Abstract
Background
Handgrip strength (HS) measures have been associated with nutritional status, morbidity, and mortality in end stage of renal disease (ESRD).
Objective
We aimed to present and discuss the HS method in ESRD patients, by reviewing published studies on the subject.
Methods
PUBMED, MEDLINE, and LILACS databases were consulted, with no filters regarding the date of publication or age of population.
Results
The terms “handgrip strength,” “end stage of renal disease,” and “nutrition status” were used, and 32 articles with publication dates from 1983 to 2017 were included. Handgrip strength is considered a simple and rapid method of assessing muscle function in chronic kidney disease and is an important predictor of nutritional status depletion, development of comorbidities, and early mortality.
Conclusion
There is a lack of studies that analyzed associations between HS and clinical and nutritional outcomes in ESRD. The establishment of HS protocols and reference values in ESRD are necessary, to assist preventive measures of unfavorable outcomes in this population.
Similar content being viewed by others
Background
Nutritional and metabolic derangements are common in end stage of renal disease (ESRD), caused by hypercatabolic status, uremic toxins, malnutrition, and inflammation. These changes in nutritional status are defined as protein energy wasting (PEW) and are strongly associated with mortality in chronic diseases, including chronic kidney disease (CKD) [1, 2].
The most common consequences of PEW are important decrease of serum proteins and progressive loss of skeletal muscle, which contribute to development of frailty, sarcopenia, and impairment of muscle functioning, particularly in ESRD patients [1, 3].
The prevalence of PEW in early stages of CKD is 20 to 25% [2] and increases with progression of the disease. Methods assessing nutritional status and body composition should be able to identify important nutritional status changes and predict risk of unfavorable clinical outcomes [4, 5].
Handgrip strength (HS) is considered a simple and rapid method of assessing muscle function. Currently, it has been used as a reliable marker of clinical prognosis in several populations due to its association with nutritional status, morbidity, and mortality [3, 6].
There are comparisons in the literature of nutritional and functional parameters, such as HS, reinforcing the importance of these indicators in clinical practice as a useful nutritional assessment instrument [7].
Recent studies relate HS measured by a dynamometer with clinical conditions and adverse outcomes such as inflammation, malnutrition, overhydration status, and higher mortality in dialytic and non-dialytic ESRD population. Because of these associations, it has been suggested that a dynamometer is a valuable tool for assessing nutritional status in clinical practice in CKD [3, 8].
Despite the use of this, measure is increasing through the years; no major developments have been done in the past 6 years [6] to develop a standardized protocol to use HS as a tool to prevent loss of muscle mass and function in ESRD population.
Objective
This paper aimed to review the use of handgrip strength in end stages of chronic kidney disease—stages G4 and G5, pre-dialytic, and in maintenance dialysis therapy individuals, by reviewing published studies in the literature.
Methodology
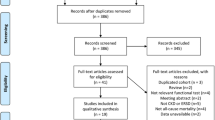
This is a narrative review. PUBMED database, which contains over 26 million citations in MEDLINE biomedical literature; LILACS, the most important and comprehensive index of scientific literature in Latin America and the Caribbean; scientific journals; and online books has been consulted. Articles were searched by hand, using the terms “handgrip strength,” “chronic kidney disease,” and “nutritional status.” There was no restriction on the basis of the language, and no filters were applied, as exclusion criteria, to the year of publication or age of the study population.
Results
There were 42 articles found with the search in databases. Thirty-one articles, published in full version, with publication between 1983 and 2017, with data about the use of HS in ESRD population, were included (Table 1). Through reading the articles, the discussion of this review was divided into four subtopics: “handgrip strength”; “sarcopenia, frailty, and chronic kidney disease”; “handgrip strength in end stage of renal disease”; and “baseline values.”
Discussion
Handgrip strength
A dynamometer is a portable and practical gauging device, which generates a reliable measure of muscular strength. It has been applied in several populations and clinical situations, including CKD. Reduced measures of HS are commonly found among ESRD patients and have strong associations with morbidity and mortality [4, 9].
