-
PDF
- Split View
-
Views
-
Cite
Cite
Fabrice Berna, Jevita Potheegadoo, Ismail Aouadi, Jorge Javier Ricarte, Mélissa C. Allé, Romain Coutelle, Laurent Boyer, Christine Vanessa Cuervo-Lombard, Jean-Marie Danion, A Meta-Analysis of Autobiographical Memory Studies in Schizophrenia Spectrum Disorder, Schizophrenia Bulletin, Volume 42, Issue 1, January 2016, Pages 56–66, https://doi.org/10.1093/schbul/sbv099
Close - Share Icon Share
Abstract
Meta-analyses and reviews on cognitive disorders in schizophrenia have shown that the most robust and common cognitive deficits are found in episodic memory and executive functions. More complex memory domains, such as autobiographical memory (AM), are also impaired in schizophrenia, but such impairments are reported less often despite their negative impact on patients’ outcome. In contrast to episodic memory, assessed in laboratory tasks, memories of past personal events are much more complex and directly relate to the self. The meta-analysis included 20 studies, 571 patients with schizophrenia spectrum disorder, and 503 comparison subjects. It found moderate-to-large effect sizes with regard to the 3 parameters commonly used to assess AM: memory specificity ( g = −0.97), richness of detail ( g = −1.40), and conscious recollection ( g = −0.62). These effect sizes were in the same range as those found in other memory domains in schizophrenia; for this reason, we propose that defective memories of personal past events should be regarded as a major cognitive impairment in this illness.
Introduction
Cognitive impairment can be considered a core feature of schizophrenia and is increasingly recognized in psychopathology research with the goal of understanding the etiology, course and outcome of the illness. 1 , 2 Most patients with schizophrenia present some decline in their neurocognitive functioning, and several meta-analyses and reviews have shown that the most robust and common deficits are found in particular areas such as processing speed, episodic memory, working memory, and executive functions. 1 , 3–10 These cognitive deficits, and in particular episodic memory deficits, are linked to poorer functioning, work performance, social adaptation, quality of life, 1 , 6 and rehabilitation outcomes. 11 , 12
In its original conceptualization, episodic memory covers a large and heterogeneous set of memory representations and includes the ability to recollect specific events located in time and place. It is opposed to semantic memory that comprises general facts relating to the world, with no reference to spatial and temporal context. 13 According to this view, recalling a past personal event or word previously learned in a wordlist refers to episodic memory. However, while most episodic memory studies have relied on laboratory tests consisting of the recall or recognition of items such as words or pictures, there is growing evidence that the cognitive processes underlying memories of personal past events, ie, autobiographical memories, cannot be merely inferred from laboratory recall and recognition tasks. 14–16 In keeping with this line of reasoning, several arguments support a distinction between episodic memory and autobiographical memory (AM). First, AM retains knowledge over retention intervals measured in weeks or decades, 15 whereas episodic memory as assessed by laboratory tasks consists of knowledge retained over intervals measured in minutes or hours. Second, autobiographical memories are highly complex mental representations of personal past events that entail sensory-perceptual details, feelings, and thoughts specific to these past events, as well as general autobiographical knowledge about, for example, life periods or places. 15 , 17 Third, in contrast to episodic memory, AM encompasses information relating directly to the self, such as self-images, values or goals, 14 , 17 which enables us, when remembering an event, to re-experience being the person we were at that time. Thus AM supports the self and provides it with a sense of continuity through time. Finally, in addition to the distinction between episodic memory and AM, a distinction must also be made within AM, between the aforementioned memories of personal past events, sometimes labeled as the episodic component of AM, and memories of personal facts (eg, one’s name), sometimes referred to as the semantic component of AM, which correspond to more abstract knowledge about the self. 14 , 17 , 18
Several additional factors demonstrate the need for a proper investigation of AM in schizophrenia. Patients often complain of impaired memories of personal past events, 19 , 20 and a better understanding of the cognitive mechanisms underlying this impairment may open the door for specific cognitive remediation. Moreover, a better understanding of AM deficits in schizophrenia may help us to better grasp of the difficulties faced by patients in their daily lives. A recent study showed that AM deficits in patients with schizophrenia spectrum disorder are stronger predictors of patients’ poor social functioning than measures of verbal episodic memory, 21 while 2 studies 21 , 22 suggested that patients’ difficulty in understanding other people’s intentions may be partially due to difficulty retrieving specific events from their past. Finally, because AM provides a sense of continuity for the self through time, patients’ AM impairment may be one of the cognitive mechanisms that accounts for the lack of cohesion of the self that is commonly reported by both patients 19 , 23 and clinicians. 24 , 25
In the context of studies conducted on AM in schizophrenia, memories of personal past events have mostly been investigated in comparison with patients’ memory for personal facts. The characteristics of their memories of past events have been assessed with the help of 3 parameters. The first of these parameters is the specificity of AM, referring to the retrieval of specific past events, ie, events that occurred at a particular time and place and lasted less than 24h. 26 The second is the richness of memory details, which gives them vividness and clarity (eg, perceptual/sensory, contextual or emotional details), and the third refers to the subjective state of consciousness associated with memory retrieval, namely conscious recollection. 27 Conscious recollection is one’s awareness of the self in time, through the ability to mentally travel back in time and to re-experience unique past personal events in the original context in which they occurred. The present article presents a meta-analysis of data published on memories of personal past events in schizophrenia, with the objective of assessing the severity and overall effect size of AM impairment in this illness.
Methods
Literature Search
MEDLINE (PubMed) and Web of Science databases were searched from inception to December 2014 to identify all studies providing data on AM in patients with schizophrenia or schizoaffective disorder. A combination of the following Medical Subject Headings (MeSH) and search terms was used: “schizophren*” OR “schizo-affective” OR “psychos*” OR “psychot*” –AND “autobiographical memory”; –AND “remote memory”; –AND “verbal memory”.
