-
PDF
- Split View
-
Views
-
Cite
Cite
H. Smith, F. Anderson, H. Raphael, P. Maslin, S. Crozier, C. Cooper, Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial, Rheumatology, Volume 46, Issue 12, December 2007, Pages 1852–1857, https://doi.org/10.1093/rheumatology/kem240
Close - Share Icon Share
Abstract
Objectives. Low trauma fractures in older people incur enormous physical, social and economic costs. Previous research indicates that an annual intramuscular injection of vitamin D may reduce fracture rates in this group. This strategy requires validation in a population setting.
Methods. Randomized, double-blind, placebo-controlled trial of 300 000 IU intramuscular (i.m.) vitamin D2 (ergocalciferol) injection or matching placebo every autumn over 3 years. 9440 people (4354 men and 5086 women) aged 75 yrs and over were recruited from general practice registers in Wessex, England. Primary outcome measure was all non-vertebral fracture. Secondary outcomes were hip and wrist fractures, and all falls.
Results. 585 subjects had incident non-spine fractures (hip 110, wrist 116, ankle 37). Hazard ratios (HRs) for fracture in the vitamin D group were: 1.09 [95% confidence interval (CI) 0.93–1.28, P = 0.29] for any first fracture, 1.49 (95% CI 1.02–2.18, P = 0.04) for hip and 1.22 (95% CI 0.85–1.76, P = 0.28) for wrist. There was no effect on falls: HR 0.98 (0.93–1.04). No protective effect was observed in any subgroup when the cohort was stratified by sex, age, previous fracture or mobility.
Conclusions. An annual i.m. injection of 300 000 IU vitamin D2 is not effective in preventing non-vertebral fractures among elderly men and women resident in the general population.
Introduction
Osteoporotic fractures are a major public health problem, and their incidence is projected to increase steeply worldwide [1]. The social and economic costs associated with osteoporotic fractures are very great [2], and reducing this burden is widely seen as a health care policy imperative. Vitamin D deficiency is common in elderly people, especially those with hip fracture, and Vitamin D supplementation, if effective, might provide a feasible and relatively cost-effective primary preventive measure against fracture among older men and women. Previous studies of vitamin D supplementation among older people in western populations have produced inconsistent results. When combined with calcium in daily oral formulations, the intervention has been shown to reduce the incidence of osteoporotic fractures among elderly nursing home residents [3]; when administered orally and without calcium supplementation, fracture prevention has been more difficult to demonstrate [4–6]. Vitamin D may also be administered by intramuscular (i.m.) injection, and a single non-randomized trial of men and women aged 85 yrs and over administered an annual i.m. injection of ergocalciferol vitamin D2 (150 000–300 000 IU) resulted in a significant reduction in the incidence of all fractures over a 5-yr period, when compared with a control series [7]. We report the results of a randomized, double-blind, placebo-controlled trial of 300 000 IU i.m. ergocalciferol injection or matching placebo on fracture rates among 9440 men and women aged 75 yrs and over, resident in the general population.
Methods
Study design
The study was a randomized, double-blind, placebo-controlled trial of 300 000 IU ergocalciferol (Evans Medical Ltd/Celltech Ltd, UK) by i.m. injection in men and women aged over 75 yrs recruited from the practice registers of 111 general practices included in the Wessex Primary Care Research Network (WReN) in central Southern England [8]. Previous studies have demonstrated that the age, gender and socioeconomic profile of practices enrolled within this network are representative of England and Wales as a whole [9]. Institutional Review Board approval for the study was obtained from the South West Multi-centre Research Ethics Committee (MREC/98/6/51).
Recruitment and randomization
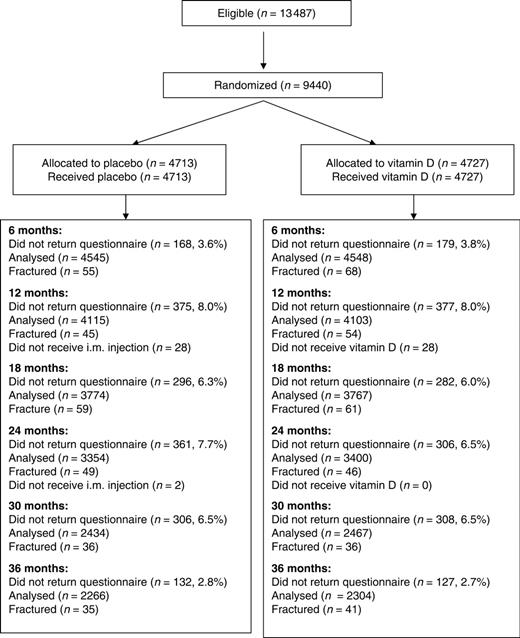
The influenza immunization programme in England and Wales offers immunization annually to all adults over 65 yrs of age; we utilized this framework to select our study sample on the principle that, were the intervention to prove effective, this would provide a cost-effective way of implementing the intervention as a public health measure. Men and women aged over 75 yrs who did not meet any of the study exclusion criteria (current cancer or any history of treated osteoporosis, bilateral total hip replacement, renal failure, renal stones, hypercalcaemia or sarcoidosis) were identified from the age–sex registers of participating practices. An information leaflet about the study with a covering letter from their practice was sent to potential participants. Those interested in participating were asked to return a form giving details of any vitamin D supplementation they took already. People taking ≥400 IU or more daily were excluded from the study. The remainder were invited to an appointment with the practice nurse, who confirmed their understanding of the study, checked for exclusion criteria not recorded in existing medical records and obtained written informed consent. A questionnaire ascertained details of previous fractures, current mobility and accommodation. The numbers of subjects invited and randomized are shown in Fig. 1.
