoylation, a cotranslational/posttranslational modification that involves the second amino acid residue, mediates binding of the kinase to the plasma membrane, thereby facilitating weak protein-protein and protein-lipid interactions (for reviews, see Refs. 333, 334). However, myristoylation alone is not enough for plasma membrane localization. The interaction of kinases with the plasma membrane is enhanced by the palmitoylation of an N-terminal Cys residue, which promotes their association with lipid rafts, specialized regions of the plasma membrane that serve as signaling platforms (for a review, see Ref. 335). Palmitoylation is also responsible for targeting SRC and FYN to endosomes (336), indicating these kinases participate in endocytosis. On the other hand, SH2 and SH3 domains interact with proline-rich regions and phospho-Tyr motifs, respectively, to regulate protein-protein interactions.
SRC kinase activity is regulated by intra- and intermolecular interactions that involve kinases and phosphatases (337). All SRC kinase family members contain 2 highly conserved Tyr residues that are critical for activity. Tyr416 is present within the kinase domain, and Tyr527 (both in chicken SRC) is present within the negative regulatory domain. The phosphorylation of SRC at Tyr527 by C-terminal SRC kinase or C-terminal SRC kinase homologous kinase brings this amino acid residue closer to the SH2 domain, rendering SRC catalytically inactive and unable to bind substrates. This closed conformation is further stabilized by the interaction of the SH3 domain with the proline-rich regions that link the SH2 domain with the kinase domain and by the dephosphorylation of Tyr416. Kinase activity increases upon the dissociation of Tyr527 from the SH2 domain, which yields an open conformation. As a result, Tyr527 becomes accessible to several phosphatases, the kinase domain can bind and phosphorylate substrates, and the SH2 and SH3 domains can interact with downstream proteins. Furthermore, the dephosphorylation of SRC at Tyr527 triggers the autophosphorylation of Tyr416 to yield a highly activated enzyme.
SRC kinase family members localize to the cytosol and plasma membrane, where they function in cell adhesion, migration, proliferation, differentiation, and survival. They also localize to the nucleus (mostly in neoplastic cells), endosomes, lysosomes, secretory granules/phagosomes, and the Golgi apparatus (328, 338–342; for reviews, see Refs. 343, 344). In the testis, SRC localizes to the blood-testis barrier, where it is postulated to function in its restructuring (for a review, see Ref. 345). Genetic models, which are available for most SRC kinase family members, provide important insights on kinase function. For example, Src-null mice die within the first few weeks of birth if weaned. However, they survive if not weaned and fed a soft-food diet (346), indicating SRC is dispensable for embryonic development. Male homozygotes display no abnormalities in the central nervous system, where Src expression is the highest, revealing other members of the SRC kinase family can functionally substitute for its loss in the brain and spinal cord. Although female homozygotes are infertile, male homozygotes display no defects in the reproductive system (347).
FAK, a nonreceptor protein tyrosine kinase, functions in cell adhesion and migration, cytoskeletal dynamics, and cell cycle progression (for reviews, see Refs. 348–350). FAK is a substrate of SRC, and this interaction between kinases is perhaps one of the most important in cell biology. In general, FAK is present at focal contacts, actin-based cell junctions that connect cells to the extracellular matrix via integrins, transmembrane receptors that facilitate inside-out and outside-in signaling (for reviews, see Refs. 351, 352). Upon ligand binding and integrin clustering, FAK is recruited to the β subunit of integrin, where it undergoes autophosphorylation at Tyr397. This phosphorylated Tyr residue becomes a docking site for SRC, which phosphorylates additional Tyr residues (ie, Tyr576 and Tyr577, which are located in the catalytic domain of FAK; Tyr861 and Tyr925, which are located in the C-terminal domain), resulting in an increase in FAK activity and the activation of other focal adhesion proteins such as paxillin, talin, and vinculin. In this way, the activated FAK-SRC complex triggers numerous signal transduction cascades to bring about changes in cell function. Integrins such as α4β1 can also activate SRC independently of FAK (353).