The systematic review was executed according to the PRISMA standard, 28 including evaluation of bias (confounding, overlapping data, publication bias). Title and abstract screening of publications was executed independently by F.B. and J.J.R. In case of disagreement or uncertainty, the full text was read and discussed until conformity was achieved. After database extraction, hand-searching for studies potentially overlooked or absent from the databases was performed by screening the references of all retrieved articles.
Inclusion/Exclusion Criteria
Inclusion criteria for the studies in the current meta-analysis were as follows: (1) to be published in an English-language peer-reviewed journal, (2) to report AM data on schizophrenia spectrum disorder according to Diagnostic and Statistical Manual of Mental Disorders ( DSM-III , 29 , 30DSM-IV31 , 32 ), or the International Classification of Diseases ( ICD-9 , 33 or ICD-1034 ) criteria, (3) to have contrasted AM performance in patients with schizophrenia spectrum disorder and healthy controls, (4) to report at least 1 measure of either memory specificity, richness of memory details or conscious recollection, and (5) to report the results with sufficient detail to allow calculation of effect sizes. Regarding the last inclusion criterion, the corresponding author of the article was contacted in the case of missing data. There were no inclusion or exclusion criteria for sample size.
Statistical Analysis
With the data reported in each study, we calculated separate effect sizes for the difference in AM performance between patients and controls for each AM parameter. To estimate effect sizes we used the standardized mean difference method adjusted for small sample bias (Hedges’s g35 ). In addition, we calculated standard error, 95% confidence interval, Z value, chi-square statistic Q to assess the homogeneity of results across studies, 36 and I2 -statistic, a percentage-based indicator of heterogeneity (with 25% denoting low, 50% moderate, and 75% high heterogeneity 37 ). We used the forest plot to determine studies that caused heterogeneity. All calculations were made according to the assumptions of a random effects model. 38
To explore the possibility of publication bias, the funnel plots were inspected graphically, and we used the trim and fill methods 39 to estimate the number of Null studies missing from the meta-analysis. We also computed a fail-safe number of studies, in other words the number of Null studies that have to be added to reduce the significance of the meta-analysis to α = 0.05.
Finally, we evaluated the potential influence of moder ator variables on effect sizes with the help of meta-regression analyses that included demographic factors (eg, age, sex ratio, level of education), psychometric (IQ), and clinical variables (duration of illness, extent of schizophrenia symptoms, and depressive symptoms). Factors relating to the task used to collect AM and the methods used to measure AM parameters were also analyzed (cf infra).
Meta and Metafor packages in R software ( http://www.r-project.org/ ) were used.
Results
Search
After the initial screening procedures, the abstracts of 638 articles were read. Then 573 articles were excluded. A total of 65 full texts were evaluated. In the case of missing baseline data or possible overlap, the authors were contacted. Finally, 23 studies comparing at least one of the previously mentioned parameters of AM could be included in the present meta-analysis. Since a few patients were the same in some studies, 40–43 the results of both studies were presented as those of 1 study. Similarly, 2 papers 44 , 45 presented different results obtained with the same sample of participants and the results from the study that included the totality of the memories collected was entered in the meta-analysis. The final number of included studies in the present meta-analysis was 20 ( Supplementary Data ). These studies are listed in the References, denoted with asterisks.
Demographics
The 20 studies covered 571 patients with schizophrenia spectrum disorder and 503 controls. The weighted mean ages in the patient and control groups were 37.3 ( SD = 9.2) and 37.1 ( SD = 9.4) years old, respectively. In both groups, most participants were male: 69.4% in the patients group and 65.1% in the control group. The weighted mean level of education in patients and controls were 12.3 ( SD = 2.5) and 12.6 ( SD = 2.3) years, respectively. These differences were not statistically significant (all P s >.05). The weighted mean duration of illness was 13.5 years ( SD = 8.0).
AM Collection Methods
The methods used to collect autobiographical memories differed across studies. Nine studies 21 , 22 , 42 , 43 , 46–50 used AM questionnaires 46 , 51–53 requiring participants to retrieve specific AM relating to specific periods of their life (eg, childhood, early adulthood, recent period). Eight 50 , 54–60 used a cueing procedure where participants had to retrieve a specific autobiographical memory in response to a cue without time period constraints. Cues consisted of words, 50 , 55–57 , 59 , 60 self-statements by the participants, 54 , 61 or autobiographical events 58 (event cueing procedure 62 ). Two studies used other methods, namely a free recall task where participants were free to retrieve up to 20 specific events from their life, 63 and a AM fluency task 64 where participants were asked to produce as many single personal events as possible in 90 s. 49 An additional 4 studies 40 , 41 , 44 , 45 collected self-defining memories that correspond to highly self-significant events, 65 and, finally, 2 studies used a diary method 66 , 67 followed by a recognition task of events that took place 1 or 2 months after the diary period. Participants narrated their memories orally, except in 5 studies which used a written procedure. 40 , 41 , 50 , 66 , 67 The number of memories collected also differed across studies, ranging from 3 to 50 ( M = 16.3, SD = 11.2) for the nondiary studies, and from 112 to 150 memories for the diary studies ( table 1 ).