Flow diagram for subjects in the Wessex Fracture Prevention Trial.
Intervention
Subjects were randomized at an individual level to either the intervention or control group. Randomization was balanced in each of the 111 practice sites by randomizing within blocks at each practice. Each participating practice was sent mixed boxes containing previously randomized, numbered ampoules of either vitamin D or placebo, which were identical in visual appearance and consistency. As each subject consented to participate in the trial, they were allocated consecutive ampoules. The number of the ampoule was then linked to the patient's name and phoned to a central location. This study number remained with the subject for the duration of the trial. The trial injection was then given i.m. into the buttock or leg. Packing and labelling were carried out by an external contractor; allocation was concealed from investigators, practice nurses and subjects. After completion of the recruitment phase, all study documentation was checked at each practice by the trial manager (HMR) and, together with any unused ampoules, returned to the central study office.
Follow-up
Active or placebo injections were administered at annual intervals and concealed in the same way as the first injection. Information on falls, current mobility and residential status was obtained at annual review (12, 24 and 36 months) by the practice nurse and on incident fractures by postal questionnaire at 6, 12, 18, 24, 30 and 36 months. Fracture history on the questionnaire was corroborated at the annual visits to the practice nurse, as well as against hospital and general practice records. Non-responders to the ‘interval’ questionnaires were sent one reminder after 4 weeks and were contacted by telephone after a further 2 weeks if necessary. Participants continued their usual drug treatment and any new drugs that were advised during routine care. Recruitment commenced in autumn 1998, and follow-up was completed in autumn 2002.
Outcome ascertainment
The principal outcome of the trial was all non-vertebral fractures. Secondary outcomes were fractures of the hip or wrist, and the frequency of falls. Study events were self-reported by subjects at 6-monthly intervals using the fracture questionnaire validated in the European Prospective Osteoporosis Study [10]. This inquires about fractures over the intervening 6 months, and is accompanied by a mannequin on which the fracture site is indicated. Any fracture reported by study participants was confirmed from hospital and practice records. Separately, practices notified any reported fracture in their patients directly to the trial office. A fracture was treated as confirmed if it met two of the three criteria: report by study subject; report by practice; and confirmation from hospital records. Fracture sites were coded using the fracture classification adopted by the International Classification of Diseases, 9th Revision. A history of falls during the preceding 6-month period was also obtained from the questionnaire.
An interviewer-administered questionnaire was performed in a randomly selected subset of 200 trial participants, which inquired about previous fracture history, dietary calcium intake, physical activity, cigarette smoking, alcohol consumption, medical and drug history and reproductive variables in women.
Statistical analyses
An intention-to-treat analysis was performed, which included all participants randomized. We compared crude incidence rates for fracture amongst those treated with vitamin D and placebo; we then estimated hazard ratios (HRs) using Cox regression in Stata 7.0, after adjustment for age. Data were censored at the time-point at which a fracture was reported, the most recent questionnaire was completed, or at the completion of study follow-up. Subjects who did not return a questionnaire at one time-point, but then returned a later questionnaire continued to contribute to earlier follow-up only. The study had 80% power to detect a difference in fracture rate of 30% (3.3% or lower, from a baseline of 4.7% [11]) at the 5% significance level, with 5000 patients in each arm. This allowed for a 20% dropout rate.
Calcium biochemistry
In a pilot of 43 subjects eligible to enter the trial (30 treated, 13 placebo), we collected blood samples for measurement of serum 25-hydroxyvitamin D (25-OHD), (1,25)-dihydroxyvitamin D (1,25-DHD) and plasma parathyroid hormone (PTH) concentrations. Samples were collected at baseline then at 1, 4, 8, 12, 13 and 16 months, covering two treatment cycles. Blood was taken without haemostasis into glass tubes containing coagulant and protease inhibitor, and then separated in a refrigerated centrifuge. Serum was transferred to cryotubes and frozen at −70°C until analysed in one batch at the end of the collection period. 1,25-DHD was assayed by RIA after immunoextraction (IDS Limited, Tyne & Wear, UK), 25-OHD by RIA (Nicholls Diagnostics, CA, USA). PTH was measured on a DPC Immulyte analyser. Biochemical studies were performed in a 2:1 ratio of treated:placebo subjects to enhance precision in the treated group; the subjects comprised consecutive eligible attendees at a single practice. Paired and non-paired t-tests (as applicable) and ANOVA were used to assess differences between groups of different time-points and changes from baseline values.
