-
PDF
- Split View
-
Views
-
Cite
Cite
Xavier Castellsagué, Mireia Díaz, Silvia de Sanjosé, Nubia Muñoz, Rolando Herrero, Silvia Franceschi, Rosanna W. Peeling, Rhoda Ashley, Jennifer S. Smith, Peter J. F. Snijders, Chris J. L. M. Meijer, F. Xavier Bosch, For the International Agency for Research on Cancer Multicenter Cervical Cancer Study Group, Worldwide Human Papillomavirus Etiology of Cervical Adenocarcinoma and Its Cofactors: Implications for Screening and Prevention, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 5, 1 March 2006, Pages 303–315, https://doi.org/10.1093/jnci/djj067
Close - Share Icon Share
Abstract
Background: Most cancers of the uterine cervix are squamous cell carcinomas. Although the incidence of such carcinomas of the uterine cervix has declined over time, that of cervical adenocarcinoma has risen in recent years. The extent to which human papillomavirus (HPV) infection and cofactors may explain this differential trend is unclear. Methods: We pooled data from eight case–control studies of cervical cancer that were conducted on three continents. A total of 167 case patients with invasive cervical adenocarcinoma (112 with adenocarcinoma and 55 with adenosquamous carcinoma) and 1881 hospital-based control subjects were included. HPV DNA was analyzed in cervical specimens with the GP5+/6+ general primer system followed by type-specific hybridization for 33 HPV genotypes. Blood samples were analyzed for chlamydial and herpes simplex virus 2 (HSV-2) serology. Multivariable unconditional logistic regression modeling was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs). All tests of statistical significance were two-sided. Results: The adjusted overall odds ratio for cervical adenocarcinoma in HPV-positive women compared with HPV-negative women was 81.3 (95% CI = 42.0 to 157.1). HPV 16 and HPV 18 were the two most commonly detected HPV types in case patients and control subjects. These two types were present in 82% of the patients. Cofactors that showed clear statistically significant positive associations with cervical adenocarcinoma overall and among HPV-positive women included never schooling, poor hygiene, sexual behavior–related variables, long-term use of hormonal contraception, high parity, and HSV-2 seropositivity. Parity had a weaker association with adenocarcinoma and only among HPV-positive women. Use of an intrauterine device (IUD) had a statistically significant inverse association with risk of adenocarcinoma (for ever use of an IUD compared with never use, OR = .41 [95% CI = 0.18 to 0.93]). Smoking and chlamydial seropositivity were not associated with disease. Conclusions: HPV appears to be the key risk factor for cervical adenocarcinoma. HPV testing in primary screening using current mixtures of HPV types and HPV vaccination against main HPV types should reduce the incidence of this cancer worldwide.
The incidence of all invasive cervical cancer and of cervical squamous cell carcinoma has been decreasing in recent years. In this context, cervical adenocarcinoma (i.e., adenosquamous carcinoma and adenocarcinoma) stands out because its incidence among young women has increased in developed countries, even those with widespread screening programs and histology-specific cancer registration ( 1 – 3 ) . In the United States, the proportion of adenocarcinoma relative to squamous cell carcinoma and to all cervical cancers doubled between 1973 and 1996, and the rate of adenocarcinoma in the population at risk also increased over this period ( 4 ) . These observations indicate that current screening practices may be insufficient to detect a substantial proportion of adenocarcinoma precursor lesions. Adenocarcinoma precursor lesions are frequently located high in the endocervical parts of the transitional zone, which may make them less accessible to the brush and less prone to be represented in a standard specimen of exfoliated cells ( 5 – 7 ) .
Previous epidemiologic studies of the association between human papillomavirus (HPV) and cervical adenocarcinoma have shown strong associations, suggesting that the relationship is causal, as is the case for the relationship between HPV and squamous cell carcinomas of the cervix ( 8 ) . The only caveat is that the numbers of cervical adenocarcinoma patients in these studies were small. The largest previous study on HPV and cervical adenocarcinoma included 124 case patients, but it was conducted entirely in the United States ( 9 , 10 ) . Little information is available about the geographic variation of HPV types in adenocarcinomas. Also, although several cofactors have been associated with the risk of squamous cell carcinomas, including smoking; endogenous and exogenous hormonal factors such as parity, oral contraceptive use, and obesity; and coinfection with other sexually transmitted infectious agents such as herpes simplex virus 2 (HSV-2) and Chlamydia trachomatis , the impact of these cofactors on the risk of adenocarcinomas is unclear. Some evidence indicates that cofactors that contribute to the progression of HPV-infected cervical cells to adenocarcinoma are distinct from those that contribute to the progression to squamous cell carcinoma. For example, smoking and high parity have been associated with increased risks of squamous cell carcinoma, but they have no or an inverse association with adenocarcinoma ( 10 – 14 ) , and obesity seems to be a risk factor for adenocarcinoma but not for squamous cell carcinoma ( 15 ) .
