-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Grobner, Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?, Nephrology Dialysis Transplantation, Volume 21, Issue 4, April 2006, Pages 1104–1108, https://doi.org/10.1093/ndt/gfk062
Close - Share Icon Share
Introduction
Nephrogenic fibrosing dermopathy (NFD) is an acquired, idiopathic disorder that is observed in patients with renal disease. Most patients with NFD have undergone dialysis for renal failure [1,2]. It tends to affect mostly the middle-aged. An association of NFD with coagulation abnormalities, recent vascular surgery or intervention (e.g. shunt/fistula and angioplasty), and presence of antiphospholipid antibodies has been discussed by several authors thus far [1,3], but the origin of the disease is still unknown. A more widespread variant of this fibrosing skin disease with involvement of other organs (e.g. lungs, liver, muscles and the heart) is described as nephrogenic systemic fibrosis (NSF) by Leboit [4], Ting et al. [5] and Daram et al. [6].
NFD is characterized clinically by thickening, induration and hardening of the skin. Distinct nodules also can be seen. The (distal) extremities are the most common area of involvement, followed by the trunk, and the face is almost never involved [1]. The diagnosis of NFD is confirmed in a skin biopsy by specific histopathologic features, namely thickened collagen bundles with surrounding clefts, mucin deposition and a proliferation of fibroblasts and elastic fibers. Signs of inflammation are absent, which makes this disorder a distinct entity [1,7].
In this report, nine end stage renal disease patients undergoing magnetic resonance (MR) angiography are presented, in five of whom skin changes of nephrogenic fibrosing dermatopathy became apparent about 2–4 weeks after the administration of gadolinium (Gd)-containing contrast agent for MR. Patients with and without NFD were compared for possible risk factors to develop this skin disease. Gd is thought to be safe as a contrast agent in renal failure. This case series however, demonstrates that Gd–DTPA possibly plays a triggering role in the development of NFD under certain circumstances.
Patients
Nine end stage renal disease patients of our dialysis unit underwent MR angiography using Gd–DTPA as contrast media over a period of approximately 2 years (mean age: 58±10.3 years; mean time on dialysis: 30.5±16.1 months). Among these patients, five were observed, all of whom developed thickening and induration of the skin, starting on the lower extremities and eventually spreading to the trunk and upper extremities (Table 1).
Characteristics of affected patients
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age (years) | 64 | 52 | 43 | 53 | 74 |
| Sex | female | female | female | male | male |
| Renal disease | chronic pyelonephritis | polycystic kidney disease | shrunken kidneys (of unknown reason) | polycystic kidney disease | vascular nephropathy |
| Duration of dialysis | 38 months | 47 months | 27 months | 58 months | 10 months |
| Medical history | arterial hypertension, repeated fistula surgery | coronary artery disease, arterial hypertension atrial flutter, status post colon carcinoma (pT3pNopMo) | arterial hypertension repeated fistula surgery | arterial hypertension atrial flutter, dilatative cardiomyopathy | arterial hyper-tension, coronary artery disease, prostatectomy |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age (years) | 64 | 52 | 43 | 53 | 74 |