In the mammalian testis, the function of the FAK-SRC complex in spermatogenesis is less defined because there are no bona fide focal contacts at the base of the Sertoli cell. Only “hemidesmosome-like attachments” associating loosely with intermediate filaments have been observed by electron microscopy (23; for a review, see Ref. 45). Instead, FAK localizes to the blood-testis barrier, where it binds and colocalizes with occludin and ZO-1 (328, 354, 355). Other studies indicate that FAK-Tyr397, which localizes to the apical ectoplasmic specialization, and FAK-Tyr407, which predominantly localizes to the blood-testis barrier, exhibit opposite effects on barrier function. In Sertoli cells, FAK-Tyr397Phe overexpression prompts junction disassembly, whereas FAK-Tyr407Phe overexpression promotes junction assembly (356). Thus, it is postulated that phosphorylated FAK acts as a molecular switch during the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier (357). However, it is difficult to interpret these findings, because FAK-Tyr397 is not present at the blood-testis barrier (356, 358), and FAK-Tyr407 is not stage-specific at the blood-testis barrier (356). In addition, FAK is highest at the blood-testis barrier during stages III–VI (354), which precedes the movement of preleptotene/leptotene spermatocytes across the barrier, and its expression is higher in germ cells than in Sertoli cells (356). Therefore, additional studies are needed to substantiate these findings.
B. Endocytosis
Endocytosis is the internalization of cargo, usually an integral membrane protein, together with a short stretch of the plasma membrane and fluid via vesicles. The key vesicles involved in this process are early, late, and recycling endosomes. Early endosomes are highly efficient sorting organelles and the site of bifurcation (ie, protein recycling or degradation) (for reviews, see Refs. 359–361). Late endosomes receive proteins, lipids, and possibly other molecules en route to lysosomes for degradation by hydrolytic enzymes, whereas recycling endosomes receive proteins and lipids en route to the plasma membrane. In the fast recycling pathway, proteins are retrieved directly from early endosomes and recycled back to the plasma membrane within approximately 2–3 minutes vs approximately 10 minutes in the slow recycling pathway, which involves recycling endosomes. In general, endocytosis occurs via dynamin dependent (ie, clathrin- and caveolin-mediated) and independent (ie, macropinocytosis and phagocytosis) pathways. Most cells, including Sertoli cells, internalize cargo by the clathrin-mediated pathway, which involves clathrin, a 3-legged protein that assembles into a curved lattice around the budding vesicle (also known as the clathrin-coated pit), as well as other clathrin-associated proteins. Clathrin-coated pits, which comprise approximately 2% of the surface area of the plasma membrane in most cells, internalize plasma membrane components, growth factors, cytokines, and hormones within minutes because clathrin-coated pits have a very short half-life (∼1 min). By contrast, caveolae are cave-like invaginations present on terminally differentiated cells such as adipocytes, fibroblasts, endothelial cells, and smooth muscle cells. They are detergent-resistant and rich in cholesterol and sphingolipids (for reviews, see Refs. 361–363). Caveolae are defined by the presence of caveolin, a cholesterol-binding protein that oligomerizes to form caveolin-rich domains that bud from the plasma membrane (364). Thus, there are apparent differences in the involvement of proteins and lipids, and in the formation of vesicles between endocytic pathways.
The cytoskeleton is essential for clathrin- and caveolin-mediated endocytosis, and actin microfilaments and actin-associated proteins participate in endosome maturation, membrane scission, and cargo sorting (for reviews, see Refs. 365, 366). For example, clathrin-mediated endocytosis has been shown to involve cortactin, which upon phosphorylation by SRC promotes actin polymerization and remodeling, resulting in the recruitment of a 7-protein complex known as the actin-related protein 2/3 complex (367, 368; for reviews, see Refs. 369, 370). The actin-related protein 2/3 complex, when activated by N-WASP (371, 372), nucleates the formation of new actin microfilaments on preexisting actin microfilaments (373), illustrating actin polymerization is important for the internalization of cargo. On the other hand, caveolin-mediated endocytosis involves filamin (374, 375), an F-actin cross-linking protein that promotes orthogonal branching of microfilaments (for reviews, see Refs. 376, 377). Caveolin is also a substrate of SRC (378, 379), suggesting phosphorylation induces the assembly of caveolin-rich domains and the internalization of caveolae. Although the actin cytoskeleton is involved in early endocytic events, the microtubule cytoskeleton is involved in late endocytic events (for a review, see Ref. 380). Microtubules are highly dynamic ultrastructures that exhibit phases of growth at plus-ends and shrinkage at minus-ends in a phenomenon known as dynamic instability (381; for reviews, see Refs. 382–384). They are essential for vesicle trafficking and intracellular organization (Figure 6) (for reviews, see Refs. 385–388).