Studies Included in the Meta-Analysis
| Name of the Studies . | Number of Participants . | Diagnosis of Patients . | Procedure to Collect Autobiographical Memories . | Parameters of Autobiographical Memories . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | Type of Task (name of the task) . | Oral/ Written . | Number of AMs . | Specificity . | Richness of Memory Detail . | Conscious Recollection . | |||
| 1 | Bennouna-Greene et al 54 | 25 | 25 | Schizophrenia | Cueing (self-statements) 61 | O | 24 | W and B | — | R |
| 2 | Berna et al 44,45 | 24 | 24 | Schizophrenia | SDM | O | 8 | W and B | — | R |
| 3 | Berna et al 67 | 10 | 10 | Schizophrenia | Diary | W | 150 | — | — | R |
| 4 | Corcoran et al 22 | 44 | 59 | Schizophrenia | AMQ (AMI) | O | 9 | — | AMI 51 | |
| 5 | Cuervo-Lombard et al 63 | 27 | 27 | Schizophrenia | Free recall | O | 20 | — | Borrini 70 | R(cont.) |
| 6 | Cuervo-Lombard et al 55 | 14 | 13 | Schizophrenia | Cueing | O | 25 | MRS 68 | Piolino 69 | R(cont.) |
| 7 | D’Argembeau et al 56 | 16 | 16 | Schizophrenia | Cueing (AMT) | O | 10 | W and B | — | — |
| 8 | Danion et al 46 | 22 | 22 | Schizophrenia | AMQ (AME) | O | 24 | W and B | Piolino 69 | R(cont.) |
| 9 | Herold et al 47 | 21 | 33 | schizophrenia and schizo- affective disorder | AMQ (E-AGI) | O | 5 | — | E-AGI 53 | — |
| 10 | Kaney et al 57 | 20 | 20 | delusional disorder | Cueing (AMT) | O | 12 | W and B | — | — |
| 11 | Mehl et al 21 | 45 | 55 | schizophrenia spectrum disorder | AMQ (AMI) | O | 9 | W and B | — | — |
| 12 | Morise et al 58 | 17 | 18 | Schizophrenia | Cueing (event cueing) 62 | O | 50 | — | — | R |
| 13 | Pernot-Marino et al 66 | 8 | 8 | Schizophrenia | Diary | W | 112 | — | — | R |
| 14 | Potheegadoo et al 42,43 | 30 | 30 | Schizophrenia | AMQ (AME) | O | 24 | MRS | Piolino 69 | R |
| 15 | Potheegadoo et al 48 | 25 | 25 | Schizophrenia | AMQ (AME) | O | 6 | MRS | Piolino 69 | — |
| 16 | Raffard et al 40,41 | 50 | 81 | Schizophrenia | SDM | W | 3 | W and B | — | — |
| 17 | Ricarte et al 59 | 31 | 31 | Schizophrenia | Cueing (AMT) | O | 12 | W and B | — | — |
| 18 | Riutort et al 49 | 24 | 24 | Schizophrenia | AMQ (AMI) + AM fluency task 64 | O | 20 | W and B | Borrini 70 | — |
| 19 | Vorontsova et al 60 | 30 | 30 | schizophrenia spectrum disorder with persecutory delusion | Cueing (AMT) | O | 12 | W and B | — | — |
| 20 | Wood et al 50 | 20 | 20 | Schizophrenia | Cueing (AMT) + AMQ (AMI) | W | 21 | W and B | AMI 51 | — |
| Name of the Studies . | Number of Participants . | Diagnosis of Patients . | Procedure to Collect Autobiographical Memories . | Parameters of Autobiographical Memories . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | Type of Task (name of the task) . | Oral/ Written . | Number of AMs . | Specificity . | Richness of Memory Detail . | Conscious Recollection . | |||
| 1 | Bennouna-Greene et al 54 | 25 | 25 | Schizophrenia | Cueing (self-statements) 61 | O | 24 | W and B | — | R |
| 2 | Berna et al 44,45 | 24 | 24 | Schizophrenia | SDM | O | 8 | W and B | — | R |
| 3 | Berna et al 67 | 10 | 10 | Schizophrenia | Diary | W | 150 | — | — | R |
| 4 | Corcoran et al 22 | 44 | 59 | Schizophrenia | AMQ (AMI) | O | 9 | — | AMI 51 | |
| 5 | Cuervo-Lombard et al 63 | 27 | 27 | Schizophrenia | Free recall | O | 20 | — | Borrini 70 | R(cont.) |
| 6 | Cuervo-Lombard et al 55 | 14 | 13 | Schizophrenia | Cueing | O | 25 | MRS 68 | Piolino 69 | R(cont.) |
| 7 | D’Argembeau et al 56 | 16 | 16 | Schizophrenia | Cueing (AMT) | O | 10 | W and B | — | — |
| 8 | Danion et al 46 | 22 | 22 | Schizophrenia | AMQ (AME) | O | 24 | W and B | Piolino 69 | R(cont.) |
| 9 | Herold et al 47 | 21 | 33 | schizophrenia and schizo- affective disorder | AMQ (E-AGI) | O | 5 | — | E-AGI 53 | — |
| 10 | Kaney et al 57 | 20 | 20 | delusional disorder | Cueing (AMT) | O | 12 | W and B | — | — |
| 11 | Mehl et al 21 | 45 | 55 | schizophrenia spectrum disorder | AMQ (AMI) | O | 9 | W and B | — | — |
| 12 | Morise et al 58 | 17 | 18 | Schizophrenia | Cueing (event cueing) 62 | O | 50 | — | — | R |
| 13 | Pernot-Marino et al 66 | 8 | 8 | Schizophrenia | Diary | W | 112 | — | — | R |
| 14 | Potheegadoo et al 42,43 | 30 | 30 | Schizophrenia | AMQ (AME) | O | 24 | MRS | Piolino 69 | R |
| 15 | Potheegadoo et al 48 | 25 | 25 | Schizophrenia | AMQ (AME) | O | 6 | MRS | Piolino 69 | — |
| 16 | Raffard et al 40,41 | 50 | 81 | Schizophrenia | SDM | W | 3 | W and B | — | — |
| 17 | Ricarte et al 59 | 31 | 31 | Schizophrenia | Cueing (AMT) | O | 12 | W and B | — | — |
| 18 | Riutort et al 49 | 24 | 24 | Schizophrenia | AMQ (AMI) + AM fluency task 64 | O | 20 | W and B | Borrini 70 | — |
| 19 | Vorontsova et al 60 | 30 | 30 | schizophrenia spectrum disorder with persecutory delusion | Cueing (AMT) | O | 12 | W and B | — | — |
| 20 | Wood et al 50 | 20 | 20 | Schizophrenia | Cueing (AMT) + AMQ (AMI) | W | 21 | W and B | AMI 51 | — |
Note : AM, Autobiographical memories; AMT, Autobiographical Memory Test 26 ; SDM, Self-Defining Memories. 65 ; AMQ, Autobiographical Memory Questionnaire; AMI, Autobiographical Memory Inventory 51 ; AME, Autobiographical Memory Enquiry 46,52 ; E-AGI, Erweitertes Autobiographisches Gedächtnisinventar 53 ; Memory specificity: W and B, Williams and Broadbent 26 ; MRS, Memory Rating Scale 68 with specific memories (scored 3–4) vs nonspecific memories (scores 0–2). Richness of memory detail: Borrini 70 ; Piolino. 69 Conscious recollection: R, Remember response of the whole memory; R(cont.), Remember response on the content of the memory (as opposed to the time and place).