Results
The 111 participating general practices invited 13 487 potential participants over a 3-yr period. Of these, 9440 people (4354 men and 5086 women) were willing to participate and were confirmed to meet the study entry criteria. 3188 subjects were recruited during the first year of the study, 4692 during the second year and 1560 during the third year. The remaining 4047 were ineligible (n = 2185; 54%) or declined to participate (n = 1862; 46%). Thus, of the 11 302 people who were eligible, 9440 (83.5%) were willing to participate. Table 1 shows the baseline characteristics of the subjects randomized. Their median age was 79.1 yrs (IQR 76.9–82.6 yrs); there were no appreciable differences between the groups in age, previous history of fracture, prevalence and frequency of falls in the last 6 months or residential status. Thirty-eight per cent of the men and women had sustained a previous clinical fracture (wrist 14%; ankle 7.4%; hip 2.8%).
Characteristics of 4354 men and 5086 women aged 75 yrs and over, randomized to treatment with vitamin D or placebo
| . | Men . | Women . | All . | |||
|---|---|---|---|---|---|---|
| Baseline characteristic . | Placebo . | Vitamin D . | Placebo . | Vit D . | Placebo . | Vitamin D . |
| No. | 2195 | 2159 | 2518 | 2568 | 4713 | 4727 |
| Median age (IQR)a | 79.1 | 79.0 | 79.2 | 79.3 | 79.1 | 79.1 |
| (76.9–82.4) | (76.8–82.1) | (77.0–82.8) | (77.1–83.1) | (76.9–82.6) | (76.9–82.7) | |
| Previous fractures | ||||||
| Any non-vert. (%) | 36.0 | 34.2 | 40.8 | 39.8 | 38.5 | 37.2 |
| Hip or femur (%) | 1.9 | 2.1 | 3.7 | 3.2 | 2.9 | 2.7 |
| Wristb (%) | 8.9 | 7.0 | 18.5 | 18.1 | 14.0 | 13.0 |
| Accommodation | ||||||
| Own/spouse (%) | 91.2 | 91.4 | 84.3 | 83.9 | 87.5 | 87.3 |
| With family (%) | 3.1 | 3.0 | 4.4 | 4.7 | 3.8 | 4.0 |
| Warden (%) | 4.2 | 4.6 | 9.3 | 9.2 | 6.9 | 7.1 |
| Residential (%) | 1.0 | 0.5 | 1.7 | 1.7 | 1.4 | 1.2 |
| Other (%) | 0.6 | 0.4 | 0.3 | 0.6 | 0.4 | 0.5 |
| . | Men . | Women . | All . | |||
|---|---|---|---|---|---|---|
| Baseline characteristic . | Placebo . | Vitamin D . | Placebo . | Vit D . | Placebo . | Vitamin D . |
| No. | 2195 | 2159 | 2518 | 2568 | 4713 | 4727 |
| Median age (IQR)a | 79.1 | 79.0 | 79.2 | 79.3 | 79.1 | 79.1 |
| (76.9–82.4) | (76.8–82.1) | (77.0–82.8) | (77.1–83.1) | (76.9–82.6) | (76.9–82.7) | |
| Previous fractures | ||||||
| Any non-vert. (%) | 36.0 | 34.2 | 40.8 | 39.8 | 38.5 | 37.2 |
| Hip or femur (%) | 1.9 | 2.1 | 3.7 | 3.2 | 2.9 | 2.7 |
| Wristb (%) | 8.9 | 7.0 | 18.5 | 18.1 | 14.0 | 13.0 |
| Accommodation | ||||||
| Own/spouse (%) | 91.2 | 91.4 | 84.3 | 83.9 | 87.5 | 87.3 |
| With family (%) | 3.1 | 3.0 | 4.4 | 4.7 | 3.8 | 4.0 |
| Warden (%) | 4.2 | 4.6 | 9.3 | 9.2 | 6.9 | 7.1 |
| Residential (%) | 1.0 | 0.5 | 1.7 | 1.7 | 1.4 | 1.2 |
| Other (%) | 0.6 | 0.4 | 0.3 | 0.6 | 0.4 | 0.5 |
aIQR, interquartile range.
bOr radius, ulna or Colles‚ fracture.