| Sex | female | female | female | male | male |
| Renal disease | chronic pyelonephritis | polycystic kidney disease | shrunken kidneys (of unknown reason) | polycystic kidney disease | vascular nephropathy |
| Duration of dialysis | 38 months | 47 months | 27 months | 58 months | 10 months |
| Medical history | arterial hypertension, repeated fistula surgery | coronary artery disease, arterial hypertension atrial flutter, status post colon carcinoma (pT3pNopMo) | arterial hypertension repeated fistula surgery | arterial hypertension atrial flutter, dilatative cardiomyopathy | arterial hyper-tension, coronary artery disease, prostatectomy |
Characteristics of affected patients
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age (years) | 64 | 52 | 43 | 53 | 74 |
| Sex | female | female | female | male | male |
| Renal disease | chronic pyelonephritis | polycystic kidney disease | shrunken kidneys (of unknown reason) | polycystic kidney disease | vascular nephropathy |
| Duration of dialysis | 38 months | 47 months | 27 months | 58 months | 10 months |
| Medical history | arterial hypertension, repeated fistula surgery | coronary artery disease, arterial hypertension atrial flutter, status post colon carcinoma (pT3pNopMo) | arterial hypertension repeated fistula surgery | arterial hypertension atrial flutter, dilatative cardiomyopathy | arterial hyper-tension, coronary artery disease, prostatectomy |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . |
|---|---|---|---|---|---|
| Age (years) | 64 | 52 | 43 | 53 | 74 |
| Sex | female | female | female | male | male |
| Renal disease | chronic pyelonephritis | polycystic kidney disease | shrunken kidneys (of unknown reason) | polycystic kidney disease | vascular nephropathy |
| Duration of dialysis | 38 months | 47 months | 27 months | 58 months | 10 months |
| Medical history | arterial hypertension, repeated fistula surgery | coronary artery disease, arterial hypertension atrial flutter, status post colon carcinoma (pT3pNopMo) | arterial hypertension repeated fistula surgery | arterial hypertension atrial flutter, dilatative cardiomyopathy | arterial hyper-tension, coronary artery disease, prostatectomy |
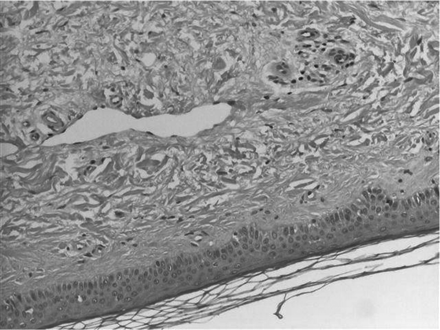
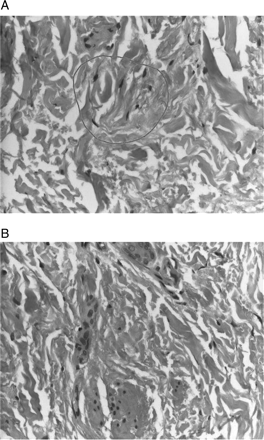
The skin was shiny and hard to the touch and the patients suffered from concomitant pain in affected areas. Conjunctival erythaema and yellowish scleral plaques could also be observed in these patients. Histologic examination of the skin biopsies taken revealed the picture of NFD. A characteristic example is given in Figures 1 and 2A,B.
Biopsy specimen of the skin of the right lower leg of patient 1. The thickened dermis demonstrates plumped collagen bundles with surrounding clefts, spindle cellproliferation. Interstitial mucin deposition is frequently present.
Results
All affected patients underwent MR examination with the use of Gd–DTPA 2–4 weeks prior to development of the skin abnormalities.
Until the development of the skin changes, their course was uneventful. The laboratory examination including antinuclear antibodies, antineutrophil cytoplasmatic antibodies, anti phospholipid antibodies, circulating immuncomplexes, anti glomerular basal membrane antibodies and complement factors C3, C4 were negative or showed normal findings. In one patient a pathological value regarding protein C and factor VIII was detected (Table 2). The mean time on dialysis of the five affected patients amounted to 36±16.5 months.