Studies Included in the Meta-Analysis
| Name of the Studies . | Number of Participants . | Diagnosis of Patients . | Procedure to Collect Autobiographical Memories . | Parameters of Autobiographical Memories . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | Type of Task (name of the task) . | Oral/ Written . | Number of AMs . | Specificity . | Richness of Memory Detail . | Conscious Recollection . | |||
| 1 | Bennouna-Greene et al 54 | 25 | 25 | Schizophrenia | Cueing (self-statements) 61 | O | 24 | W and B | — | R |
| 2 | Berna et al 44,45 | 24 | 24 | Schizophrenia | SDM | O | 8 | W and B | — | R |
| 3 | Berna et al 67 | 10 | 10 | Schizophrenia | Diary | W | 150 | — | — | R |
| 4 | Corcoran et al 22 | 44 | 59 | Schizophrenia | AMQ (AMI) | O | 9 | — | AMI 51 | |
| 5 | Cuervo-Lombard et al 63 | 27 | 27 | Schizophrenia | Free recall | O | 20 | — | Borrini 70 | R(cont.) |
| 6 | Cuervo-Lombard et al 55 | 14 | 13 | Schizophrenia | Cueing | O | 25 | MRS 68 | Piolino 69 | R(cont.) |
| 7 | D’Argembeau et al 56 | 16 | 16 | Schizophrenia | Cueing (AMT) | O | 10 | W and B | — | — |
| 8 | Danion et al 46 | 22 | 22 | Schizophrenia | AMQ (AME) | O | 24 | W and B | Piolino 69 | R(cont.) |
| 9 | Herold et al 47 | 21 | 33 | schizophrenia and schizo- affective disorder | AMQ (E-AGI) | O | 5 | — | E-AGI 53 | — |
| 10 | Kaney et al 57 | 20 | 20 | delusional disorder | Cueing (AMT) | O | 12 | W and B | — | — |
| 11 | Mehl et al 21 | 45 | 55 | schizophrenia spectrum disorder | AMQ (AMI) | O | 9 | W and B | — | — |
| 12 | Morise et al 58 | 17 | 18 | Schizophrenia | Cueing (event cueing) 62 | O | 50 | — | — | R |
| 13 | Pernot-Marino et al 66 | 8 | 8 | Schizophrenia | Diary | W | 112 | — | — | R |
| 14 | Potheegadoo et al 42,43 | 30 | 30 | Schizophrenia | AMQ (AME) | O | 24 | MRS | Piolino 69 | R |
| 15 | Potheegadoo et al 48 | 25 | 25 | Schizophrenia | AMQ (AME) | O | 6 | MRS | Piolino 69 | — |
| 16 | Raffard et al 40,41 | 50 | 81 | Schizophrenia | SDM | W | 3 | W and B | — | — |
| 17 | Ricarte et al 59 | 31 | 31 | Schizophrenia | Cueing (AMT) | O | 12 | W and B | — | — |
| 18 | Riutort et al 49 | 24 | 24 | Schizophrenia | AMQ (AMI) + AM fluency task 64 | O | 20 | W and B | Borrini 70 | — |
| 19 | Vorontsova et al 60 | 30 | 30 | schizophrenia spectrum disorder with persecutory delusion | Cueing (AMT) | O | 12 | W and B | — | — |
| 20 | Wood et al 50 | 20 | 20 | Schizophrenia | Cueing (AMT) + AMQ (AMI) | W | 21 | W and B | AMI 51 | — |
| Name of the Studies . | Number of Participants . | Diagnosis of Patients . | Procedure to Collect Autobiographical Memories . | Parameters of Autobiographical Memories . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls . | Patients . | Type of Task (name of the task) . | Oral/ Written . | Number of AMs . | Specificity . | Richness of Memory Detail . | Conscious Recollection . | |||
| 1 | Bennouna-Greene et al 54 | 25 | 25 | Schizophrenia | Cueing (self-statements) 61 | O | 24 | W and B | — | R |
| 2 | Berna et al 44,45 | 24 | 24 | Schizophrenia | SDM | O | 8 | W and B | — | R |
| 3 | Berna et al 67 | 10 | 10 | Schizophrenia | Diary | W | 150 | — | — | R |
| 4 | Corcoran et al 22 | 44 | 59 | Schizophrenia | AMQ (AMI) | O | 9 | — | AMI 51 | |
| 5 | Cuervo-Lombard et al 63 | 27 | 27 | Schizophrenia | Free recall | O | 20 | — | Borrini 70 | R(cont.) |
| 6 | Cuervo-Lombard et al 55 | 14 | 13 | Schizophrenia | Cueing | O | 25 | MRS 68 | Piolino 69 | R(cont.) |
| 7 | D’Argembeau et al 56 | 16 | 16 | Schizophrenia | Cueing (AMT) | O | 10 | W and B | — | — |