Characteristics of 4354 men and 5086 women aged 75 yrs and over, randomized to treatment with vitamin D or placebo
| . | Men . | Women . | All . | |||
|---|---|---|---|---|---|---|
| Baseline characteristic . | Placebo . | Vitamin D . | Placebo . | Vit D . | Placebo . | Vitamin D . |
| No. | 2195 | 2159 | 2518 | 2568 | 4713 | 4727 |
| Median age (IQR)a | 79.1 | 79.0 | 79.2 | 79.3 | 79.1 | 79.1 |
| (76.9–82.4) | (76.8–82.1) | (77.0–82.8) | (77.1–83.1) | (76.9–82.6) | (76.9–82.7) | |
| Previous fractures | ||||||
| Any non-vert. (%) | 36.0 | 34.2 | 40.8 | 39.8 | 38.5 | 37.2 |
| Hip or femur (%) | 1.9 | 2.1 | 3.7 | 3.2 | 2.9 | 2.7 |
| Wristb (%) | 8.9 | 7.0 | 18.5 | 18.1 | 14.0 | 13.0 |
| Accommodation | ||||||
| Own/spouse (%) | 91.2 | 91.4 | 84.3 | 83.9 | 87.5 | 87.3 |
| With family (%) | 3.1 | 3.0 | 4.4 | 4.7 | 3.8 | 4.0 |
| Warden (%) | 4.2 | 4.6 | 9.3 | 9.2 | 6.9 | 7.1 |
| Residential (%) | 1.0 | 0.5 | 1.7 | 1.7 | 1.4 | 1.2 |
| Other (%) | 0.6 | 0.4 | 0.3 | 0.6 | 0.4 | 0.5 |
| . | Men . | Women . | All . | |||
|---|---|---|---|---|---|---|
| Baseline characteristic . | Placebo . | Vitamin D . | Placebo . | Vit D . | Placebo . | Vitamin D . |
| No. | 2195 | 2159 | 2518 | 2568 | 4713 | 4727 |
| Median age (IQR)a | 79.1 | 79.0 | 79.2 | 79.3 | 79.1 | 79.1 |
| (76.9–82.4) | (76.8–82.1) | (77.0–82.8) | (77.1–83.1) | (76.9–82.6) | (76.9–82.7) | |
| Previous fractures | ||||||
| Any non-vert. (%) | 36.0 | 34.2 | 40.8 | 39.8 | 38.5 | 37.2 |
| Hip or femur (%) | 1.9 | 2.1 | 3.7 | 3.2 | 2.9 | 2.7 |
| Wristb (%) | 8.9 | 7.0 | 18.5 | 18.1 | 14.0 | 13.0 |
| Accommodation | ||||||
| Own/spouse (%) | 91.2 | 91.4 | 84.3 | 83.9 | 87.5 | 87.3 |
| With family (%) | 3.1 | 3.0 | 4.4 | 4.7 | 3.8 | 4.0 |
| Warden (%) | 4.2 | 4.6 | 9.3 | 9.2 | 6.9 | 7.1 |
| Residential (%) | 1.0 | 0.5 | 1.7 | 1.7 | 1.4 | 1.2 |
| Other (%) | 0.6 | 0.4 | 0.3 | 0.6 | 0.4 | 0.5 |
aIQR, interquartile range.
bOr radius, ulna or Colles‚ fracture.
Table 2 shows the number of subjects with an incident fracture in the placebo and treated groups. Eighty-five men and 194 women in the placebo group sustained an incident fracture over the 3-yr follow-up period. These contrasted with 68 men and 238 women in the vitamin D-treated group. There was no evidence for a protective effect of vitamin D against fracture at any site: all fractures [HR 1.09; 95% confidence interval (CI) 0.93–1.28], wrist (HR 1.22; 95% CI 0.85–1.76), ankle (HR 1.63; 95% CI 0.84–3.17). There was a small, but statistically significant (P = 0.04) excess risk of hip fracture associated with allocation to treatment with vitamin D (HR 1.49; 95% CI 1.02–2.18). When the genders were analysed separately, the tendency for an increase in fracture risk was particularly observed among women, in whom there was a 59% increase in hazard at the proximal femur or distal forearm among those treated with vitamin D compared with placebo (P = 0.003). There was no significant effect of vitamin D treatment on the frequency of falls (HR 0.98; 95% CI 0.93–1.04).