Further characteristics of NFD – patients
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Duration of observation with the disease | 3 months | 5 months | 16 months | 12 months | 10 months | ||||
| Abnormal laboratory findings | protein C 55% (70–130%), factor VIII 279% (60–150%) | none | none | none | none | ||||
| Medication – patients have in common | there could be no medication identified that all patients have in common | ||||||||
| Angiotensin converting enzyme inhibitors | − | − | − | + | − | ||||
| Angiotensin II receptor blockers | + | + | + | − | − | ||||
| Administration of Gd–DTPA iv. | + | + | + | + | + | ||||
| Typical histopathology for NFD | + | + | + | + | + | ||||
| Clinical symptoms within 2–4 weeks after Gd–DTPA iv. | + | + | + | + | + | ||||
| pH value | 7.35 | 7.30 | 7.23 | 7.31 | 7.30 | ||||
| Actual bicarbonate | 20.7 mmol/l | 19.6 mmol/l | 16.3 mmol/l | 20.3 mmol/l | 20.6 mmol/l | ||||
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Duration of observation with the disease | 3 months | 5 months | 16 months | 12 months | 10 months | ||||
| Abnormal laboratory findings | protein C 55% (70–130%), factor VIII 279% (60–150%) | none | none | none | none | ||||
| Medication – patients have in common | there could be no medication identified that all patients have in common | ||||||||
| Angiotensin converting enzyme inhibitors | − | − | − | + | − | ||||
| Angiotensin II receptor blockers | + | + | + | − | − | ||||
| Administration of Gd–DTPA iv. | + | + | + | + | + | ||||
| Typical histopathology for NFD | + | + | + | + | + | ||||
| Clinical symptoms within 2–4 weeks after Gd–DTPA iv. | + | + | + | + | + | ||||
| pH value | 7.35 | 7.30 | 7.23 | 7.31 | 7.30 | ||||
| Actual bicarbonate | 20.7 mmol/l | 19.6 mmol/l | 16.3 mmol/l | 20.3 mmol/l | 20.6 mmol/l | ||||
Further characteristics of NFD – patients
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Duration of observation with the disease | 3 months | 5 months | 16 months | 12 months | 10 months | ||||
| Abnormal laboratory findings | protein C 55% (70–130%), factor VIII 279% (60–150%) | none | none | none | none | ||||
| Medication – patients have in common | there could be no medication identified that all patients have in common | ||||||||
| Angiotensin converting enzyme inhibitors | − | − | − | + | − | ||||
| Angiotensin II receptor blockers | + | + | + | − | − | ||||
| Administration of Gd–DTPA iv. | + | + | + | + | + | ||||
| Typical histopathology for NFD | + | + | + | + | + | ||||
| Clinical symptoms within 2–4 weeks after Gd–DTPA iv. | + | + | + | + | + | ||||
| pH value | 7.35 | 7.30 | 7.23 | 7.31 | 7.30 | ||||
| Actual bicarbonate | 20.7 mmol/l | 19.6 mmol/l | 16.3 mmol/l | 20.3 mmol/l | 20.6 mmol/l | ||||
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Duration of observation with the disease | 3 months | 5 months | 16 months | 12 months | 10 months | ||||
| Abnormal laboratory findings | protein C 55% (70–130%), factor VIII 279% (60–150%) | none | none | none | none | ||||
| Medication – patients have in common | there could be no medication identified that all patients have in common | ||||||||
| Angiotensin converting enzyme inhibitors | − | − | − | + | − | ||||
| Angiotensin II receptor blockers | + | + | + | − | − | ||||
| Administration of Gd–DTPA iv. | + | + | + | + | + | ||||
| Typical histopathology for NFD | + | + | + | + | + | ||||
| Clinical symptoms within 2–4 weeks after Gd–DTPA iv. | + | + | + | + | + | ||||
| pH value | 7.35 | 7.30 | 7.23 | 7.31 | 7.30 | ||||
| Actual bicarbonate | 20.7 mmol/l | 19.6 mmol/l | 16.3 mmol/l | 20.3 mmol/l | 20.6 mmol/l | ||||
Data of the four patients not affected by skin disease is given in Table 3. Their mean time on dialysis amounted to 23.75±12.5 months. No other differences were found with respect to age, sex, medication, underlying renal disease, dialysis modalities and comorbidities; only the mean time on dialysis seemed to be longer in affected patients.