| 8 | Danion et al 46 | 22 | 22 | Schizophrenia | AMQ (AME) | O | 24 | W and B | Piolino 69 | R(cont.) |
| 9 | Herold et al 47 | 21 | 33 | schizophrenia and schizo- affective disorder | AMQ (E-AGI) | O | 5 | — | E-AGI 53 | — |
| 10 | Kaney et al 57 | 20 | 20 | delusional disorder | Cueing (AMT) | O | 12 | W and B | — | — |
| 11 | Mehl et al 21 | 45 | 55 | schizophrenia spectrum disorder | AMQ (AMI) | O | 9 | W and B | — | — |
| 12 | Morise et al 58 | 17 | 18 | Schizophrenia | Cueing (event cueing) 62 | O | 50 | — | — | R |
| 13 | Pernot-Marino et al 66 | 8 | 8 | Schizophrenia | Diary | W | 112 | — | — | R |
| 14 | Potheegadoo et al 42,43 | 30 | 30 | Schizophrenia | AMQ (AME) | O | 24 | MRS | Piolino 69 | R |
| 15 | Potheegadoo et al 48 | 25 | 25 | Schizophrenia | AMQ (AME) | O | 6 | MRS | Piolino 69 | — |
| 16 | Raffard et al 40,41 | 50 | 81 | Schizophrenia | SDM | W | 3 | W and B | — | — |
| 17 | Ricarte et al 59 | 31 | 31 | Schizophrenia | Cueing (AMT) | O | 12 | W and B | — | — |
| 18 | Riutort et al 49 | 24 | 24 | Schizophrenia | AMQ (AMI) + AM fluency task 64 | O | 20 | W and B | Borrini 70 | — |
| 19 | Vorontsova et al 60 | 30 | 30 | schizophrenia spectrum disorder with persecutory delusion | Cueing (AMT) | O | 12 | W and B | — | — |
| 20 | Wood et al 50 | 20 | 20 | Schizophrenia | Cueing (AMT) + AMQ (AMI) | W | 21 | W and B | AMI 51 | — |
Note : AM, Autobiographical memories; AMT, Autobiographical Memory Test 26 ; SDM, Self-Defining Memories. 65 ; AMQ, Autobiographical Memory Questionnaire; AMI, Autobiographical Memory Inventory 51 ; AME, Autobiographical Memory Enquiry 46,52 ; E-AGI, Erweitertes Autobiographisches Gedächtnisinventar 53 ; Memory specificity: W and B, Williams and Broadbent 26 ; MRS, Memory Rating Scale 68 with specific memories (scored 3–4) vs nonspecific memories (scores 0–2). Richness of memory detail: Borrini 70 ; Piolino. 69 Conscious recollection: R, Remember response of the whole memory; R(cont.), Remember response on the content of the memory (as opposed to the time and place).
Methods Used to Measure AM Parameters
Memory specificity data were reported in 14 studies ( table 1 ). The proportion of specific memories vs nonspecific memories was included in the meta-analysis. Memory specificity was assessed in 10 studies 21 , 41 , 45 , 49 , 50 , 54 , 56 , 57 , 59 , 60 according to the criteria defined by Williams and Broadbent, 26 such that memories of single events situated in a particular time and place and lasting less than 24h were considered specific, whereas memories of repeated events and events lasting more than 24h were considered nonspecific. In the 4 remaining studies 43 , 46 , 48 , 55 that used memory rating scales, 68 specific memories corresponded to those scoring 3 or 4 and nonspecific memories to those scoring 0–2.
Data pertaining to the richness of memory detail were reported in 9 studies. 22 , 42 , 46–50 , 55 , 63 These studies used 4 distinct rating scales to assess the number of memory details. 51 , 53 , 69 , 70
Conscious recollection data were reported in 9 studies and 2 methods were used. In the first case participants were asked to assess the presence or absence of the experience of conscious recollection associated with either whole memory retrieval, 43 , 44 , 54 , 58 , 66 , 67 or retrieval of 3 different memory components (content, place, and time). 46 , 55 , 63 In the second case, the proportion of autobiographical memories associated with conscious recollection of the content was used.