Incidence of first fractures and age-adjusted HRs (Cox regression) of treatment with vitamin D compared with treatment with placebo
| Fractures . | Placebo . | Vitamin D . | HRa (95% CI) . | P-value . |
|---|---|---|---|---|
| All | ||||
| No. | 4713 | 4727 | ||
| Any non-vertebral | 279 | 306 | 1.09 (0.93–1.28) | 0.29 |
| Hip or femur | 44 | 66 | 1.49 (1.02–2.18) | 0.04 |
| Wristb | 52 | 64 | 1.22 (0.85–1.76) | 0.28 |
| Hip, femur or wristb | 92 | 129 | 1.40 (1.07–1.82) | 0.02 |
| Falls | 2577 | 2544 | 0.98 (0.93–1.04) | 0.50 |
| Men | ||||
| No. | 2195 | 2159 | ||
| Any non-vertebral | 85 | 68 | 0.81 (0.59–1.11) | 0.20 |
| Hip or femur | 18 | 18 | 1.02 (0.53–1.97) | 0.94 |
| Wristb | 8 | 4 | 0.50 (0.15–1.66) | 0.26 |
| Hip, femur or wristb | 26 | 22 | 0.86 (0.49–1.52) | 0.61 |
| Falls | 1145 | 1092 | 0.97 (0.89–1.05) | 0.46 |
| Women | ||||
| No. | 2518 | 2568 | ||
| Any non-vertebral | 194 | 238 | 1.21 (1.00–1.47) | 0.05 |
| Hip or femur | 26 | 48 | 1.80 (1.12–2.90) | 0.02 |
| Wristb | 44 | 60 | 1.34 (0.91–1.98) | 0.14 |
| Hip, femur or wristb | 66 | 107 | 1.59 (1.17–2.16) | 0.003 |
| Falls | 1432 | 1452 | 0.99 (0.92–1.06) | 0.78 |
| Fractures . | Placebo . | Vitamin D . | HRa (95% CI) . | P-value . |
|---|---|---|---|---|
| All | ||||
| No. | 4713 | 4727 | ||
| Any non-vertebral | 279 | 306 | 1.09 (0.93–1.28) | 0.29 |
| Hip or femur | 44 | 66 | 1.49 (1.02–2.18) | 0.04 |
| Wristb | 52 | 64 | 1.22 (0.85–1.76) | 0.28 |
| Hip, femur or wristb | 92 | 129 | 1.40 (1.07–1.82) | 0.02 |
| Falls | 2577 | 2544 | 0.98 (0.93–1.04) | 0.50 |
| Men | ||||
| No. | 2195 | 2159 | ||
| Any non-vertebral | 85 | 68 | 0.81 (0.59–1.11) | 0.20 |
| Hip or femur | 18 | 18 | 1.02 (0.53–1.97) | 0.94 |
| Wristb | 8 | 4 | 0.50 (0.15–1.66) | 0.26 |
| Hip, femur or wristb | 26 | 22 | 0.86 (0.49–1.52) | 0.61 |
| Falls | 1145 | 1092 | 0.97 (0.89–1.05) | 0.46 |
| Women | ||||
| No. | 2518 | 2568 | ||
| Any non-vertebral | 194 | 238 | 1.21 (1.00–1.47) | 0.05 |
| Hip or femur | 26 | 48 | 1.80 (1.12–2.90) | 0.02 |
| Wristb | 44 | 60 | 1.34 (0.91–1.98) | 0.14 |
| Hip, femur or wristb | 66 | 107 | 1.59 (1.17–2.16) | 0.003 |
| Falls | 1432 | 1452 | 0.99 (0.92–1.06) | 0.78 |
aHRs adjusted for age.
bOr radius, ulna or Colles.
Incidence of first fractures and age-adjusted HRs (Cox regression) of treatment with vitamin D compared with treatment with placebo
| Fractures . | Placebo . | Vitamin D . | HRa (95% CI) . | P-value . |
|---|---|---|---|---|
| All | ||||
| No. | 4713 | 4727 | ||
| Any non-vertebral | 279 | 306 | 1.09 (0.93–1.28) | 0.29 |
| Hip or femur | 44 | 66 | 1.49 (1.02–2.18) | 0.04 |
| Wristb | 52 | 64 | 1.22 (0.85–1.76) | 0.28 |
| Hip, femur or wristb | 92 | 129 | 1.40 (1.07–1.82) | 0.02 |
| Falls | 2577 | 2544 | 0.98 (0.93–1.04) | 0.50 |
| Men | ||||
| No. | 2195 | 2159 | ||
| Any non-vertebral | 85 | 68 | 0.81 (0.59–1.11) | 0.20 |
| Hip or femur | 18 | 18 | 1.02 (0.53–1.97) | 0.94 |
| Wristb | 8 | 4 | 0.50 (0.15–1.66) | 0.26 |
| Hip, femur or wristb | 26 | 22 | 0.86 (0.49–1.52) | 0.61 |
| Falls | 1145 | 1092 | 0.97 (0.89–1.05) | 0.46 |
| Women | ||||
| No. | 2518 | 2568 | ||
| Any non-vertebral | 194 | 238 | 1.21 (1.00–1.47) | 0.05 |
| Hip or femur | 26 | 48 | 1.80 (1.12–2.90) | 0.02 |
| Wristb | 44 | 60 | 1.34 (0.91–1.98) | 0.14 |
| Hip, femur or wristb | 66 | 107 | 1.59 (1.17–2.16) | 0.003 |
| Falls | 1432 | 1452 | 0.99 (0.92–1.06) | 0.78 |
| Fractures . | Placebo . | Vitamin D . | HRa (95% CI) . | P-value . |
|---|---|---|---|---|
| All | ||||
| No. | 4713 | 4727 | ||
| Any non-vertebral | 279 | 306 | 1.09 (0.93–1.28) | 0.29 |
| Hip or femur | 44 | 66 | 1.49 (1.02–2.18) | 0.04 |
| Wristb | 52 | 64 | 1.22 (0.85–1.76) | 0.28 |
| Hip, femur or wristb | 92 | 129 | 1.40 (1.07–1.82) | 0.02 |
| Falls | 2577 | 2544 | 0.98 (0.93–1.04) | 0.50 |
| Men | ||||
| No. | 2195 | 2159 | ||