Characteristics of unaffected patients
| . | Patient A . | Patient B . | Patient C . | Patient D . |
|---|---|---|---|---|
| Age (years) | 49 | 75 | 54 | 58 |
| Sex | male | male | female | male |
| Renal disease | polycystic kidney disease | vascular nephropathy | polycystic kidney disease | diabetic nephropathy |
| Duration of dialysis | 3 months | 26 months | 36 months | 30 months |
| Medical history | arterial hypertension | coronary artery disease, arterial hypertension | arterial hypertension | coronary artery disease diabetic polyneuropathy |
| Angiotensin converting enzyme inhibitor | + | + | − | + |
| Angiotensin II receptor blockers | − | − | − | − |
| pH-value | 7.38 | 7.40 | 7.40 | 7.39 |
| Actual bicarbonate | 22.0 mmol/l | 23.2 mmol/l | 23.6 mmol/l | 23 mmol/l |
| . | Patient A . | Patient B . | Patient C . | Patient D . |
|---|---|---|---|---|
| Age (years) | 49 | 75 | 54 | 58 |
| Sex | male | male | female | male |
| Renal disease | polycystic kidney disease | vascular nephropathy | polycystic kidney disease | diabetic nephropathy |
| Duration of dialysis | 3 months | 26 months | 36 months | 30 months |
| Medical history | arterial hypertension | coronary artery disease, arterial hypertension | arterial hypertension | coronary artery disease diabetic polyneuropathy |
| Angiotensin converting enzyme inhibitor | + | + | − | + |
| Angiotensin II receptor blockers | − | − | − | − |
| pH-value | 7.38 | 7.40 | 7.40 | 7.39 |
| Actual bicarbonate | 22.0 mmol/l | 23.2 mmol/l | 23.6 mmol/l | 23 mmol/l |
Characteristics of unaffected patients
| . | Patient A . | Patient B . | Patient C . | Patient D . |
|---|---|---|---|---|
| Age (years) | 49 | 75 | 54 | 58 |
| Sex | male | male | female | male |
| Renal disease | polycystic kidney disease | vascular nephropathy | polycystic kidney disease | diabetic nephropathy |
| Duration of dialysis | 3 months | 26 months | 36 months | 30 months |
| Medical history | arterial hypertension | coronary artery disease, arterial hypertension | arterial hypertension | coronary artery disease diabetic polyneuropathy |
| Angiotensin converting enzyme inhibitor | + | + | − | + |
| Angiotensin II receptor blockers | − | − | − | − |
| pH-value | 7.38 | 7.40 | 7.40 | 7.39 |
| Actual bicarbonate | 22.0 mmol/l | 23.2 mmol/l | 23.6 mmol/l | 23 mmol/l |
| . | Patient A . | Patient B . | Patient C . | Patient D . |
|---|---|---|---|---|
| Age (years) | 49 | 75 | 54 | 58 |
| Sex | male | male | female | male |
| Renal disease | polycystic kidney disease | vascular nephropathy | polycystic kidney disease | diabetic nephropathy |
| Duration of dialysis | 3 months | 26 months | 36 months | 30 months |
| Medical history | arterial hypertension | coronary artery disease, arterial hypertension | arterial hypertension | coronary artery disease diabetic polyneuropathy |
| Angiotensin converting enzyme inhibitor | + | + | − | + |
| Angiotensin II receptor blockers | − | − | − | − |
| pH-value | 7.38 | 7.40 | 7.40 | 7.39 |
| Actual bicarbonate | 22.0 mmol/l | 23.2 mmol/l | 23.6 mmol/l | 23 mmol/l |
Strikingly, all affected patients had metabolic acidosis, while all unaffected patients showed normal findings regarding pH- value and actual bicarbonate at time of MR angiography. The mean age of the affected five patients was 57.2±10.7 years and their mean time on dialysis amounted to 36±16.5 months. The mean time of observation with the disease was 9.2±4.7 months. The mean age of the four patients without development of the skin changes was 59±9.7 years. Their mean time on dialysis amounted to 23.75±12.5 months. Comparing the two groups of patients, the mean time on dialysis was longer in affected patients. Age, sex, underlying renal disease, drug therapy including angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, dialysis modalities and comorbid conditions showed no correlation with the development of the skin disease.
However, the mean pH-value of the affected patients amounted to 7.29±0.04 and the mean actual bicarbonate value was 19.5±1.7 mmol/l. The mean pH-value of the four unaffected patients was 7.39±0.01 and the mean value of actual bicarbonate was 22.95±0.58 mmol/l.