Effect Sizes and Meta-Analytical Results
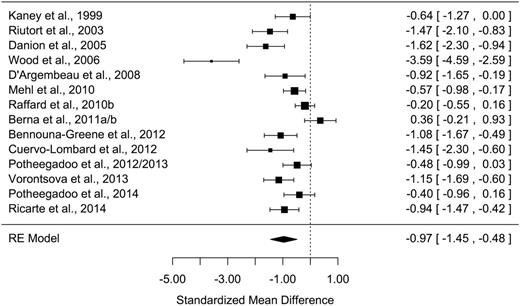
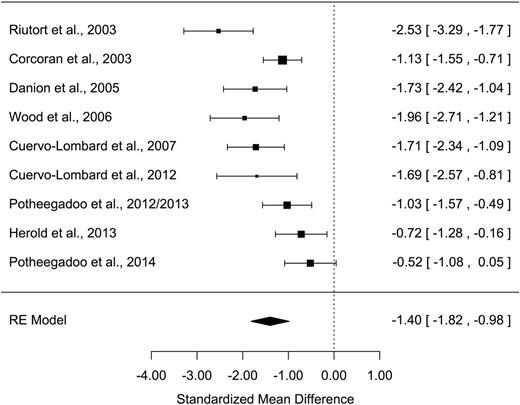
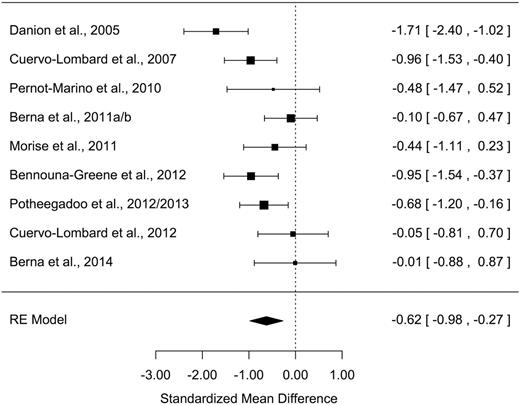
All measures of AM parameters were found to be significantly altered in patients with schizophrenia ( Z values > 2.48; P s < .013) with moderate-to-large effect sizes ( table 2 ). The grand mean weighted effect size was 0.98 ( SD = 0.11, Z = –9.15, P < .001), and a significant difference was observed between the effect sizes of the 3 AM parameters ( Q = 8.15, P = .017). Post hoc analyses revealed a significantly larger effect size for richness of memory detail than for conscious recollection ( Q = 8.14, P = .004), with other direct comparisons not being significant ( Q s < 2.44, P s > .11). Overall, a significant moderate-to-high heterogeneity was observed in the studies, ranging from 60.7% to 90.0%. Forest plots are shown in figures 1–3 (for funnel plots, see Supplementary Data ).
Effect Sizes for Memory Specificity, Richness of Memory Detail, and Conscious Recollection in Patients With Schizophrenia Spectrum Disorder
| . | k . | Number of Participants (Pn/Cn) . | ES ( g ) . | SD . | 95% CI . | Z Value . | Homogeneity Q . | I2 (%) . | Fail-Safe Number . | Number of Missing Null Studies . |
|---|---|---|---|---|---|---|---|---|---|---|
| Memory specificity | 14 | 416/376 | −0.97 | 0.25 | −1.45/−0.48 | −3.94*** | 75.9*** | 90.0 | 610 | 5 |
| Richness of memory detail | 9 | 253/227 | −1.40 | 0.21 | −1.82 /−0.98 | −6.57*** | 30.0*** | 75.7 | 553 | 3 |
| Conscious recollection | 9 | 177/177 | −0.62 | 0.18 | −0.98/−0.27 | −3.46** | 19.8* | 60.7 | 116 | 1 |
| . | k . | Number of Participants (Pn/Cn) . | ES ( g ) . | SD . | 95% CI . | Z Value . | Homogeneity Q . | I2 (%) . | Fail-Safe Number . | Number of Missing Null Studies . |
|---|---|---|---|---|---|---|---|---|---|---|
| Memory specificity | 14 | 416/376 | −0.97 | 0.25 | −1.45/−0.48 | −3.94*** | 75.9*** | 90.0 | 610 | 5 |
| Richness of memory detail | 9 | 253/227 | −1.40 | 0.21 | −1.82 /−0.98 | −6.57*** | 30.0*** | 75.7 | 553 | 3 |
| Conscious recollection | 9 | 177/177 | −0.62 | 0.18 | −0.98/−0.27 | −3.46** | 19.8* | 60.7 | 116 | 1 |
Note : k , number of studies; Pn, number of patients; Cn, number of controls; ES ( g ), effect size (Hedges’s g ) 35 ; SD , Standard Deviation of effect size. 95% CI: 95% Confidence Interval; Q , Homogeneity statistics 36 ; I2 , Heterogeneity statistics. 37
*P < .05, **P < .01, ***P < .001.
Effect Sizes for Memory Specificity, Richness of Memory Detail, and Conscious Recollection in Patients With Schizophrenia Spectrum Disorder
| . | k . | Number of Participants (Pn/Cn) . | ES ( g ) . | SD . | 95% CI . | Z Value . | Homogeneity Q . | I2 (%) . | Fail-Safe Number . | Number of Missing Null Studies . |
|---|---|---|---|---|---|---|---|---|---|---|
| Memory specificity | 14 | 416/376 | −0.97 | 0.25 | −1.45/−0.48 | −3.94*** | 75.9*** | 90.0 | 610 | 5 |
| Richness of memory detail | 9 | 253/227 | −1.40 | 0.21 | −1.82 /−0.98 | −6.57*** | 30.0*** | 75.7 | 553 | 3 |
| Conscious recollection | 9 | 177/177 | −0.62 | 0.18 | −0.98/−0.27 | −3.46** | 19.8* | 60.7 | 116 | 1 |
| . | k . | Number of Participants (Pn/Cn) . | ES ( g ) . | SD . | 95% CI . | Z Value . | Homogeneity Q . | I2 (%) . | Fail-Safe Number . | Number of Missing Null Studies . |
|---|---|---|---|---|---|---|---|---|---|---|
| Memory specificity | 14 | 416/376 | −0.97 | 0.25 | −1.45/−0.48 | −3.94*** | 75.9*** | 90.0 | 610 | 5 |
| Richness of memory detail | 9 | 253/227 | −1.40 | 0.21 | −1.82 /−0.98 | −6.57*** | 30.0*** | 75.7 | 553 | 3 |
| Conscious recollection | 9 | 177/177 | −0.62 | 0.18 | −0.98/−0.27 | −3.46** | 19.8* | 60.7 | 116 | 1 |
Note : k , number of studies; Pn, number of patients; Cn, number of controls; ES ( g ), effect size (Hedges’s g ) 35 ; SD , Standard Deviation of effect size. 95% CI: 95% Confidence Interval; Q , Homogeneity statistics 36 ; I2 , Heterogeneity statistics. 37
*P < .05, **P < .01, ***P < .001.