| Any non-vertebral | 85 | 68 | 0.81 (0.59–1.11) | 0.20 |
| Hip or femur | 18 | 18 | 1.02 (0.53–1.97) | 0.94 |
| Wristb | 8 | 4 | 0.50 (0.15–1.66) | 0.26 |
| Hip, femur or wristb | 26 | 22 | 0.86 (0.49–1.52) | 0.61 |
| Falls | 1145 | 1092 | 0.97 (0.89–1.05) | 0.46 |
| Women | ||||
| No. | 2518 | 2568 | ||
| Any non-vertebral | 194 | 238 | 1.21 (1.00–1.47) | 0.05 |
| Hip or femur | 26 | 48 | 1.80 (1.12–2.90) | 0.02 |
| Wristb | 44 | 60 | 1.34 (0.91–1.98) | 0.14 |
| Hip, femur or wristb | 66 | 107 | 1.59 (1.17–2.16) | 0.003 |
| Falls | 1432 | 1452 | 0.99 (0.92–1.06) | 0.78 |
aHRs adjusted for age.
bOr radius, ulna or Colles.
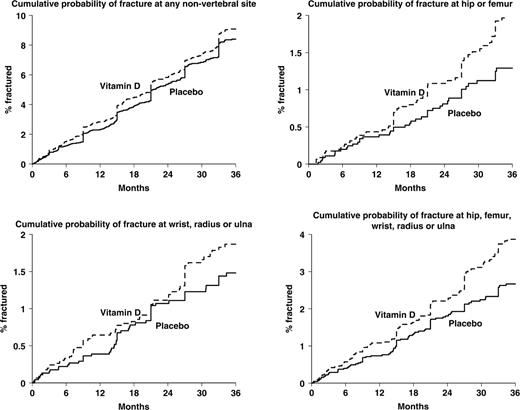
Figure 2 shows the cumulative probability of fracture at various skeletal sites according to treatment group. It shows steps between 6-monthly assessments, as unknown fracture time-points were interpolated in this manner. For all non-spine fractures, there was almost no divergence in the cumulative incidence pattern between the vitamin D and placebo-treated patients over the 3 yrs of the study. When the analyses were confined to fractures at the wrist, hip or both of these sites, fracture incidence tended to be higher in the vitamin D-treated arm when compared with those subjects using placebo. However, these differences were not statistically significant for the wrist (HR = 1.22, 95% CI 0.85–1.76).
Cumulative probability of fracture at various skeletal sites, according to treatment with vitamin D or placebo.
There were no statistically significant interactions between age, previous fracture history or functional status and the effects of vitamin D treatment on fracture risk; these tests of interaction were exploratory and not pre-planned. There was, however, a significant interaction between gender and treatment group on the risk of any non-spine fracture such that a detrimental effect of vitamin D was seen amongst women, but not among men (P = 0.03). Among the 200 trial participants completing the more detailed interviewer-administered questionnaire, 22 subjects (11%) had sustained a previous hip or wrist fracture; 48 (24%) reported <30 min weight-bearing activity each day; 74 (37%) had ever smoked; and only four consumed greater than 20 units of alcohol each week. The mean dietary calcium intake of these 200 subjects was 625 mg daily (s.d. 231).
In the biochemistry study, measurements were obtained at baseline, and at 1, 4, 8, 12, 13 and 16 months of follow-up. Among the 43 trial participants included, mean plasma 25-OHD concentration at baseline was 56.5 ng/ml (s.d. 23.7); PTH concentration 4.83 pmol/l (IQR 3.92–5.63); and 1,25-DHD concentration 74.3 pmol/l (s.d. 27.5). Increases from baseline were observed in 25-OHD and 1,25-DHD concentrations among those subjects receiving active treatment, but not in those on placebo. For 25-OHD, the actively treated group showed a 21% increase at 4 months, but this was not statistically significant (P = 0.15). There was a more pronounced increase in 1,25-DHD concentration over this period (35.7%) and this attained statistical significance (P = 0.02). At this time-point, the difference between absolute 1,25-DHD concentrations (but not 25-OHD concentrations) among actively treated and placebo subjects was also statistically significant (P < 0.002). These changes in vitamin D status were accompanied by only modest (17%) suppression of PTH in the treated subjects at this time-point, a level which did not attain statistical significance. By the eighth month after injection, these changes had drifted towards basal values, although the significantly higher 1,25-DHD levels in the active group persisted (P = 0.04). Observations during the second year of follow-up (after a second vitamin D or placebo injection during the fall) broadly resembled those in the first year of the study.
