-
PDF
- Split View
-
Views
-
Cite
Cite
Kathryn L. Mills, François Lalonde, Liv S. Clasen, Jay N. Giedd, Sarah-Jayne Blakemore, Developmental changes in the structure of the social brain in late childhood and adolescence, Social Cognitive and Affective Neuroscience, Volume 9, Issue 1, January 2014, Pages 123–131, https://doi.org/10.1093/scan/nss113
Close - Share Icon Share
Abstract
Social cognition provides humans with the necessary skills to understand and interact with one another. One aspect of social cognition, mentalizing, is associated with a network of brain regions often referred to as the ‘social brain.’ These consist of medial prefrontal cortex [medial Brodmann Area 10 (mBA10)], temporoparietal junction (TPJ), posterior superior temporal sulcus (pSTS) and anterior temporal cortex (ATC). How these specific regions develop structurally across late childhood and adolescence is not well established. This study examined the structural developmental trajectories of social brain regions in the longest ongoing longitudinal neuroimaging study of human brain maturation. Structural trajectories of grey matter volume, cortical thickness and surface area were analyzed using surface-based cortical reconstruction software and mixed modeling in a longitudinal sample of 288 participants (ages 7–30 years, 857 total scans). Grey matter volume and cortical thickness in mBA10, TPJ and pSTS decreased from childhood into the early twenties. The ATC increased in grey matter volume until adolescence and in cortical thickness until early adulthood. Surface area for each region followed a cubic trajectory, peaking in early or pre-adolescence before decreasing into the early twenties. These results are discussed in the context of developmental changes in social cognition across adolescence.
INTRODUCTION
Mentalizing, the ability to infer the intentions, beliefs and desires of others to predict their behavior, is fundamental to human development. In a large number of functional neuroimaging studies using a wide variety of mentalizing tasks, the process of mental state attribution has been associated with a network of brain regions that include the medial prefrontal cortex (mPFC), temporoparietal junction (TPJ), posterior superior temporal sulcus (pSTS) and anterior temporal cortex (ATC; Frith and Frith, 2003; Blakemore, 2012). Developmental functional magnetic resonance imaging (fMRI) studies have shown changes in recruitment within this ‘social brain network’ across adolescence (see Burnett et al., 2011 for review). However, little is known about the way in which these regions of the social brain network develop structurally during this period of life. In this study, we investigated structural development in regions of the social brain network from ages 7 to 30 years using data from the longest ongoing longitudinal pediatric neuroimaging sample (Giedd et al., 1996).
From infancy, humans display signs of social cognition (Striano and Reid, 2006; Kovács et al., 2010). For many years it was assumed that social cognitive development was mostly complete in childhood (Wimmer and Perner, 1983). However, recent behavioral research demonstrates that online social cognitive skills improve across adolescence (Dumontheil et al., 2010a). Several fMRI studies have shown differences in functional recruitment of the social brain network between adolescence and adulthood during social cognitive tasks (Blakemore, 2008, 2010). Despite the variety of paradigms used, from understanding irony (Wang et al., 2006) to thinking about social emotions like guilt (Burnett et al., 2009), many developmental fMRI studies of social cognition to date have reported decreased recruitment of dorsal mPFC in adolescents as compared with adults (Wang et al., 2006; Blakemore et al., 2007; Pfeifer et al., 2007, 2009; Burnett et al., 2009; Sebastian et al., 2011; Goddings et al., 2012). In some studies, higher activity in more posterior regions, such as the pSTS/TPJ (Blakemore et al., 2007), and in the ATC (Burnett et al., 2009), was observed in adults compared to adolescents. These changes in functional recruitment have been hypothesized to reflect changes in neurocognitive strategy and/or neuroanatomy (Blakemore, 2008). As decreases in functional activity can co-occur with reductions in grey matter volume (Lu et al., 2009; Cohen Kadosh et al., 2012), it is informative to describe the typical structural developmental trajectories of social brain regions.