Nutritional assessment and muscle function evaluation enable the diagnosis of nutritional and functional impairments and, consequently, allow early interventions to avoid unfavorable outcomes, such as reduced quality of life, sarcopenia, frailty, and early death [10, 11].
Although a dynamometer is an apparatus currently used in several clinical situations, some factors may influence the measurement, such as age, gender, body mass, dominant hand, and the manipulator position [8].
Studies show that there is a predominance of higher values of muscular strength in males, in individuals aged between 30 and 45 years and in individuals who are overweight and obese, according to the body mass index (BMI). Values tend to decrease with advancing age and the presence of PEW [11].
According to Schlüssel et al., there is strength variation also between the sides of the body, which can vary between 5 and 30% [7]. Higher absolute values were obtained by measurements performed with standing individuals [12,13,14]. Clinical procedure and hand’s dominance may influence the measurement, as described in some studies [15,16,17], and others only observed a significant difference between the right and left sides [18, 19]. Innes affirms that shoulder flexed at 180° yields better results when compared to 90° flexion or standard position 0° [14].
Verbal encouragement, as described more than 20 years ago by Johansson et al., results in 8% higher values of muscle contraction strength, statistically significant difference. In this study, a higher voice volume for transmitting instructions to men between 18 and 30 years was related to greater motivation and strength [20].
Besides the use of average of several HS readings, it is possible to use the measurement of only one reading, the largest between two or three, or the average of the two largest readings between three readings. According to Innes, no significant differences were observed between them, being the evaluator discretion the most appropriate method [14].
This same author suggests that a period of muscular contraction of 3 s is enough to record the greatest measure of HS. A rest period between measurements is recommended and may vary from 2, 5, or 15 s to 1 min [14].
There are differences of measures performed before and after hemodialysis session: Pinto et al. showed that there was a significant reduction of HS observed after the session, when compared to the measure performed in initial minutes. The authors affirm that hemodialysis procedure affects negatively the HS [15].
Considering the possible interferences exposed, it is necessary to establish a protocol in each clinic or institution and maintain the procedure consistency to ensure reliable measurements provided by HS [7, 9, 21] (Fig. 1).
Non-dialytic ESRD individuals have several factors that contribute to the decline of muscle strength and function, such as anemia, decreased serum albumin and hemoglobin levels, presence and severity of proteinuria, decreased renal function, protein hypercatabolism, advanced age, and inflammation. These factors are more strongly contributors to decline in strength of ESRD individuals (CKD stages 4 and 5) than when compared to those of individuals in CKD stages 2 and 3 [10].
Handgrip strength in ESRD
Due to associations with morbidity and mortality, investigation of nutritional disorders in ESRD population is extremely important [6, 11]. Reduced values of HS are often found in patients undergoing dialysis therapy and reflect muscle mass depletion [9].
A recent systematic review of 18 studies in populations submitted to hemodialysis and peritoneal dialysis has described associations of reduced HS with dialysis and clinical and nutritional parameters. Associations with reduced levels of serum hemoglobin, presence of diabetes mellitus, decreased renal function, inflammation, carnitine deficiency, and varying degrees of PEW were found. Higher survival in these populations was related to values of HS above the average obtained [6].
Vogt et al. established cutoffs of HS to identify all-cause mortality risk in dialysis patients, most of them on hemodialysis, in different genders. The authors showed that PEW has been independently associated with poor outcomes, such as longer hospitalization and mortality. Moreover, HS cutoffs that predicted mortality were higher for men (22.5 kg) than for women (7.0 kg) [22].
Levels of testosterone in uremia are additional factors that contribute to decline of muscle mass and strength in men with CKD [23]. Low muscle strength is associated with aging, PEW, and physical inactivity, according to Isoyama et al. [24]. End stages of renal disease individuals have, commonly, high levels of physical inactivity that contribute to decrease functional capacity and decreased HS values [25].