Endogenous and exogenous hormones have traditionally been related to the development of cervical adenocarcinomas, although few studies have been carried out among HPV-positive women, and those that have been carried out are of limited statistical power ( 9 , 10 , 13 , 15 , 16 ) . The lack of adequately powered studies in HPV-positive women limits the understanding of the cofactors for cervical adenocarcinoma because evaluating the role of cofactors in the presence of a necessary cause such as HPV is best achieved in analyses restricted to HPV-positive women ( 17 , 18 ) .
To better describe the distribution of HPV types in cervical adenocarcinoma and assess the roles of HPV infection and cofactors in the development of cervical adenocarcinoma, we carried out a pooled analysis of data from a series of case–control studies of adenocarcinoma of the cervix. The studies were conducted on three continents and coordinated by the International Agency for Research on Cancer (IARC). Some of the associations with cofactors examined in this study (i.e., smoking, parity, HSV2, and C. trachomatis ) have been assessed in subsets of the subjects included in this analysis ( 13 , 19 – 21 ) . For this report, we included more studies, and we present the full data on HPV in cervical adenocarcinoma case patients and control subjects and on associations with all cofactors considered in the IARC case–control studies that included patients with cervical adenocarcinoma.
M ETHODS
Case–Control Studies
From 1985 through 1997, eight case–control studies of cervical cancer that included adenocarcinomas and adenosquamous carcinomas were conducted in eight countries with a broad range of the incidence of cervical cancer. Regions covered included North Africa [Algeria ( 22 ) and Morocco ( 23 ) ], South America [Brazil ( 24 ) , Paraguay ( 25 ) , and Peru ( 26 ) ], and Southeast Asia [India ( 27 ) , Thailand ( 28 ) , and the Philippines ( 29 ) ]. Detailed information about the methods of selection of case patients and control subjects can be found in the original papers. In brief, case patients were women with incident histologically confirmed invasive adenocarcinoma or adenosquamous carcinoma of the cervix who had not received previous treatment. Control subjects were hospital or clinic based and they were frequency matched to case patients by 5-year age groups in all studies. All protocols were approved by the IARC and local ethics committees. Written informed consent was obtained from all study subjects.
Data and Specimen Collection
All women were interviewed at hospitals by trained interviewers using a standardized questionnaire to elicit information on potential risk factors for cervical cancer (i.e., sociodemographic variables, sexual behavior–related variables, history of sexually transmitted diseases, tobacco smoking, reproductive variables, use of contraception methods, personal hygiene–related variables, and history of use of Pap smear testing). In some centers the main questionnaire was locally adapted and slightly modified. In Paraguay, a simplified version of the original questionnaire was used. Thus, a few questionnaire items differed or were not collected in some centers. After the interview, all women had a pelvic examination performed by a gynecologist or nurse, and two cervical scrapes were collected for cytology and HPV DNA detection. A tumor biopsy specimen was also taken from case patients and frozen. The histologic diagnosis of each woman's cervical cancer was based on the pathology review of the original slides, which was performed by the local pathologist at each participating center.
HPV DNA Detection and Typing
Cervical scrapes and biopsy specimens were analyzed for HPV DNA in a central laboratory (Department of Pathology, UV Medical Center, Amsterdam, The Netherlands, by investigators blinded to case–control status. Polymerase chain reaction (PCR)–based assays were used to detect HPV in crude extracts, as described previously ( 30 ) . In brief, HPV DNA was detected by amplification with GP5+/6+ general PCR primers, hybridizing the PCR products with mixtures of HPV-specific digoxigenin-labeled oligonucleotide probes, and subjecting the samples to enzyme immunoassay detection ( 23 ) . Subsequently, GP5+/6+ PCR was repeated on positive samples in triplicate to generate sufficient products for further typing. These products were then pooled and typed by consecutive hybridization to HPV type–specific oligonucleotide probes for 33 different HPV types (both high risk and low risk) in three hybridization rounds. The first hybridization round included probes for HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 54, and 56. The second round included probes for HPV types 6, 11, 26, 34, 40, 42, 43, 44, 53, 58, 59, and 68. The third round included probes for HPV types 57, 61, 66, 70, 72, 73, 81, 82 (both the W13B/MM4 subtype and the IS39 subtype), 83, and cand89 (equivalent to CP6108). Also, HPV positivity was assessed by low-stringency Southern blot analysis of the GP5+/6+ PCR products with a probe consisting of a mixture) of HPV-specific DNA fragments. PCR products that were positive by low-stringency Southern blot analysis but not by enzyme immunoassay were coded as HPV X, indicating that these represent HPV (sub)types, either high risk or low risk, that are not detectable with any of the 33 specific probes mentioned above. To assess the quality of target DNA, a 209-bp fragment of the β-globin gene was amplified using the primers BGPCO3 and BGPCO5, as described previously ( 31 ) . For specimens from case patients that were negative for β-globin DNA and either negative for HPV DNA or positive for HPV X, DNA was isolated from the crude extracts and reanalyzed.