The human brain undergoes profound changes in anatomy across the first decades of life (Webb et al., 2001; Petanjek et al., 2011). Neuroimaging methods, such as MRI, have enabled the investigation of these anatomical changes in large, longitudinal samples (Giedd et al., 1996). These studies have consistently shown continuing neuroanatomical development in grey and white matter (Giedd et al., 1999; Sowell et al., 2003; Brain Development Cooperative Group, 2012), with association cortices reducing in grey matter volume across adolescence. Whereas previous studies have investigated changes in structure across the entire brain, we have limited our analysis to four a priori regions of interest (ROIs) within the social brain network. Additionally, we have utilized surface-based cortical reconstruction software to distinguish grey matter volume, cortical thickness and surface area trajectories in our ROIs. As previous studies using vertex-wise analysis, lobar-level analysis or large numbers of ROIs encompassing the whole brain have typically shown inverted u-shaped trajectories, or a general decrease, in grey matter volume across the frontal, temporal and parietal cortices, we predicted similar trajectories in all regions examined in our analysis. Previous studies investigating cortical thickness across late childhood have shown diverse patterns, including increasing cortical thickness in medial prefrontal and anterior temporal cortices (Sowell et al., 2004), decreasing cortical thickness in the frontal pole (van Soelen et al., 2012) and decreasing cortical thickness in lateral parietal lobes (Sowell et al., 2004; van Soelen et al., 2012). Investigations of cortical thickness across the second decade and into adulthood have shown increasing cortical thickness in the temporal lobe until mid-adolescence (Shaw et al., 2008), and decreasing cortical thickness in medial frontal and lateral parietal lobes (Shaw et al., 2008; Tamnes et al., 2010). Based on these findings, we predicted either an inverted u-shaped pattern or general decrease in cortical thickness for all the regions in this study. However, changes in surface area across development have been less well characterized, although investigators are beginning to include analyses of surface area in studies (Ostby et al., 2009; Raznahan et al., 2011; Shaw et al., 2012). We predicted that the surface area would show an inverted u-shaped trajectory reaching a peak in early/pre-adolescence, similar to what was found by Raznahan et al. (2011) for the entire cortex. Although we did not predict sex differences in these regions based on differential social cognitive development between females and males, we did predict that females would reach a peak in grey matter volume and surface area, but not in cortical thickness, earlier than males (Raznahan et al., 2011).
METHODS
Participants
The sample consisted of 288 unrelated individuals (124 females, 164 males), each of whom had undergone two or more high-quality MRI scans ∼2 years apart over the age range of 7–30 years. The average age that participants displayed the earliest signs of pubertal onset (Tanner Stage 2), as measured by a self-report (parents or participants) Tanner stage diagrams (Taylor et al., 2001), in a subset of individuals (41 females, 51 males), was 10.01 years (9.94 for females, 10.06 for males). This subset was chosen based on how many individuals were participants in the project while in Tanner Stage 2, and the availability of data. As adolescence is defined as the time period between puberty and relative self-sufficiency, when we refer to the period of adolescence in the context of this study, we are referring to roughly 10–18 years of age. Of the 288 individuals, 126 were members of twin pairs (55 females, 71 males); only one member per twin pair was included in the analysis. There were no significant differences between males and females in socioeconomic status (SES), handedness, race, intelligence quotient (IQ) or number of scans (Table 1). The absence of neurological or psychiatric illness was established through a telephone screening interview and completion of a parent-report screening questionnaire (Childhood Behavior Checklist). Handedness was established using Physical and Neurological Examination of Soft Signs inventory (Denckla, 1985). All participants had a full-scale IQ >80 (IQ was estimated using age-appropriate Wechsler Intelligence Scales). SES was quantified using Hollingshead Scales (Hollingshead, 1975). Sample characteristics are detailed in Table 1.
Participant characteristics
| . | Group . | Sex difference . | ||
|---|---|---|---|---|
| Characteristic . | All . | Female . | Male . | . |
| Number of individuals | 288 | 124 | 164 | n.s. |
| Singleton | 162 | 69 | 93 | |
| Member of twin pair | 126 | 55 | 71 | |
| Handedness, no. | n.s. | |||
| Right | 255 | 107 | 148 | |
| Mixed | 21 | 10 | 11 | |
| Left | 11 | 7 | 4 | |
| Race, no. | n.s. | |||
| Caucasian | 250 | 107 | 143 | |
| African-American | 21 | 10 | 11 | |
| Asian | 2 | 1 | 1 | |
| Hispanic | 8 | 4 | 4 | |
| Other | 7 | 2 | 5 | |
| IQ | n.s. | |||
| Mean (s.d.) | 114 (12.0) | 113 (12.5) | 115 (11.5) | |
| SES | n.s. | |||
| Mean (s.d.) | 41 (18.2) | 42 (17.5) | 40 (18.7) | |
| Number of scans, no. | n.s. | |||
| 2 scans | 138 | 60 | 78 | |
| 3 scans | 73 | 36 | 37 | |
| 4 scans | 40 | 11 | 29 | |
| 5 scans | 23 | 12 | 11 | |
| 6 scans | 11 | 5 | 6 | |
| 7 scans | 3 | 0 | 3 | |
| Total | 857 | 362 | 495 | |
| Age distribution of scans (years) | ||||
| Mean (s.d.) | 14.9 (4.9) | 14.4 (4.9) | 15.2 (4.8) | |
| Range | 7.0–30.6 | 7.1–29.5 | 7.0–30.6 | |
| . | Group . | Sex difference . | ||
|---|---|---|---|---|
| Characteristic . | All . | Female . | Male . | . |
| Number of individuals | 288 | 124 | 164 | n.s. |
| Singleton | 162 | 69 | 93 | |
| Member of twin pair | 126 | 55 | 71 | |
| Handedness, no. | n.s. | |||
| Right | 255 | 107 | 148 | |
| Mixed | 21 | 10 | 11 | |
| Left | 11 | 7 | 4 | |
| Race, no. | n.s. | |||
| Caucasian | 250 | 107 | 143 | |
| African-American | 21 | 10 | 11 | |
| Asian | 2 | 1 | 1 | |
| Hispanic | 8 | 4 | 4 | |
| Other | 7 | 2 | 5 | |
| IQ | n.s. | |||
| Mean (s.d.) | 114 (12.0) | 113 (12.5) | 115 (11.5) | |
| SES | n.s. | |||
| Mean (s.d.) | 41 (18.2) | 42 (17.5) | 40 (18.7) | |
| Number of scans, no. | n.s. | |||
| 2 scans | 138 | 60 | 78 | |
| 3 scans | 73 | 36 | 37 | |
| 4 scans | 40 | 11 | 29 | |
| 5 scans | 23 | 12 | 11 | |
| 6 scans | 11 | 5 | 6 | |
| 7 scans | 3 | 0 | 3 | |
| Total | 857 | 362 | 495 | |
| Age distribution of scans (years) | ||||
| Mean (s.d.) | 14.9 (4.9) | 14.4 (4.9) | 15.2 (4.8) | |
| Range | 7.0–30.6 | 7.1–29.5 | 7.0–30.6 | |
n.s., not statistically significant at P < 0.05.