The data analyzed suggests that a dynamometer can be considered a useful tool in evaluation of muscle function related to the nutritional status of dialysis patients [6]. There are, to date, few studies that discuss associations of HS measurement and clinical outcomes in patients with non-dialytic ESRD.
Five observational, transverse, and longitudinal studies were found in the literature, which described associations between dynamometry and the occurrence, prevalence, and predictive power of sarcopenia mortality, and a literature review that addressed aspects of combating severe mass loss and muscle strength in uremic non-dialytic ESRD individuals [6, 10, 21, 26, 27].
Of the most relevant nephro-protective clinical strategies in ESRD, we highlight the nutritional and body compartment evaluation. Depleted nutritional status and decrease in muscle mass contribute to an accelerated loss of renal function and an increased risk of early death in pre-dialytic ESRD patients [21].
A study performed by Amparo et al. observed associations of HS measurement in CKD stages 2 to 5 patients with some parameters: lower HS were older, lower renal function and lower serum albumin, and worse evaluation by the Malnutrition-Inflammation Score [3]. Malnutrition-Inflammation Score (MIS) is a nutritional assessment tool capable to predict negative clinical outcomes, proposed by Kalantar-Zadeh et al. in 2001 [28].
Hiraki et al. investigated physical functionality of non-dialytic patients according to CKD stage. Some parameters of physical evaluation were used, among them, the dynamometer. All parameters showed worse results with disease progression, being more significant in period before dialysis therapy initiation [10].
Zhou et al. showed that two important markers of physical functionality, balance, and strength tests were significantly related to muscle mass and glomerular filtration rate decline, demonstrating the development risk of sarcopenia during CKD evolution [26].
A recent review discusses strategic hypotheses of mass loss attenuation and decreased muscular function related to age and uremic state. Authors affirm that after establishing strategies to increase muscle mass, such as adequate nutritional support, metabolic acidosis correction, and resistance exercises, other strategies can be considered, such as testosterone and growth hormone replacement, stimulation of mitochondrial biogenesis, and stem cells [8].
Sarcopenia and frailty in CKD
The term “sarcopenia” has recently been redefined as a syndrome of progressive decline in age-related mass and muscle function and associated with an increased risk of frailty, physical disability, and mortality risk [4, 29].
Sarcopenia is associated to functional impairment and worsening quality of life, especially in ESRD, influenced by aging, sedentary lifestyle, low vitamin D levels, high circulating potassium levels, arterial hypertension, insulin resistance, deficient macronutrients, and low socioeconomic level, as demonstrated by the NHANES III study [30].
Definition of sarcopenia in this population depends on the method applied. When defined by reduced values of HS and skeletal muscle mass, evaluated by electric bioimpedance test, Pereira et al. found a higher predictive power of mortality in this population [29].
Frailty is defined as a physiological state of greatest vulnerability to stress present in the elderly, characterized by a significant deterioration in cognitive, functional, and health status. It was initially described in geriatric population, but currently, there is a high prevalence in young and old CKD individuals, reaching from 26 to 68% in ESRD [31].
Sarcopenia and frailty have been associated to increased risk of hospitalizations, falls, and mortality in CKD [11, 31]. Diagnostic methods and interventions can improve quality of life, minimize functional disabilities, and reduce unfavorable clinical outcomes [31].
Although it is not considered a unique method of diagnosis of sarcopenia by International Society of Renal Nutrition and Metabolism, researchers have used the HS in patients submitted to dialysis therapy to aid in their diagnosis and treatment [6].
A recent study estimated the prevalence of 6 to 10% of sarcopenic stages 3 and 5 CKD patients aged 18 to 80 years old. Diagnostic criteria used in this study were HS measurements by the dynamometer (< 30th percentile of a population-based reference) and other anthropometric assessments [29].
In a systematic review, Leal et al. showed that HS is widely used as a method of diagnostic criteria of sarcopenia, and its results are similar to general population: HS values are associated with age and gender, capable to predict clinical complications. However, the authors highlighted that it is necessary to standardize the techniques used for HS, the position of measurement, and reference values for this population [6].