A large effect size ( g = −0.97) was found for memory specificity of past events. Secondary analyses showed that factors relating to the task did not significantly influence effect size. According to the meta-regression, the number of memories recalled had no significant influence ( F [1,12] = 1.41, P = .26). There was no significant difference in effect sizes between the 11 studies that used an oral procedure and the 3 that used a written procedure (χ 2 = 0.05, P = .82), nor between the 7 studies that used a cueing method and 5 involving AM questionnaires (χ 2 = 0.80, P = .37). However, group differences in memory specificity were not significant in the 2 self-defining memory studies 41 , 45 as opposed to the studies using other methods (AM questionnaires and cueing methods; χ 2 = 4.8, P = .03). Without these 2 studies, 41 , 45 the memory specificity effect size increased to −1.14 ( P < .0001).
Regarding the method used to assess memory specificity, there was no significant difference in effect size between the 10 studies that used the method devised by 26 and the 4 that used memory rating scales 69 (χ 2 = 0.0, P = 1.0).
The meta-regressions performed on memory specificity showed that effect size was not significantly influenced by patients’ age, the duration of their illness, sex ratio, IQ, or level of education (all F s < 3.49, P s > .09; see Supplementary Data ).
Regarding symptoms of schizophrenia, 9 studies 21 , 41 , 43 , 45 , 48 , 54–56 , 59 reported symptoms measured with the Positive And Negative Syndrome Scale, 70 3 46 , 49 , 60 used other scales (Brief Psychiatric Rating Scale, 71 Scale for the Assessment of Negative Symptoms and Scale for the Assessment of Positive Symptoms, 72 or Psychotic Symptom Rating Scales 73 ) and, given the lack of equivalence across scales, were not included in the meta-regression, and 2 50 , 57 did not measure schizophrenia symptoms. The meta-regression performed on these 9 studies failed to show any significant influence of either positive, negative, or total symptoms on memory specificity ( F s < 1.94, P s > . 21).
Furthermore, 9 studies 21 , 41 , 43 , 48 , 50 , 56 , 57 , 59 , 60 measured depressive symptoms (using the Beck Depression Inventory 74 ), 2 45 , 54 excluded patients with a diagnosis of current depression, 75 and 3 46 , 49 , 55 provided no information on depression. The meta-regression performed on these 9 studies failed to show any significant influence of the level of depression on effect sizes ( F [1,7] = 0.31, P = .60).
A large ( g = −1.40) and moderate effect size ( g = −0.62) were found for richness of memory detail and conscious recollection, respectively.
Secondary analyses showed that factors relating to the task or the method used to assess richness of memory detail and conscious recollection did not significantly influence effect sizes in either case (for more details see Supplementary Data ). Patients’ age, the duration of their illness, their sex ratio, IQ, and level of education had no significant effect on effect size either ( F s < 0.92, P s > .36; Supplementary Data ).
The extent to which schizophrenia symptoms influenced the richness of memory detail could not be assessed owing to insufficient data (3 studies out of 9), and their influence with respect to conscious recollection was not significant ( F s < 1.90, P s > .22; Supplementary Data ). Due to insufficient data, it was not possible to assess the influence of symptoms of depression in terms of either richness of memory detail or conscious recollection.
Discussion
This is the first meta-analysis to address AM impairment in patients with schizophrenia-spectrum disorder. Our results revealed large effect sizes for both specificity and richness of detail of autobiographical memories, and a moderate effect size for conscious recollection. Therefore, they show that schizophrenia is associated with an impaired ability to retrieve memories of unique personal past events, access memory details related to these events, and to mentally re-live these events. While these results are in keeping with evidence that episodic memory is impaired in schizophrenia, 3 , 5 , 7 , 10 they indicate that memory deficits are not limited to a reduced capacity to retrieve items previously learned in a laboratory setting using recall and recognition tasks. Memory deficits extend to autobiographical memories, ie, memories involving information related to the self, and are more complex and remote than information presented in episodic memory tasks. 14 , 16
The number of studies included in our meta-analysis was relatively small, although comparable to that of previous meta-analyses of AM in patients with depression (14 studies 76 and 22 studies 77 ). The analysis of publication bias and sensitivity suggests the results obtained are reliable, with a fail-safe number index ranging from 116 to 610 for the different AM measures. Moderate to large heterogeneity was found across studies for all measures of AM, which led us to use a more conservative random effect model. As highlighted above, the methods used to assess AM parameters differed across studies, but this did not significantly influence measures of effect size. The most important methodological differences between studies concerned the instructions given to participants in order to collect autobiographical memories and the number of memories collected across studies. These factors did not significantly influence the results, however, save for the self-defining memories procedure, for which the differences in memory specificity between groups were not significant. This was probably because the instructions for retrieving self-defining memories do not require memories to be specific 65 and generally mean a lower proportion of specific memories are retrieved than with other studies involving explicit instructions to recall specific memories. Finally, the meta-regressions analyses showed that neither age, illness duration, sex ratio, IQ, level of education nor symptoms of illness or depressive symptoms significantly influenced the results, although they are limited by the relatively small number of studies reporting the same moderator, which prevented us from examining possible interactions between the moderators. Nevertheless, it is reasonable to consider that the effect size obtained in this meta-analysis reflects the overall AM deficit characterizing most patients with schizophrenia regardless of the methods used to collect and analyze memories.