Demographic characteristics for the sample. There were no significant differences in IQ, handedness, SES, race or number of scans between female and male participants.
Participant characteristics
| . | Group . | Sex difference . | ||
|---|---|---|---|---|
| Characteristic . | All . | Female . | Male . | . |
| Number of individuals | 288 | 124 | 164 | n.s. |
| Singleton | 162 | 69 | 93 | |
| Member of twin pair | 126 | 55 | 71 | |
| Handedness, no. | n.s. | |||
| Right | 255 | 107 | 148 | |
| Mixed | 21 | 10 | 11 | |
| Left | 11 | 7 | 4 | |
| Race, no. | n.s. | |||
| Caucasian | 250 | 107 | 143 | |
| African-American | 21 | 10 | 11 | |
| Asian | 2 | 1 | 1 | |
| Hispanic | 8 | 4 | 4 | |
| Other | 7 | 2 | 5 | |
| IQ | n.s. | |||
| Mean (s.d.) | 114 (12.0) | 113 (12.5) | 115 (11.5) | |
| SES | n.s. | |||
| Mean (s.d.) | 41 (18.2) | 42 (17.5) | 40 (18.7) | |
| Number of scans, no. | n.s. | |||
| 2 scans | 138 | 60 | 78 | |
| 3 scans | 73 | 36 | 37 | |
| 4 scans | 40 | 11 | 29 | |
| 5 scans | 23 | 12 | 11 | |
| 6 scans | 11 | 5 | 6 | |
| 7 scans | 3 | 0 | 3 | |
| Total | 857 | 362 | 495 | |
| Age distribution of scans (years) | ||||
| Mean (s.d.) | 14.9 (4.9) | 14.4 (4.9) | 15.2 (4.8) | |
| Range | 7.0–30.6 | 7.1–29.5 | 7.0–30.6 | |
| . | Group . | Sex difference . | ||
|---|---|---|---|---|
| Characteristic . | All . | Female . | Male . | . |
| Number of individuals | 288 | 124 | 164 | n.s. |
| Singleton | 162 | 69 | 93 | |
| Member of twin pair | 126 | 55 | 71 | |
| Handedness, no. | n.s. | |||
| Right | 255 | 107 | 148 | |
| Mixed | 21 | 10 | 11 | |
| Left | 11 | 7 | 4 | |
| Race, no. | n.s. | |||
| Caucasian | 250 | 107 | 143 | |
| African-American | 21 | 10 | 11 | |
| Asian | 2 | 1 | 1 | |
| Hispanic | 8 | 4 | 4 | |
| Other | 7 | 2 | 5 | |
| IQ | n.s. | |||
| Mean (s.d.) | 114 (12.0) | 113 (12.5) | 115 (11.5) | |
| SES | n.s. | |||
| Mean (s.d.) | 41 (18.2) | 42 (17.5) | 40 (18.7) | |
| Number of scans, no. | n.s. | |||
| 2 scans | 138 | 60 | 78 | |
| 3 scans | 73 | 36 | 37 | |
| 4 scans | 40 | 11 | 29 | |
| 5 scans | 23 | 12 | 11 | |
| 6 scans | 11 | 5 | 6 | |
| 7 scans | 3 | 0 | 3 | |
| Total | 857 | 362 | 495 | |
| Age distribution of scans (years) | ||||
| Mean (s.d.) | 14.9 (4.9) | 14.4 (4.9) | 15.2 (4.8) | |
| Range | 7.0–30.6 | 7.1–29.5 | 7.0–30.6 | |
n.s., not statistically significant at P < 0.05.
Demographic characteristics for the sample. There were no significant differences in IQ, handedness, SES, race or number of scans between female and male participants.