Discussion
All haemodialysis patients with NFD had undergone MR angiography within 2–4 weeks before the onset of the skin changes. Gd–DTPA was administered intravenously as a contrast agent. A literature survey revealed no reports on comparable findings in Gd exposed patients.
Gd–DTPA introduced in 1988 is the first paramagnetic contrast agent approved for clinical use in MR imaging. The frequency of adverse reactions (headache, nausea, pain and sensation of cold at the site of injection, taste perversion, dizziness, vasodilation and reduced threshold for seizures) is low. Gd-containing contrast agents are widely used as contrast media and are thought to be safe, even in patients with impaired renal function. The Gd–DTPA is a small complex and diffuses easily through the pores of the vessels. Although Gd-containing contrast agents are rapidly cleared with a half-life of about 2 h in patients with normal renal function, in chronic renal failure half-life is prolonged and may exceed 30–120 h. Lack of immediate adequate dialysis after MR angiography significantly prolongs Gd clearance [8]. Possible side effects occur due to dissociation of the Gd–ligand complex into metal ion and ligand. This process is facilitated both by endogenous metals like zinc, copper, and iron, further calcium, and endogenous acids, destabilizing the complex and leading to its dissociation [9]. In renal failure the combination of metabolic acidosis and the absence of adequate clearance of Gd-containing agent is present [10].
Free Gd ion solubility is poor and can form precipitates of salts with anions like phosphate [10] – that is usually elevated in dialysis population – and with carbonate or hydroxyl, which are deposited in the interstium of muscle, bone, liver and skin and other organs and may cause a sparse initial infiltration of inflammatory cells with expression of TGF-beta.
MR imaging with use of gadolinium is an examination that is not done very often in the entire cohort of dialysis patients. During the 2 years of observation, nine patients were exposed to Gd. Comparing the two groups of patients, the only differences observed were that the mean time on dialysis was longer in affected patients and that they were acidotic at the time of using Gd.
NFD is a rare, relatively newly described disease, and its exact pathogenesis is not well understood. Because of the association with tissue injury, including vascular tissue injury (e.g. vascular surgery) and thrombotic episodes in some cases of NFD, endothelial damage with elevated levels of cytokines may be partially responsible for the development of NFD. Data from the registry suggests that thrombotic complications could trigger the skin changes [3].
It has been suggested that the spindle cells involved in NFD/NSF are circulating fibrocytes (CD 34/procollagen I positive cells) that are normally present in the blood and are involved in wound repair and tissue remodelling. These circulating fibrocytes would be aberrantly recruited to the dermis in the absence of overt tissue injury [3,12,13].
Mackay–Wiggan et al. [14] described elevated anticardiolipin antibodies in some of their affected patients. Not all our patients underwent surgery, or had thrombotic episodes in a close time relation to the development of the skin changes, and have not shown elevated anticardiolipin antibodies. Another hypothesis regarding angiotensin-converting enzyme inhibitors as a trigger [15] could not be confirmed in our dialysis unit.
Other investigators observed the expression of transforming growth factor beta 1, which is a potent stimulus for the production of collagen I by some cell types and mediates interstitial fibrosis [11].
NFD was unknown before March 1997 and some authors suggest that the sudden occurrence of the disease in the last 8 years makes it likely that a new agent or technique of examination causes NFD/NSF [1,3,4,6,16].
So far more than 170 cases have been identified worldwide. There seems to be no gender predilection.
Typical is the unique histopathology of NFD that includes thickened collagen bundles with surrounding clefts, increased dermal mucin deposition, proliferation of dendritic cells and increased elastic fibers [1]. Multinucleated cells positive for CD 68 and XIIIa and an increased expression of TGF-beta1 have also been observed [1,11]. Some of the clinical manifestations of NFD are similar to other fibrosing disorders like morphea, systemic sclerosis, eosinophilic fasciitis, β2-microglobulin amyloidosis, scleromyxedema, fibroblastic rheumatism, Morbus Köhlmeier–Degos, necrobiosis lipoidica and eosiniphilia–myalgia syndrome.