Endocytosis is known to regulate the gate and fence functions of TJs. For example, different cells internalize occludin and claudin, usually in response to an external stimulus, via clathrin- and caveolin-mediated pathways, resulting in a disruption of TJ function (389, 390; for reviews, see Refs. 391–393). Specifically, caveolin-1 regulates Ras-related protein Rab-5A (RAB5), a small GTPase that localizes to TJs (394) and controls the localization of occludin (395). Claudin uses a distinct mechanism of internalization, with adjoining claudin molecules internalizing as an entity into one cell (396; for a review, see Ref. 393). Connexin 43 also employs this mechanism (397, 398; for reviews, see Refs. 399, 400). In Sertoli cells, testosterone promotes the recycling of endocytosed occludin back to the plasma membrane via the slow recycling pathway, which supports TJ function (401). Testosterone also increases the levels of clathrin and caveolin-1 (402), suggesting androgens facilitate endocytosis. It is postulated that testosterone facilitates transcytosis, the transport of cargo across a polarized cell (for a review, see Ref. 403), during the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier. However, there is no in vitro model of germ cell movement across the Sertoli cell barrier and little evidence that TJ proteins are transcytosed from in front of migrating spermatocytes to behind these germ cells. By contrast, cytokines such as TGFβ target TJ proteins to late endosomes for degradation (401, 402). These events are partly mediated by kinases because Src knockdown affects the endocytosis of CAR and JAM-A by decelerating their kinetic profiles and decreasing their internalization levels (328). Furthermore, endocytosed proteins are trafficked to late endosomes for degradation, resulting in a disruption of TJ function. Src knockdown also affects the actin cytoskeleton, which can also affect protein internalization and trafficking (328). Thus, it is not known whether SRC directly functions in endocytosis. Nevertheless, clathrin knockdown disrupts the delivery of proteins to the basolateral, but not the apical, domain in MDCK cells (404). Similarly, the loss of caveolin-1 affects cell polarity and directional cell movement (405, 406), illustrating endocytosis also regulates the fence function of TJs.
Interestingly, the testis uses a unique mechanism of internalization defined as the “junction internalization hypothesis” to bring about the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier. It states tubulobulbar complexes internalize intact junctions into one cell via the clathrin-mediated pathway (407, 408; for a review, see Ref. 409), similar to the way in which claudin and connexin are endocytosed in epithelial cells. Tubulobulbar complexes are testis-specific, actin-rich ultrastructures that consist of a long tube and a large bulb near the end of the tube. Tubulobulbar complexes are capped at the distal end by a clathrin-coated pit that eventually buds from the ultrastructure and enters the endocytic pathway. Tubulobulbar complexes between Sertoli cells are defined as basal tubulobulbar complexes, and they are present in areas of the plasma membrane that are occupied by ectoplasmic specializations. Tight and gap junctions are interspersed between basal tubulobulbar complexes (407, 410; for a review, see Ref. 409), indicating tubulobulbar complexes internalize these junctions. Indeed, claudin 11, connexin 43, and nectin-2, as well as with RAB5 and early endosome antigen 1, associate with basal tubulobulbar complexes (407, 411). Cortactin also associates with the basal tubulobulbar complex (324). It is postulated that basal tubulobulbar complexes reduce junction size and number at stages IV and V to prepare for the movement of preleptotene/leptotene spermatocytes across the blood-testis barrier (407). Presently, it is not known whether Sertoli cells internalize proteins via other mechanisms.
V. Future Directions in the Study of the Blood-Testis Barrier and Concluding Remarks on the Status of Male Contraceptive Research
We have reviewed here the biology and regulation of the mammalian blood-tests barrier and discussed studies that may trigger new questions, foster new ideas, and drive the field forward. Throughout the review, we have emphasized the uniqueness of the blood-testis barrier, providing examples of how it is similar and dissimilar from other blood-tissue barriers. Lastly, we have highlighted specific research areas that require additional studies. For example, it is not known whether basal tubulobulbar complexes internalize the proteins of the tricellular TJ. Although pioneering observations on the blood-testis barrier were made more than 5 decades ago, the most important questions we ask today are: why study the blood-testis barrier and why is it important? An intact blood-testis barrier is critical for spermatogenesis, and subfertility or infertility can ensue if its function is perturbed. Fertility can also be affected if Sertoli-germ cell interactions are disrupted because undeveloped germ cells cannot fertilize an egg. Many investigators, including us, are purposefully affecting cell-cell interactions in the testis to determine whether this approach can result in the development of a safe and reversible nonhormonal male contraceptive. Thus, it is important to understand the biology of the mammalian blood-testis barrier so that this information can be applied to male reproduction and contraceptive development. We must also be assured that nonhormonal male contraceptives will be desired by men in the long term.