Participants were recruited from the community through local advertisement and paid for their participation in the study (Giedd et al., 1996). The institutional review board of the National Institutes of Health approved the research protocol employed in this study and written informed consent and assent to participate in the study were obtained from parents/adult participants and children, respectively.
Image acquisition
All MRI scans were T1-weighted images with contiguous 1.5 mm axial slices and 2.0 mm coronal slices, obtained on the same 1.5-T General Electric Signa scanner (Milwaukee, WI, USA) using a three-dimensional spoiled gradient-recalled echo sequence with the following parameters: echo time, 5 ms; repetition time, 24 ms; flip angle, 45°; acquisition matrix, 256 × 192; number of excitations, 1; and field of view, 24 cm. A clinical neuroradiologist evaluated all scans for gross abnormalities.
Image processing
Cortical reconstruction was performed with the Freesurfer5.1 image analysis suite, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Dale et al., 1999; Fischl et al., 1999a). The processing stream for structural images includes motion correction (Reuter et al., 2010), removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, intensity normalization (Sled et al., 1998), tessellation of the grey matter white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007) and surface deformation following intensity gradients to optimally place the grey/white and grey/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale and Sereno, 1993; Dale et al., 1999; Fischl and Dale, 2000). Only one anatomical scan was processed per scanning session (timepoint). For the purposes of this analysis, we have averaged together these two surface area measurements to obtain a measurement of the middle grey surface area. Each cortical reconstruction was visually inspected (by author K.L.M.), and unsuccessful cortical reconstructions were identified and excluded from the present analyses. This left a sample of 288 individuals (857 scans) for the analyses of mBA10, TPJ and pSTS, and a sample of 221 individuals (447 scans) for the analysis of the ATC. Each cortical model was registered to a spherical atlas using individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., 1999b). Measurements of mean cortical thickness (mm), middle grey surface area (mm2) and grey matter volume (mm3) were extracted for each region of interest.
Regions of interests
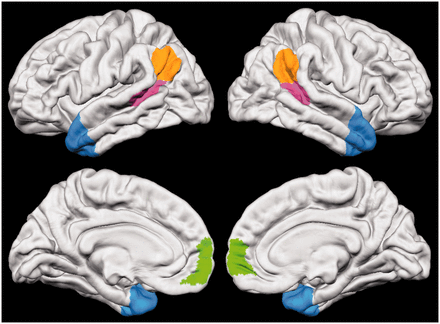
Four regions of interest (ROIs) were created for each hemisphere using an averaged template in Freesurfer. These ROIs, illustrated in Figure 1, include medial Brodmann Area 10 (mBA10), TPJ, pSTS and ATC. As this analysis necessitated well-defined borders for all ROIs, we chose mBA10 as our proxy for dorsal mPFC. The mBA10 itself has been characterized in meta-analyses as a neural correlate of mentalizing (Amodio and Frith, 2006; Gilbert et al., 2006). Structural measurements for each hemisphere were combined to produce one value for each ROI. Posthoc analyses for the left and right hemispheres were performed to examine hemispheric differences. We describe how we defined each ROI below.
The social brain. Social brain regions of interest include medial Brodmann Area 10 (mBA10; green), temporoparietal junction (TPJ; orange), posterior superior temporal sulcus (pSTS; pink) and anterior temporal cortex (ATC; blue).
mBA10
The medial portion of BA10 was defined using the PALS B12 Brodmann atlas projected onto an averaged Freesurfer template (Van Essen, 2005). From this atlas, the BA10 label was selected, and any area of this label located outside of the medial cortex was eliminated.
TPJ
The TPJ was defined using the border coordinates of a functional subdivision generated in a previous study (Mars et al., 2012). This study defined three consistent functional subregions within a larger TPJ area in the right hemisphere, each with unique functional and structural connectivity profiles. Of the three subregions, we chose to use the posterior TPJ subregion, as it was functionally connected to other areas of the social brain, such as mBA10 and ATC (Mars et al., 2012).
pSTS
The pSTS region was created by extending the Desikan-Killiany Atlas defined bank STS (Fischl et al., 2004; Desikan et al., 2006) to the border of the TPJ.
ATC
The ATC was defined by extending the temporal pole, defined as Brodmann Area 38 in the PALS B12 Brodmann atlas, back to include areas of the ATC recruited during mentalizing tasks in previous fMRI studies (see Olson et al., 2007 for a review).
Statistical analysis
We used mixed models (Pinheiro and Bates, 2000) to estimate the fixed effects of age on each measure, with nested random effects terms modeled for within person dependence of observations. For example, an equation for a model using cubic grey matter volume (GM) growth for ith individual’s jth timepoint is: GMij = Intercept + di + β1(age) + β2(age2) + β3(age3) + εij. Age terms were centered to the mean age (i.e. the mean age, 14.89 years, was subtracted from each age) to reduce the correlation between the age, age-squared and age-cubed terms. For each measure, an F-test was used to determine whether a cubic, quadratic or linear growth model best fit the entire sample.