HPV was detected in biopsy samples by using the sandwich method ( 32 ) . In brief, a series of sections was cut, the outer of which were used for hematoxylin–eosin staining and histomorphologic assessment. The inner sections were pooled and used for PCR. Biopsy samples that were negative by GP5+/6+ PCR were subjected to PCR amplification with HPV E7 type-specific primers for 14 HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) that are classified as high risk or probable high risk ( 30 ) . This approach was taken to exclude the possibility of false-negative findings that could have resulted from viral integration events that may have affected the GP5+/6+ primer region, which is within the viral L1 open reading frame ( 32 ) .
Case patients were scored HPV positive if HPV DNA was detected in exfoliated cells, biopsy samples, or both. For control subjects, HPV DNA prevalence was determined only in exfoliated cells. When we repeated the analyses using the HPV DNA prevalence in exfoliated cells for case subjects, the results were virtually identical (data not shown).
HSV-2 and Chlamydial Serology
The HSV-2 serologic assay used has already been described in a previous report based on samples from the same study population ( 19 ) . In brief, the University of Washington Virology Laboratory HSV western blot analysis procedure ( 33 ) was used to detect type-specific HSV-2 antibodies in serum samples from subjects from Morocco, Peru, and Thailand. Serum samples from subjects from Brazil and The Philippines was screened for HSV-2 immunoglobulin G (IgG) antibodies by using the Gull/Pre-Meridian HSV-2 enzyme-linked immunosorbent assay (Gull Laboratories, Salt Lake City, UT) according to the manufacturer's instructions. All serum samples with positive, equivocal, or borderline negative enzyme-linked immunosorbent assay results were retested with the western blot assay to obtain HSV-2 type-specific results ( 34 ) . HSV-2 testing was not performed in subjects from Paraguay, Algeria, or India.
C. trachomatis seropositivity was analyzed in women from Brazil, Morocco, Peru, the Philippines, and Thailand by using the strategy and original data reported in two previous reports based on samples from the same study population ( 20 , 35 ) . In brief, IgG-class antibodies against C. trachomatis were assayed by a microimmunofluorescescence assay ( 36 ) . The antigen panel consisted of purified elementary bodies of C. trachomatis (from serovar A and from serovar groups BDE, CJHI, and FGK) and of C. pneumoniae (to monitor cross-reactive genus-specific antibody responses against all chlamydial species). Serum samples were diluted 1 : 8 for screening for C. trachomatis antibodies and then further diluted (1 : 16, 1 : 32, 1 : 128, etc.) to define the titer (i.e., the endpoint at which antibody response was lost). An IgG titer of 1 : 8 or greater against any C. trachomatis serovar group was considered evidence of seropositivity. An IgG titer of 1 : 16 or greater against C. pneumoniae was considered evidence for past C. pneumoniae respiratory infection.
All serologic assays were conducted by investigators blinded to the subject's case–control status.
Statistical Analysis
Unconditional logistic regression methods were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of cervical adenocarcinoma associated with various cofactors and HPV types. For analyses of HPV associations we coded, in a single variable, categories for each HPV type for single infections and categories of multiple types for combinations of multiple infections. HPV-negative subjects were used as the referent group. Unless otherwise specified, all logistic regression models were adjusted for age in tertiles (18–42 years, 45–53 years, ≥54 years), country, years of schooling in quartiles (none, 1–4, 5–9, ≥10), age at first sexual intercourse in quartiles (≥23 years, 20–22 years, 18–19 years, ≤17 years), and number of previous screening Pap smears the woman had until 12 months before enrollment in the study (none, 2–5, ≥6). All tertiles and quartiles used for a given variable were based on the distribution of the variable in all subjects in the study.
To assess the potential effects of confounding by other cofactors, a simpler model that included only the design variables (country and age), years of schooling, and age at first sexual intercourse was also fitted, and the results were compared with those from the fully adjusted model. Several variables related to sexual behavior were associated with cancer risk (data not shown), but only age at first sexual intercourse was used in the final analyses because it showed the best fit in the logistic regression models. HPV genotype distribution was calculated as the percentage of women infected with each type relative to the total number of HPV-positive women. For instance, a woman positive for HPV 16 and 18 would contribute to the calculated percentage of both HPV types. Analyses of potential cofactors were restricted to HPV-positive case patients and control subjects. Finally, we performed a multivariable analysis among HPV-positive subjects that included, in addition to the covariates mentioned above, number of pregnancies, history of sexually transmitted disease, number of baths or showers per week, and ever use of an intrauterine contraceptive device (IUD). All P values are two-sided.