Discussion
This large, pragmatic, population-based, randomized, double-blind, placebo-controlled trial found that an annual i.m. injection of 300 000 IU vitamin D did not reduce fracture risk when compared with placebo. This is the largest trial to date addressing the primary prevention of osteoporotic fractures in older people and the largest trial of i.m. vitamin D. The trial involved over 9000 men and women aged 75 yrs and over; it was adequately powered to detect a 30% difference in fracture rate between treatment and placebo arms. The baseline rate of all non-osteoporotic fractures was close to that expected using routinely derived epidemiological data for England and Wales [11]. There were, however, fewer hip fractures than anticipated, reflecting the relatively healthy elderly population who chose to take part in the study. The groups were well balanced at entry to the trial, and individuals who previously used supplements containing 400 IU vitamin D or more daily were excluded from entry. The method of ascertaining non-spine fractures has been well validated previously, and the vast majority of these could be corroborated from a second source (hospital notes or radiographic records).
This was a large, pragmatically designed, randomized controlled trial. As a consequence, the principal issue addressed was the capacity of a public health programme of annual i.m. vitamin D injections in the elderly, to reduce fracture incidence. This had already been suggested by a smaller, non-randomized study. The major deficiency of the study was the relative paucity of explanatory measures such as assessment of vitamin D status in the enrolled subjects, as well as other determinants of fracture risk such as dietary calcium intake, smoking, alcohol consumption and physical activity. We were able to recruit a small sample of participants in whom circulating levels of 25-OHD, 1,25-DHD and PTH were able to be evaluated over a protracted period within the study. Although too small to allow detailed inference, this subset clearly demonstrated that our subjects were relatively vitamin D replete, with only around 25% falling into the range of modest or severe deficiency (<30 ng/ml). With hindsight, it would have been preferable to increase the size of this biochemical subset, but the available data suggest that this intervention was ineffective in reducing fractures in a relatively vitamin D replete population. Additionally, the 25-OHD assays that were utilized may have been less reliable in the estimation of vitamin D2. We did obtain more detailed risk factor information in 200 participants that were in accord with the biochemical subset, in revealing calcium intakes around the values previously reported in the healthy UK elderly, and certainly well above those found among previously published studies reporting a benefit of calcium and vitamin D supplementation. These deficiencies limited our ability to perform subgroup analyses adjusting for the effects of calcium intake, vitamin D status or PTH. A third caveat is that our intervention was vitamin D2, and data published after the inception of this trial confirm that vitamin D2 has an attenuated effect when compared with vitamin D3 [12–15]. The relative potency of the plant sterol ergocalciferol (vitamin D2) and the animal form, cholecalciferol (vitamin D3) is disputed; comparison of D2 and D3 potency in humans has given conflicting results [12, 13]. The most recent study contrasting the effectiveness of vitamin D2 with that of vitamin D3 suggested that both calciferols produced similar rises in serum 25-OHD concentration over 3 days, but that levels declined much more rapidly in subjects treated with D2 [14]. We opted to use the identical formulation of ergocalciferol that was utilized in the previous Finnish study [7]. However, our dosing frequency was annual and the pharmacokinetics of i.m. vitamin D might differ if administered more frequently. Although the age, gender and socioeconomic profile of the practices in the Wessex Primary Care Network was representative of England and Wales, our study participants were likely to have had higher functional capacity than the older population from which they were drawn; they were more likely to live in their own homes, and had a slightly lower than expected hip fracture rate. Finally, information on falls was collected retrospectively by practice nurse interview or a postal questionnaire enquiring about falls over the past 6 months. Although falls were only a secondary outcome measure of the trial, it has been established that prospective collection of falls data at frequent intervals is more reliable than retrospective assessment over long periods [16].
Vitamin D insufficiency is widespread in the elderly population of northern Europe and the USA [17–19]. Supplementation is an effective way of preventing this. A daily oral dose of 400–800 IU has been shown to elevate serum 25-OHD levels, reduce serum PTH and suppress markers of bone turnover in vulnerable older people [5, 6, 20] but its cost utility in fracture prevention remains uncertain. Giving periodic vitamin D supplementation by the i.m. route, while somewhat invasive, has advantages over daily oral supplements in cost-effectiveness and compliance. However, fewer data are available on the effect of i.m. dosing on circulating vitamin D metabolites. One study comparing i.m. with i.v. and oral vitamin D showed a delayed rise in serum 25-OHD levels with the i.m. route. The study also showed that in a rat model about half of the vitamin D administered by i.m. injection remained unaltered in situ [21], providing an additional reason why this particular mode of delivery proved ineffective. A further possible problem is the potential absorption of vitamin D2 on plastic syringes, although quality assurance on the ampoules used was undertaken at a national reference laboratory.