After determining the best growth model for the entire sample, we conducted likelihood-ratio (LR) tests to determine if either the growth curve height or shape were statistically different between sexes. First, to establish if growth curve height was significantly different between males and females, we conducted a LR test to determine whether the best fitted model including age and a main effect of sex predicted significantly more variance in the measure of interest compared with the model including age terms alone. Second, to establish if the growth curve shape for the measure of interest was significantly different between males and females, we conducted a LR test to determine whether the best fitted model including interactions between age terms and sex predicted significantly more variance in the measure of interest compared with a simpler model including only age terms and a main effect of sex. For measures of interest following non-linear growth curves, age-at-peak was calculated by solving the first-order derivative of the growth trajectory equation that had been defined for that measure using mixed modeling.
RESULTS
Medial BA10
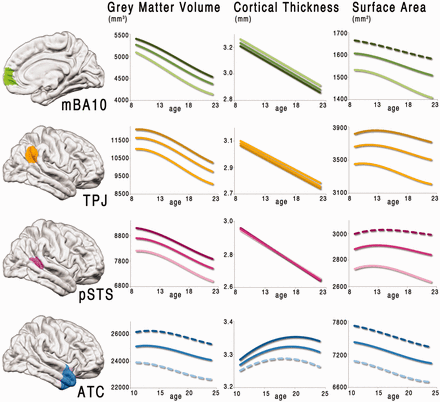
Grey matter volume for mBA10 followed a cubic trajectory (F(1,566) = 9.51, P < 0.003) decreasing steadily from age 9 to 22 years. Cortical thickness for mBA10 decreased linearly from childhood into the early twenties (F(1,568) = 329.34, P < 0.001), whereas the surface area followed a cubic trajectory (F(1,566) = 5.67, P < 0.018), peaking at 8.0 years and decreasing into the early twenties (Figure 2). Grey matter volume for the left mBA10 followed a linear trajectory (F(1,568) = 219.46, P < 0.0001), and right mBA10 followed a cubic trajectory (F(1,566) = 12.72, P < 0.0005). Cortical thickness for the left mBA10 followed a quadratic trajectory (F(1,567) = 7.93, P < 0.006), and right mBA10 followed a cubic trajectory (F(1,566) = 7.18, P < 0.008). Surface area for the left mBA10 followed a linear trajectory (F(1,568) = 32.97, P < 0.0001), and right mBA10 followed a linear trajectory (F(1,568) = 89.11, P < 0.0001) (Supplementary Figures S1 and Supplementary Data).
Fitted models of structural development across adolescence. The best fitting models for all participants are shown for each region of interest (combined hemispheres). Models are fitted to the middle 80% of the sample (ages 9–22 years for mBA10, TPJ and pSTS; ages 11–24 years for ATC). The lighter lines show the fitted models applied to females only, and the darker lines show the fitted models applied to males only. Solid lines indicate the fitted model was significant P < 0.05, whereas dashed lines indicate the fitted model was not significant (P ≥ 0.05).
TPJ
Grey matter volume for the TPJ followed a cubic trajectory (F(1,566) = 44.06, P < 0.0001) peaking at 9.3 years and then decreasing into the early twenties. Cortical thickness for the TPJ decreased linearly from childhood into the early twenties (F(1,568) = 475.81, P < 0.0001), whereas the surface area followed a cubic trajectory (F(1,566) = 14.73, P < 0.0002) peaking at 11.5 years and decreasing into the early twenties. Grey matter volume for the left TPJ followed a cubic trajectory (F(1,566) = 20.00, P < 0.0001), and right TPJ followed a cubic trajectory (F(1,566) = 37.46, P < 0.0001). Cortical thickness for the left TPJ followed a linear trajectory (F(1,568) = 341.44, P < 0.0001), and right TPJ followed a linear trajectory (F(1,568) = 354.93, P < 0.0001). Surface area for the left TPJ followed a cubic trajectory (F(1,566) = 10.73, P < 0.002), and right TPJ followed a cubic trajectory (F(1,566) = 6.306, P < 0.02).
pSTS
Grey matter volume for the pSTS followed a cubic trajectory (F(1,566) = 25.90, P < 0.0001) peaking at 8.7 years and then decreasing into the early twenties. Cortical thickness for the pSTS decreased linearly from childhood into the early twenties (F(1,568) = 574.07, P < 0.0001), whereas the surface area followed a cubic trajectory (F(1,568) = 6.98, P < 0.009) peaking at 12.9 years and decreasing into the early twenties. Grey matter volume for the left pSTS followed a cubic trajectory (F(1,568) = 16.39, P < 0.0002), and right pSTS followed a cubic trajectory (F(1,566) = 14.98, P < 0.0002). Cortical thickness for the left pSTS followed a linear trajectory (F(1,568) = 444.21, P < 0.0001), and right pSTS followed a linear trajectory (F(1,568) = 335.75, P < 0.0001). Surface area for the left pSTS followed a linear trajectory (F(1,568) = 12.17, P < 0.0006), and right pSTS followed a cubic trajectory (F(1,566) = 4.82, P < 0.03).