Reference values
Although dynamometry is currently used in several clinical situations and is capable of assisting clinical interventions, one of the major obstacles to its use is the lack of cutoff points, both in healthy population and in populations with comorbidities, including ESRD [6].
The proposal to define cutoff points or reference values of HS in different populations is important for real and reliable comparisons between obtained and normative values [3, 7]. Calibration protocols must be followed to maintain the measurement consistency.
Few studies have developed cutoff points for HS, but they have portrayed its importance in classifying muscle mass and function loss degrees, predicting unfavorable outcomes and designing interventions that may improve muscle function [3, 7, 9, 21].
Pereira et al., in 2015, evaluated sarcopenia prevalence in 287 individuals with stages 3 to 5 CKD, according to HS measurements and others parameters of muscle mass reduction. In this study, in absence of reference values for non-dialytic ESRD population, reference values of a population study were considered [7, 29].
The establishment of measurement protocols that ensure the definition of cutoff points for ESRD individuals is necessary to better evaluate muscle function and reduce unfavorable clinical event occurrence.
Conclusions
Handgrip strength is one of the most widely used muscle functional evaluation methods currently available and has been considered a practical and reliable measure of skeletal muscle function in the general population and also in ESRD patients.
The early nutritional and muscle function diagnosis, obtained by the nutritional and muscle strength assessment, is important to avoid unfavorable consequences such as reduced quality of life, PEW, and early death.
In this review, we presented studies that dealt with occurrence, prevalence, and predictive power of poor clinical outcomes, mortality, and aspects to combat the marked loss of muscle mass and strength in uremic individuals. These topics are important and may base greater studies.
The measure of HS are influenced by age, gender, body mass, presence of sarcopenia and uremia, the stage of CKD, hand’s dominance, body position, verbal encouragement, and dialysis procedure. It can be considered to clinical and research practice the average of three HS readings, or the average of two higher readings, between three readings, or the higher reading, between readings.
Further studies are needed to standardize the techniques used for HS, establishment protocols, and reference values to assist preventive measures of unfavorable outcomes in ESRD population.
Abbreviations
- BMI:
-
Body mass index
- CKD:
-
Chronic kidney disease
- ESRD:
-
End stage of renal disease
- HS:
-
Handgrip strength
- MIS:
-
Malnutrition-Inflammation Score
- PEW:
-
Protein energy wasting
References
Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–8.
Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Management of protein-energy wasting in nondialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163–77.
Amparo FC, Cordeiro AC, Carrero JJ, Cuppari L, Lindholm B, Amondeo C, Kamimura MA. Malnutrition-Inflammation score is associated with handgrip strength in nondialysis-dependent CKD patients. J Ren Nutr. 2013;23(N 4):283–7.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–23.
Borges MCC, Vogt BP, Martin LC, Caramori JCT. Malnutrition Inflammation score CUT-off predicting mortality in maintenance hemodialysis patients. Clin Nutr ESPEN. 2017;17:63-67.
Leal OV, Mafra D, Fouque D, Anjos LA. Use of handgrip strength in the assessment of the muscle function of chronic kidney disease patients on dialysis: a systematic review. Nephrol Dial Transplant. 2011;26:1354–60.
Schlüssel MM, Anjos LA, Kac G. A Dinamometria atual e seu uso na avaliação nutricional. Rev Nutr, Campinas. 2008;21(2):223–35.
Stenvinkel P, Barany P, Chung SH, et al. A comparative analysis of nutritional parameters as predictor of outcome in male and female ESRD patients. Nephrol Dial Transplant. 2002;17:1266–74.
Hasheminejad N, Namdari M, Mahmoodi MR, Bahrampour A, Jalal Azmandian J. Association of Handgrip Strength With Malnutrition- Inflammation Score as an Assessment of Nutritional Status in Hemodialysis Patients. Iran J Kidney Dis. 2016;10(1):30-5.