The effect of vitamin D supplementation on the risk of non-vertebral fracture is unclear despite several large, well-designed placebo-controlled trials. Two studies of daily oral supplementation using vitamin D combined with calcium demonstrated significant decreases in non-vertebral fractures among 3270 female nursing home residents in France [3] and in 445 relatively healthy men and women in the USA [22]. In the first of these, supplementation appeared to have its principal effect on fracture incidence within 18–24 months of commencing therapy. In the second, the placebo fracture rate was among the highest recorded in controlled trials of osteoporosis interventions (around 22%, compared with 12% in other studies of anti-resorptive treatment). In contrast, studies of daily oral vitamin D without calcium supplementation have reported less or no benefit on fracture rates. Examples include a study of 2578 elderly independent men and women from Holland randomized to 400 IU vitamin D3 or placebo [5], or one of 1100 residents of Norwegian nursing homes randomized to 5 ml cod liver oil or placebo [6]. Trials using intermittent dosing of vitamin D have been more likely to show a reduction in fracture risk. A trial of 100 000 IU oral cholecalciferol or placebo every 4 months in 2686 community-dwelling British men and women aged ≥65 yrs found significant reductions in the relative risk of any fracture (HR 0.78; 95% CI 0.61–0.99), or fracture at the hip, wrist, forearm or spine (HR 0.67; 95% CI 0.48–0.93) [4]. The study that inspired this current trial utilized an annual i.m. injection of 150 000–300 000 IU ergocalciferol in 479 Finnish men and women aged >85 yrs who were living in their own home and 320 subjects aged 75–84 yrs living in a home for aged people. Although treatment allocation was inadequately randomized by current standards (using alternate allocation without blinding), the group of subjects administered i.m. vitamin D had a significantly (P = 0.03) lower rate of all fractures. This was particularly marked (P = 0.02) for fractures of the upper limb, but was not apparent for lower limb sites [7]. The present study was considerably larger, subjects were randomized to treatment or placebo and outcomes were assessed in a blind fashion using validated measures. Our results clearly do not support those of the Finnish study and suggest that an annual i.m. injection of vitamin D does not result in a measurable reduction in the risk of non-vertebral fractures, perhaps due to insufficient suppression of PTH. Two recent studies of intermittent oral vitamin D administration, in contrast, have failed to reveal a reduction in fracture risk [23, 24], Finally, the recently published Medical Research Council Randomized Evaluation of Calcium or Vitamin D (RECORD) Trial evaluated daily oral calcium, vitamin D, both interventions combined, and double placebo, in 5292 men and women aged 70 yrs and over, who had sustained a low trauma fracture [25]. In accordance with the results of our study, vitamin D whether alone or in combination with calcium supplementation was ineffective in reducing the incidence of fracture. These complex findings have been thoroughly reviewed with the recent publication of the calcium and vitamin D component of the Women's Health Initiative [26]. Our data are also in accordance with recent randomized controlled trials of calcium supplementation in elderly women [27, 28], in which significant reductions in fracture risk were not observed. As with one of these studies, we also found a statistically significant excess risk of hip fracture in the intervention group [27]; we are not aware of any biologically plausible reason why calcium or vitamin D supplementation should increase hip fracture risk and we concur with the authors of that report that these observations are likeliest due to chance. A remote possibility is that if a small number of participants had vitamin D deficiency and osteomalacia, treatment with vitamin D might have led to a rapid improvement in myopathy, but the mineralization deficit might have persisted for several months. In this situation, improved mobility might have been associated with an increased risk of hip fracture. The latest Cochrane Review addressing this issue [29], and a recent meta-analysis [30] all concur that vitamin D alone is ineffective in preventing fractures among older people, but that combined calcium and vitamin D supplementation may decrease the incidence of hip and other non-vertebral fractures, especially in care home residents.
In conclusion, we have performed a large, pragmatic, randomized, controlled trial comparing the anti-fracture effectiveness of a single i.m. injection of 300 000 IU vitamin D2 annually with placebo over a 3-yr period. Although acceptable and safe, this intervention was not effective in reducing the incidence of fractures among men and women aged over 75 yrs resident in the general population.

Acknowledgements
We thank the General Practitioners and patients who participated. Julie Smith and Tanya Humphreys provided administrative support; Vanessa Cox managed the data. The report was prepared by Gill Strange. We are also grateful to the external Advisory Group: Dr S Drew, Dr S Clayton, Mr D Matthews, Mrs B Hughes and Mrs Sue O’Regan.
Funding: The study was supported by the Medical Research Council of Great Britain, the South West NHS Research and Development Directorate, the National Osteoporosis Society and Celltech UK plc.
Disclosure Statement: F. A. has received honoraries from Shire Pharmaceuticals and Prostrakan ltd for lecturing at educational meetings. All other authors have declared no conflicts of interest.




Comments