ATC
Grey matter volume for the ATC followed a cubic trajectory (F(1,223) = 6.31, P < 0.02) peaking at age 12.6 years and then decreasing into the mid-twenties. Cortical thickness increased from childhood following a quadratic trajectory (F(1,224) = 9.56, P < 0.002) peaking at 19.4 years, whereas surface area followed a cubic trajectory (F(1,224) = 3.91, P < 0.05) peaking at 8.3 years and decreasing into the mid-twenties. Grey matter volume for the left ATC followed a linear trajectory (F(1,225) = 13.96, P < 0.0003), and right ATC followed a cubic trajectory (F(1,223) = 7.12, P < 0.009). Cortical thickness for the left ATC followed a quadratic trajectory (F(1,224) = 5.10, P < 0.03), and right ATC followed a linear trajectory (F(1,225) = 3.96, P < 0.05). Surface area for the left ATC followed a linear trajectory (F(1,225) = 32.22, P < 0.0001), and right ATC followed a cubic trajectory (F(1,223) = 4.60, P < 0.04).
Sex differences
Sex differences were observed in grey matter volume and surface area, but not in cortical thickness, for all ROIs. Grey matter volume trajectory heights were significantly different between females and males in mBA10 (LR = 34.68, P < 0.0001), TPJ (LR = 33.41, P < 0.0001), pSTS (LR = 38.10, P < 0.0001) and ATC (LR = 67.43, P < 0.0001), with females displaying smaller volumes than males across ages 7–30 years. Surface area trajectory heights were significantly different between females and males in mBA10 (LR = 53.83, P < 0.0001), TPJ (LR = 42.26, P < 0.0001), pSTS (LR = 41.30, P < 0.0001) and ATC (LR = 54.43, P < 0.0001), with females displaying smaller surface areas than males across ages 7–30 years. Additionally, a significant difference in trajectory shape was observed in TPJ surface area (LR = 9.89, P < 0.02), with females reaching a peak in surface area earlier than males (10.2 years vs. 12.6 years, respectively). No differences in cortical thickness were observed between the sexes in the combined hemispheres and in the individual hemispheres. Left and right hemispheres showed the same effects for all measurements as the combined hemispheres for mBA10 and pSTS. The interactive effect for surface area in the TPJ did not hold in each individual hemisphere, but grey matter volume and surface area trajectory heights were significantly different between females and males. Grey matter volume for the ATC in the left hemisphere showed a significant interactive effect with sex (LR = 4.60, P < 0.04), whereas grey matter volume for the ATC in the right hemisphere, as well as surface area for both hemispheres, displayed a main effect of sex, as was found in the combined hemispheres.
DISCUSSION
The aim of this study was to investigate the structural development of the social brain network across adolescence using data from the longest ongoing longitudinal pediatric neuroimaging sample (288 participants; 857 scans; ages 7–30 years) (Giedd et al., 1996). While grey matter volume in mBA10, TPJ and pSTS reached a peak in late childhood before decreasing into adulthood, the ATC increased in grey matter volume until adolescence (∼12 years), decreasing thereafter. Cortical thickness in mBA10, TPJ and pSTS decreased linearly across adolescence, largely consistent with results in previous studies (Ostby et al., 2009; Tamnes et al., 2010; van Soelen et al., 2012; but see Shaw et al., 2008). However, cortical thickness in the ATC followed a quadratic trajectory, increasing until early adulthood. Surface area for each region followed a cubic trajectory, reaching a peak in early or pre-adolescence before decreasing into the early twenties, whereas previous lobar-level studies have shown general decreases for the frontal, temporal and parietal cortices (Ostby et al., 2009). Differences in grey matter volume and surface area, but not in cortical thickness, were observed between female and male participants. Males displayed larger cortical volumes and greater surface areas than females across all ROIs. Differences in age-at-peak were only observed for the surface area of the TPJ, which reached a peak 2 years earlier in females than in males. Although we report peak ages in this study, these ages should be interpreted with caution since factors such as age range can affect estimated peaks when using global models (i.e. linear, quadratic and cubic models) (Fjell et al., 2010). As grey matter volume is the product of surface area and cortical thickness, it may be that the sex differences observed in grey matter volumes are driven by differences in surface area rather than cortical thickness.