There is no effective treatment for NFD. Physical therapy should be started to improve the range of motion of contracted joints. Various medical therapies including topical and systemic steroids, immunosuppressive therapy have been ineffective. Plasmapheresis have shown some benefit in some patients, and recently improvement was reported after photopheresis in three patients [17,18]. Thalidomide has also shown some improvement in three patients [19]. The influence of erythropoietin dosage has also been investigated. It was suggested that decreasing the erythropoietin dose might improve NFD in some patients because recombinant erythropoietin has potential fibrogenic properties [4]. Significant improvement was seen in several patients with a return to normal kidney function, either spontaneously or as a result of renal transplantation [3].
In our center the course of the patients was different. Patient 1 improved as a result of renal transplantation, patient 2 died a few weeks later due to myocardial infarction, patient 3 refused any additional therapy and is waiting for renal transplantation. Patients 4 and 5 are also awaiting renal transplantation. As photopheresis was under investigation at the time of diagnosis, we decided to treat with pentoxyfyllin, a substance with anti- tumour necrosis factor activity that has shown some benefits in other fibrotic disorders [20,21]. A treatment with 1200 mg pentoxifyllin orally per day was established and the extension of skin changes of patient 4, who was in a later phase of the disease, seemed to slow or arrest. Patient 5 was treated in an early phase of the disease and there a stabilization and a slight reversal of the process has been observed. Regarding pentoxifyllin, it is unclear what contribution vasodilation with possible ameliorating renal perfusion and antifibrotic activity had in the clinical stabilization of the disease. Controlled studies of therapy for NFD must be done.
Conclusion
In end stage renal disease patients undergoing magnetic resonance imaging with the use of Gd-DTPA, attention should be drawn to the correction of metabolic acidosis, as acidosis and Gd-DTPA could play a triggering role for the development of nephrogenic fibrosing dermopathy (NFD).
The author is grateful to the pathologist Dr. Eva Markis from the General Hospital of Wiener Neustadt for the pathology examinations of skin biopsies and Figures 1 and 2.
Conflict of interest statement. I do not declare any conflict of interest.
References
Cowper SE, Su L, Robin H, Bhawan J, LeBoit PE. Nephrogenic fibrosing dermopathy.
Swartz RD, Crofford LJ, Phan SH, Ike RW, Su LD. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure.
Cowper SE. Nephrogenic fibrosing dermopathy: the first six Years.
Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement.
Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review.
McNeill AM, Barr RJ. Scleromyxedema-like fibromucinosis in a patient undergoing hemodialysis.
Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis.
Mann JS. Stability of gadolinium complexes in vitro and in vivo.
Vorobiov M, Basok A, Tovbin D, Shnaider A, Katchko L, Rogachev B. Iron-mobilizing properties of the gadolinium – DTPA complex: clinical and experimental observations.
Jimenez SA, Artlett CM, Sandorfi N et al. Nephrogenic fibrosing dermopathy: study of inflammatory cells and transforming growth factor beta1 expression in affected skin.
Cowper SE, Bucala R. Nephrogenic fibrosing dermopathy: suspect identified, Motive Unclear. [Letter].
Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen – secreting cells of the peripheral blood.
Mackay-Wiggan JM, Cohen DJ, Hardy MA, Knobler EH, Grossman ME. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease).
Fazeli A, Lio PA, Liu V. Nephrogenic fibrosing dermopathy: are ACE inhibitors the missing link? [Letter].
Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy.
Baron PW, Cantos K, Hillebrand DJ et al. Nephrogenic fibrosing dermopathy after liver transplantation successfully treated with plasmapheresis.
Gilliet M, Cozzio A, Burg G, Nestle FO. Successful treatment of three cases of nephrogenic fibrosing dermopathy with extracorporal photopheresis.
Moschella SL, Kay J, Mackool BT, Liu V. Case 35–2004. A 68-year-old man with end stage renal disease and thickening of the skin.
Vicktor C., Schultz-Ehrenburg U. Papulosis maligna atrophicans (Köhlmeier – Degos)





Comments