Previous meta-analyses carried out with respect to memory domains other than AM in schizophrenia reported moderate-to-large effect sizes. 3 , 5 , 7 , 10 This was particularly true for episodic verbal memory, which is one of the most impaired memory domains in schizophrenia. 7 , 78 The effect sizes obtained for AM measures were in the same range, 3 , 5 , 7 , 10 suggesting that AM deficits must be regarded as a major domain of cognitive impairment in schizophrenia. This new finding has probably been under-acknowledged until today, despite that studies have shown that AM deficits are more reliable predictors of patients’ reduced social performance than episodic memory (as assessed by traditional laboratory tasks) or clinical symptoms. 21 Other studies have also shown that AM deficits in schizophrenia may underlie patients’ impaired theory of mind abilities 21 , 22 and altered sense of self. 41 , 54 , 63 Taken together, these aspects underscore the clinical relevance of AM for gaining a better understanding of the psychopathology of schizophrenia.
The memory specificity effect size was also in the range reported in 2 recent meta-analyses of patients with depression. 76 , 77 As mentioned above, and unlike studies conducted on patients with depression, the level of depressive symptoms was not significantly correlated with AM impairment in patients with schizophrenia, which suggests that cognitive deficits unrelated to mood disorder may account for the deficits observed in schizophrenia. There are other clinical factors, such as trauma history, however, that may also contribute to reduced memory specificity in patients 79 , 80 and to the heterogeneity we found in our meta-analysis. It is a fact that people with trauma history usually employ emotional regulation processes, such as cognitive avoidance, 79 , 81 that prevent memory retrieval processes from accessing specific memories. Although patients with schizophrenia have trauma history more frequently than controls, 82 , 83 several studies have supplied evidence against the role of cognitive avoidance processes in patients’ reduced memory specificity. 48 , 59 , 84 Finally, previous meta-analyses of cognition in domains other than AM have reported significant correlations between cognitive deficits and the level of negative symptoms. 3 , 8 , 85 We found 3 AM studies that showed negative correlations between memory specificity and negative 41 , 84 or positive 56 symptom scores. However, our meta-regressions found that neither positive nor negative symptoms of illness significantly influenced AM parameters. These conflicting results raise the question of which symptoms might be most correlated with AM deficits, and future studies could explore the relationship between AM deficits and self-disorders further, since self-disorders are certainly the dimension of illness most closely related to the “memory of self” that is AM. 17
Regarding the neurocognitive mechanisms underlying these alterations, the effortful process of generative retrieval, which allows individuals to access highly specific memory details relating to a particular past event and to retain them in working memory, relies heavily on executive functioning. However, due to prefrontal cortex dysfunctions at both structural and molecular levels, executive functions are markedly impaired in schizophrenia, which may be a critical factor accounting for AM impairment in patients. Although studies involving measures of executive functions failed to find any significant correlations between patients’ executive abilities and memory specificity, 21 , 44 , 54 , 59 1 study 48 provided convincing evidence that executive dysfunction is a direct contributing factor. This study showed that patients’ reduced capacity to retrieve specific memories during an initial effortful retrieval phase could be alleviated, by giving them suitable strategies involving specific cues to retrieve memory details. In addition, a functional neuroimaging study 55 demonstrated that the brain network involved in AM retrieval was impaired in patients with schizophrenia who exhibited reduced activation in the left lateral prefrontal cortex, anterior cingulate cortex, and right cerebellum.
Besides impaired retrieval processes, deficient encoding of AM information may also contribute to deficits observed in patients. Research on episodic memory has provided evidence of defective strategic processes (due to executive dysfunction) during information encoding in patients with schizophrenia. 78 , 86 This may reflect patients’ difficulty with spontaneously linking and organizing the different aspects of the material to be remembered. 87 These relational memory impairments described in schizophrenia 88 , 89 point to defective functioning of the hippocampus. 90 However, while with episodic memory research the material to be encoded can be controlled, it is difficult to experimentally check the “defective strategic encoding” assumption of AM deficits in schizophrenia, since personal events generally happened several years before the AM test. Finally, neuroimaging studies have largely demonstrated structural and molecular hippocampal abnormalities 91 , 92 and although patients did not display any abnormal activation of the hippocampus during AM retrieval, 55 a significant positive correlation was observed between left hippocampal volume and the number of AM details recalled, 47 suggesting that alteration of the hippocampus may also contribute to AM impairments observed in patients.
This meta-analysis suffers from a number of limitations. First, our conclusions are restricted to memories of personal past events due to the fact that very few studies have investigated memories of personal facts. 47 , 49 , 50 , 93 Second, it is difficult to check material to be encoded in AM, and furthermore many AM methods rely on self-reporting and cannot be assessed for accuracy of recall, so that differences in the approach to self-reporting rather than AM itself may also affect the results. This limitation concerns memory specificity and richness of memory detail, the ratings for which are based on an analysis of the participants’ verbatim report. Conscious recollection, however, relates exclusively to the participant’s subjective experience, not his or her verbal report of the event. 27
To conclude, AM deficits must be considered a major cognitive impairment in schizophrenia, but it is also worth mentioning that such deficits can be compensated for if patients receive appropriate treatments targeting AM. 94–97 As we look ahead to future experimental research into AM and improvements in therapies targeting memory recall for patients with schizophrenia, wearable digital cameras 98 may provide a better way of checking the accuracy of events experienced by patients and represent a promising tool for improving AM, as already shown in certain groups of patients. 99 , 100
Funding
Institut National de la Santé et de la Recherche Médicale (INSERM).
Acknowledgments
We thank Gillian Wakenhut and Sarah King for proofreading the manuscript. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References