The social brain network
While the co-activation of regions examined in this study has been demonstrated in many social cognitive fMRI experiments, the individual contributions of these anatomically distinct regions to social cognitive processes is still under debate. Electrophysiological and fMRI studies consistently report the involvement of the pSTS in the perception of biological motion and eye gaze (Puce and Perrett, 2003), and in grasping the intentionality and appropriateness of biological motion (Pelphrey et al., 2004). It may be that the pSTS is involved in decoding complex social gestures conveyed through eye gaze and body movement. The TPJ, while in close anatomical proximity to the pSTS, is involved in different aspects of social cognition. It is suggested that the TPJ is activated specifically in situations when one is inferring the mental states of others, rather than just information known about another (Saxe and Kanwisher, 2003; Saxe et al., 2009). In contrast, mBA10 is activated in multiple conditions: when inferring the mental states of others, when reflecting on knowledge of another’s traits, and when reflecting on the traits of oneself (Frith, 2007). Frith (2007) has proposed that the underlying similarity between tasks that activate mBA10 is their involvement in handling communicative intentions, which requires a second order representation of mental state, whether our own or another’s. A combination of lesion, non-human primate and fMRI studies has prompted researchers to theorize the involvement of the ATC in interpreting social narratives (Olson et al., 2007), and processing social scripts (Frith and Frith, 2003; Frith 2007).
Many of the ROIs followed similar structural trajectories, with mBA10, TPJ and pSTS reaching a peak in grey matter volume during childhood, followed by a steady decrease in adolescence before leveling off in the early twenties. However, the ATC showed a different trajectory, increasing in grey matter volume until adolescence, before gradually decreasing until leveling off in the mid-twenties. It is unclear why the ATC would continue to increase in grey matter volume so late in development, reaching a peak in grey matter volume later than the onset of puberty. This area of the cortex contains important linkages to both the mPFC and limbic structures (e.g. amygdala, hippocampus) via the uncinate fasciculus, which is one of the last white matter tracts to reach maturity (Lebel et al., 2008). Perhaps the relatively late myelination of ATC projections allows a longer window for learning social scripts. Future investigations that measure white matter in social brain regions are needed to determine the role of white matter maturity (i.e. axonal caliber, myelination) in the development of these regions. Despite differences in the timing and tempo of grey matter volume decline between regions of the social brain, each region continues to change across adolescence before relatively stabilizing in the early twenties.
Underlying anatomy and histology
Until recently, most structural MRI studies of the developing brain have examined only grey and white matter volumes in relatively large regions. Grey matter volume itself is the product of cortical thickness and surface area, which are influenced by distinct genetic (Panizzon et al., 2009; Winkler et al., 2010), evolutionary (Rakic, 1995) and cellular (Chenn and Walsh, 2002) processes, in addition to being phenotypically distinct (Winkler et al., 2010). Grey matter volume is more correlated with, and genetically and environmentally related to, surface area than cortical thickness (Winkler et al., 2010). Differences in surface area are pronounced across species (Rakic, 1995; Hill et al., 2010), whereas cortical thickness is highly conserved (Roth and Dicke, 2005; Fish et al., 2008). Interestingly, areas similar to regions explored in this study have greatly expanded in surface area across evolution (Hill et al., 2010). Furthermore, many of these regions showed relatively greater surface area expansion between infancy and adulthood, although mBA10 is a notable exception (Hill et al., 2010).
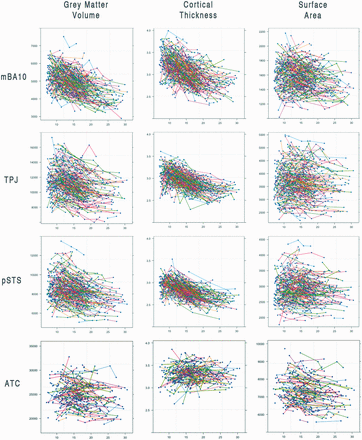
The dissimilarity of cortical thickness and surface area growth across late childhood and adolescence is observable in the results of this study. Surface area is much more variable than cortical thickness between individuals (Figure 3). Perhaps the combination of decreasing linear trajectories in cortical thickness and subtle cubic trajectories in surface area observed in mBA10, TPJ and pSTS contribute to the early-peaking (and more profoundly decreasing) grey matter volume trajectories observed in this study. In contrast, the late-peaking cortical thickness and gradually decreasing surface area of the ATC shapes the grey matter trajectory to resemble the surface area trajectory with a later peak around 12 years.
Scatter plots of all participants for each measure of each region of interest. There are 288 individuals and 857 scans represented in the mBA10, TPJ and pSTS graphs, and 221 individuals and 447 scans represented in the ATC graphs.
The underlying mechanisms associated with a reduction in grey matter volume are still debated (see Paus et al., 2008), and we are unaware of any studies that have tested the relationship between developmental changes in underlying cellular or synaptic anatomy and structural MRI measures. Despite these limitations, it is thought that reductions in grey matter volume may reflect synaptic reorganization and/or increases in white matter integrity (Paus et al., 2008). Histological studies of postmortem human brain tissue support the idea that association cortices continue to undergo synaptic pruning across adolescence (Huttenlocher and Dabholkar, 1997; Petanjek et al., 2011), although the regions examined in these studies do not include the regions included in the present analysis. There is also histological evidence for an extended period of myelination in association cortices, continuing well into adolescence (Yakovlev and Lecours, 1967).