A recent study conducted in Pakistan by our organization, with the support of the World Bank through the Bank-Netherlands Partnership Program, shows a concrete change in the attitude of men toward family planning. This study reveals that men are enthusiastic in receiving more information on contraception and in playing a greater role in family planning. This gradual change in attitude is likely due to the enormous financial difficulties that men face in raising large families in Pakistan. However, a lack of affordable contraceptive methods and family planning services impede men from playing a greater role. In light of these findings, there is a great need to introduce male contraceptives that are safe, effective, reversible, and affordable. However, no compound has met the strict guidelines of the United States Food and Drug Administration and reached the consumer market. Healthy individuals would use male contraceptives for extended lengths of time. Thus, there can be no/few short- and long-term side effects caused by their use, no/minimal adverse interactions with other drugs that treat serious and life-threatening diseases, and absolutely no deaths. For example, liver enzymes such as cytochrome P-450 eliminate from the body most cholesterol-lowering drugs (ie, statins), which are taken by many men in their mid/late forties and early fifties. Any drug that blocks the action of these liver enzymes will increase the level of the statin in the blood, which may cause severe skeletal muscle damage and other serious conditions. Other contraindications may exist with male contraceptive use as well, and additional studies are needed once the candidate compound shows promise in clinical trials.
In addition, there are other issues that need to be addressed, because they will partly determine marketability, profit, and overall patient satisfaction. For example, will men from different ethnic backgrounds respond equally to a given male contraceptive? How will contraceptive efficacy (ie, the period of complete to reversible infertility) be established in different individuals without having to visit a urologist or clinic, especially for nonhormonal agents? How will long-term infertility affect Sertoli cell and sperm function should a man decide that he wants to father a child/children? Would reversibility be immediate and complete? Finally, will successful antifertility tests in animals translate to successful antifertility tests in humans? The United States Food and Drug Administration reports that approximately 92% of drugs approved for testing in humans never receive approval for human use. Of those that are approved, more than half are later recalled due to serious or lethal effects in humans. For example, the rheumatoid arthritis drug, rofecoxib (Vioxx; Merck), was safe in animal studies and approved in 1999 for human use, but it was eventually withdrawn from the consumer market in 2004, because it doubled the risk of myocardial infarction and stroke, resulting in the death of more than 60 000 individuals in the United States alone. Thus, any male contraceptive that is approved for human use will require stringent and careful testing.
Abbreviations
- aPKC
atypical protein kinase C
- CAR
coxsackievirus and adenovirus receptor
- CDC42
cell division cycle 42
- CRB
Crumbs
- DLG
discs-large
- ES
ectoplasmic specialization
- FAK
focal adhesion kinase
- JAM-A
junctional adhesion molecule-A
- LGL
lethal giant larvae
- LIN-7
lin-7 homolog A
- LSR
lipolysis-stimulated lipoprotein receptor
- MAGUK
membrane-associated guanylate kinase
- MARVEL
MAL and related proteins for vesicle trafficking and membrane link
- N-WASP
neuronal Wiskott-Aldrich syndrome protein
- PALS
protein associated with Lin-7
- PAR
partitioning defective
- PATJ
Pals-associated TJ protein
- PDZ
postsynaptic density 95, discs large, and ZO-1 proteins
- PHLPP
pleckstrin homology domain leucine-rich repeat protein phosphatase
- PI3K
phosphoinositide-3-kinase
- PKB
protein kinase B
- PKC
protein kinase C
- RAB5
Ras-related protein Rab-5A
- RAC
ras-related C3 botulinum toxin
- RHO
ras homolog
- SCRIB
Scribble
- SH
Src homology
- TJ
tight junction
- TJP
tight junction protein
- ZO
zona occludens
- ZONAB
ZO-1-associated nucleic acid binding protein.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD; 2R01 HD056034 and U54 HD029990 Project 5).