Hiraki K, Yasuda T, Hotta C, Izawa KP, Morio Y, Watanabe S, Sakurada T, Shigagaki Y, Kimura K. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17:225–31.
Souza VA, Oliveira D, Mansur HN, Fernandes NMS, Bastos MG. Sarcopenia na Doença Renal Crônica. J Bras Nefrol. 2015;37(1):98–105.
Boadella JM, Kuijer PP, Sluiter JK, Frings-Dresen MH. Effect of self-selected handgrip position on maximal handgrip strength. Arch Phys Med Rehab. 2005;86(2):328–31.
Carrero JJ, Qureshi AR, Axelsson J, et al. Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am J Clin Nutr. 2007;85:695–701.
Innes E. Handgrip strength testing: a review of literature. Aust Occup Ther J. 1999;46(3):120–40.
Pinto AP, Ramos CI, Meireles MS, Kamimura MA, Cuppari L. Impacto da sessão de hemodiálise na força de preensão manual. J Bras Nefrol. 2015;37(4):00.
Crosby CA, Wehbé MA, Mawr B. Hand strength: normative values. J Hand Surg. 1994;19(4):665–70.
Incel NA, Ceceli E, Durukan PB, Erdem HR, Yorgancioglu ZR. Grip strength: effect of hand dominance. Singap Med J. 2002;43(5):234–7.
Hanten WP, Chen WY, Austin AA, Brooks RE, Carter HC, Law CA, et al. Maximum grip strength in normal subjects from 24 to 64 years of age. J Hand Ther. 1999;12:193–200.
Johansson CA, Kent BE, Shepard KF. Relationship between verbal command volume and magnitude of muscle contraction. Phys Ther. 1983;63(8):1260–5.
Chang YT, Wu HL, Guo HR, Cheng YY, Tseng CC, Wang MC, Lin MC, Sung JM. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26:3588–95.
Vogt BP, Borges MCC, Góes CR, Caramori JCT. Handgrip strenght is an independent predictor of allcause mortality in maintenance dialysis patients. Clin Nutr. 2016;35(6):1429-1433.
Cigarrán S, Pousa M, Castro MJ, González B, Martínez A, Barril G, Aguilera A, Coronel F, Stenvinkel P, Carrero JJ. Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J Ren Nutr. 2013;23(5):e89–95.
Isoyama N, Qureshi AR, Avesani CA, Lindholm B, Bàràny P, Heimbürger O, Cederholm T, Peter Stenvinkel P, Juan Jesús Carrero JJ. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9:1720–8.
Broers NJH, Martens RJH, Cornelis T, van der Sande FM, Diederen NMP, Hermans MMH, et al. Physical activity in end-stage renal disease patients: the effects of starting dialysis in the first 6 months after transition period. Nephron. 2017;137(1):47–56.
Stenvinkel P, Carrero JJ, Walden F, Ikizler TA, Nader GA. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant. 2015;0:1–8.
Zhou Y, Hellberg M Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2018;33(2):342–348.
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–63.
Pereira RA, Cordeiro AC, Avesani CM, Carrero JJ, Lindholm B, Amparo FC, Amodeo C, Cuppari L, Kamimura MA. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;0:1–7.
Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–86.
Heimburguer O, Qureshi AR, Blaner WS, et al. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am J Kidney Dis. 2000;36:1213–25.
Bohm C, Storsley L, Navdeep T. The assessment of frailty in older people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:498–504.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
MCO contributed to the conception and design of the study, acquisition and interpretation of data, and drafting the article. MNBB and ALB contributed to the conception and design of the study, in drafting the article, revising it critically for important intellectual content, and final approval of the version. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oliveira, M.C., Bufarah, M.N.B. & Balbi, A.L. Handgrip strength in end stage of renal disease—a narrative review. Nutrire 43, 14 (2018). https://doi.org/10.1186/s41110-018-0073-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-018-0073-2