Relationship between structure and function
While this study describes how areas of the social brain develop structurally from late childhood into early adulthood, there have been a number of fMRI studies that show functional changes in the social brain during this period (see Burnett et al., 2011 for review). Many of these studies report decreases in dorsal mPFC recruitment between adolescence and adulthood during social cognitive tasks (Wang et al., 2006; Blakemore et al., 2007; Pfeifer et al., 2007, 2009; Burnett et al., 2009; Sebastian et al., 2011). Why adolescents recruit the mPFC, an area involved in decoding communicative intent and second order mental state representation, more than adults in social cognitive tasks is still an open question. It has been suggested that the decrease in recruitment of the mPFC across adolescence may relate to changes in neuroanatomy or maturing neurocognitive strategies (Blakemore, 2008). Likewise, the protracted structural development of the PFC is often interpreted as reflecting the relative neuroplasticity of this region during adolescence (Giedd et al., 1999). This suggests that structural and functional changes in similar brain regions are co-occurring across development. This idea is supported by a study that correlated cortical grey matter thickness and functional brain activity in typically developing children performing an orthographic processing task (Lu et al., 2009). The authors of this study found that increased activation correlated with mature brain morphology in the same region, and that both of these measures correlate with performance even after accounting for age. However, this study did not involve social cognition, and future developmental studies may begin to characterize the relationship between structural and functional changes by recording structural measures as a potential covariate in fMRI analysis, as some have already begun to do (Cohen Kadosh et al., 2012). This study demonstrated that both age-related and performance-related fMRI activation during a face processing task correlated with structural changes in some, but not all, of the same brain regions (Cohen Kadosh et al., 2012). Another study by Dumontheil et al. (2010b), found that individual differences in grey and white matter volumes could not account for the age-related changes in fMRI activation during a relational reasoning task. These mixed results suggest that age-related changes in blood oxygen level dependent signal do not entirely reflect structural maturation, and may instead reflect the maturation of neurocognitive strategies.
Future directions and limitations
This analysis did not correlate brain structure with social cognitive skill. By correlating structural brain development trajectories in the social brain with social cognitive skills, future studies could characterize inter-individual differences in brain development as they relate to social cognition. It may be that extreme variations in tempo or timing of social brain development correlate with disorders of social cognition (e.g., autism spectrum disorders). However, as it is currently difficult to disentangle genetically pre-programmed developmental changes from those that are triggered by changes in the environment, it may not be possible to speculate on how social cognitive development influences the developmental changes occurring in the brain across adolescence and vice versa.
We also do not address how puberty may affect the observed structural brain development trajectories, as we do not have pubertal measures on all participants in this sample. Pubertal maturation does seem to correlate with brain structure (Neufang et al., 2009; Peper et al., 2009; Bramen et al., 2011), and it would be informative to explore the influence of puberty on the structural trajectories of these social brain regions.
To further describe how regions of the social brain develop across adolescence, it would be beneficial to utilize structural and functional connectivity analytic methods. These methods illustrate how each region within the network communicates with the others, and changes in connectivity strength may reflect maturing neurocognitive strategies. Although previous fMRI studies have shown that recruitment of specific regions of the social brain changes across development, it is unclear how these regions interact during this period of time. As various brain networks have been shown to change in both organization and connectivity strength across adolescence (Fair et al., 2007, 2008, 2009, 2010), the social brain network may also undergo reorganization during this period.
Finally, we would like to address the issue of quality control in structural MRI studies. This large sample included only high-quality structural MRI volumes that had each been visually inspected and rated for motion and artefacts. However, many of the cortical reconstructions for these high-quality scans failed the Freesurfer pipeline for one region only, the ATC. It is important to note that this is not a problem with Freesurfer alone, but also other cortical reconstruction software, and future studies investigating the ATC would benefit from visual inspection of this area for quality control purposes. While this analysis uses the standard Freesurfer pipeline, we acknowledge that the new longitudinal Freesurfer pipeline could improve the cortical reconstruction process, and are excited to implement the longitudinal pipeline in future studies.
CONCLUSION
The social brain network continues to develop structurally across adolescence before relatively stabilizing in the early twenties. This protracted development lends support to the theory that areas of the brain involved in social cognition are maturing from late childhood into early adulthood. It is likely that convergence across multiple methodologies will help us to understand how the social brain develops across adolescence.
FUNDING
This study was supported by the National Institutes of Health, National Institute of Mental Health Intramural Research Program and the NIH Graduate Partnership Program. S.J.B. is funded by a Royal Society University Research Fellowship.
The authors gratefully acknowledge the continued participation of all families and individuals involved in this longitudinal study. We thank A.L. Goddings, M. Lombardo, R. Mars and B. Dickerson for valuable discussion concerning the definition of the temporoparietal junction for this project; and the Athinoula Martinos Center for Biomedical Imaging for providing software used for the analysis.