Disclosure Summary: The authors have nothing to disclose.
Reference



![An electron micrograph of the intermediate compartment in the adult rat testis. This image shows 2 leptotene spermatocytes connected by intercellular bridges and enclosed within the intermediate compartment at stages IX–XI of the seminiferous epithelial cycle. The blood-testis barrier, which is created by Sertoli cells (SCs), is visible by the precipitation of lanthanum nitrate (arrowheads), which fails to advance beyond the TJs. Scale bar, 1 μm. (Reproduced from figure 1(e) of Mruk and Cheng [233] and used with permission.)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/edrv/36/5/10.1210_er.2014-1101/3/m_zef9991529060001.jpeg?Expires=1716326603&Signature=MaIwKNA7QnjjKK3EUBlBAd-peCP7KN-zIIlIMPL987fRzS~fy6P-sOImh220h~0DdN4R5r3pA3wv38iIpj2Mw0OpUBtGTBqx6-5o0FunrJlw~lkxw7Q-lJQUTmPj2UyRgJPqWerjRv9yhliXwcsTX6vNRsXamXSdraygL1xckYLP0DkkvTGN8r6CxqJFqq2z7~34oTzo4f5dM1zhaZOKFJVihqi7IDdX68Vez4vpeXDrEMvPz3KyYVnCzi3PUCyc-lKgHzJEfV7FoLEZu7~FOjPkMpNpxhNYdZwCy3T3lDB~lj1VPKIMOjP6Snx7-5-YVYDL08shbsjfWwTLmmXHZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
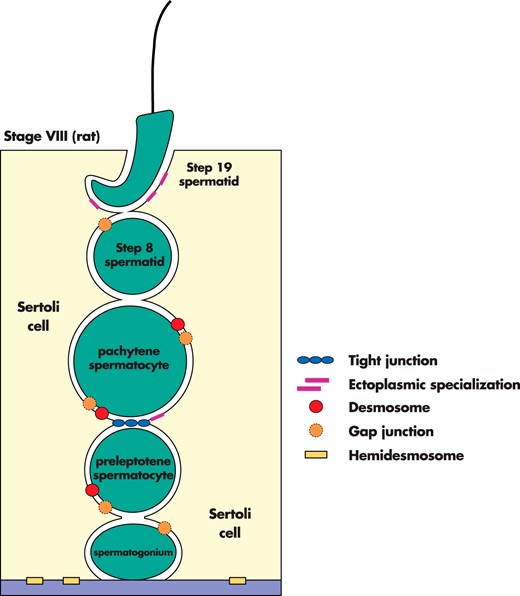
![An electron micrograph of the blood-testis barrier in the adult rat testis. The blood-testis barrier is comprised of TJs, basal ectoplasmic specializations (ESs), desmosomes (DSs), and gap junctions (data not shown). TJs are typified by “kisses,” regions of contact between Sertoli cell plasma membranes (arrowheads). The basal ES is characterized by bundles of actin microfilaments (asterisks) positioned between the Sertoli cell plasma membrane and cisternae of endoplasmic reticulum (er). DSs are typified by electron dense material between Sertoli cells. Scale bar, 0.75 μm. (Reproduced from figure 4A of Sarkar et al [267] and used with permission).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/edrv/36/5/10.1210_er.2014-1101/3/m_zef9991529060003.jpeg?Expires=1716326603&Signature=XWugiBoXFm3lEawhPgc6LDLiUeq9RNKE2P1SI8zCh2SvnHq0odXq1gO3fboEmGo7eWZfGe96EoBy8kzyGW4tM4APmX2xcE1vtXx~YOBrL9dgJk5PeuipzY23ZjY6maugA7aFNzcY~yh3OPiljRTaCDeMHMAJ5OE--cMK9Sr52FiQ7~9TXbKaLP7IHbZ5WfWksczDZbp7IVU2C-f~I78Np77oBdT4zT3wU-aMBjuwDvWZLGGCxua1LfRfRvUkIl4eUNP~OlMWewO5Rf~FzlBsvIYawJk1rdaaoLYzfQlmBIvsaa6n8gf1hXEBCVFm04SRuvveV-KEwUms~~lyjvjMzg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)