R ESULTS
Subjects' Characteristics
A total of 167 case patients with cervical adenocarcinoma (112 [67%] with a histologic diagnosis of invasive adenocarcinoma and 55 [33%] with a histologic diagnosis of invasive adenosquamous carcinoma) and 1881 control subjects were included in the pooled analyses. Table 1 summarizes the main sociodemographic, reproductive, and lifestyle characteristics of study subjects by case and control status and the association of these characteristics with cervical adenocarcinoma risk after various adjustments. In the fully adjusted model, the following variables showed positive and statistically significant associations with adenocarcinoma risk: never schooling, several variables related to sexual behavior, long-term use of hormonal contraceptives, and HSV-2 seropositivity. Use of an IUD, having had at least one Pap smear before 12 months before enrollment, and a large number (i.e., six or more) of baths or showers per week were all inversely related to the risk of adenocarcinoma. Lifetime number of pregnancies, history of tobacco smoking, and C. trachomatis seropositivity were not statistically significantly associated with adenocarcinoma risk. There were no marked differences in the magnitude of most associations between the simpler model and the fully adjusted model, except for overall larger odds ratios in the latter model for anal intercourse, years of use of hormonal contraceptives, and numbers of baths or showers per week.
Distribution of case patients and control subjects by selected sociodemographic, reproductive, and lifestyle characteristics and odds ratios for cervical adenocarcinoma *
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR1 † . | OR2 ‡ (95% CI) . |
|---|---|---|---|---|
| Total | 1881 (100) | 167 (100) | ||
| Country and world region | ||||
| North Africa | 405 (21.5) | 30 (18.0) | ||
| Algeria | 202 (10.7) | 14 (8.4) | ||
| Morocco | 203 (10.8) | 16 (9.6) | ||
| South America | 522 (27.7) | 50 (29.9) | ||
| Brazil | 225 (12.0) | 18 (10.8) | ||
| Paraguay | 101 (5.4) | 7 (4.2) | ||
| Peru | 196 (10.4) | 25 (15.0) | ||
| Southeast Asia | 954 (50.7) | 87 (52.1) | ||
| The Philippines | 387 (20.6) | 34 (20.4) | ||
| Thailand | 354 (18.8) | 41 (24.5) | ||
| India | 213 (11.3) | 12 (7.2) | ||
| Age group, y | ||||
| 18–42 | 640 (34.0) | 49 (29.3) | ||
| 43–53 | 617 (32.8) | 63 (37.7) | ||
| ≥54 | 624 (33.2) | 55 (32.9) | ||
| Years of schooling | ||||
| None | 460 (24.5) | 62 (37.1) | 1.00 | 1.00 (Referent) |
| 1–4 | 499 (26.6) | 43 (25.7) | 0.45 | 0.57 (0.31 to 1.05) |
| 5–9 | 452 (24.1) | 38 (22.7) | 0.49 | 0.56 (0.29 to 1.09) |
| ≥10 | 468 (24.9) | 24 (14.4) | 0.34 | 0.42 (0.19 to 0.92) |
| Unknown | 2 | 0 | ||
| Ptrend | <.001 | .03 | ||
| Age at first sexual intercourse, y | ||||
| ≥23 | 464 (25.0) | 19 (11.4) | 1.00 | 1.00 (Referent) |
| 20–22 | 405 (21.8) | 30 (18.1) | 1.69 | 1.49 (0.73 to 3.04) |
| 18–19 | 379 (20.4) | 40 (24.1) | 2.46 | 2.68 (1.32 to 5.42) |
| ≤17 | 611 (32.9) | 77 (46.4) | 2.88 | 2.08 (1.05 to 4.12) |
| Unknown | 22 | 1 | ||
| Ptrend | <.001 | .03 | ||
| Lifetime No. of sexual partners | ||||
| 1 | 1444 (78.0) | 100 (60.2) | 1.00 | 1.00 (Referent) |
| 2–3 | 352 (19.0) | 54 (32.5) | 1.85 | 1.56 (0.95 to 2.57) |
| ≥ 4 | 54 (2.9) | 12 (7.2) | 2.86 | 2.18 (0.80 to 5.94) |
| Unknown | 31 | 1 | ||
| Ptrend | <.001 | .04 | ||
| Anal intercourse § | ||||
| Never | 1611 (91.3) | 132 (82.5) | 1.00 | 1.00 (Referent) |
| Rarely | 76 (4.3) | 12 (7.5) | 2.14 | 3.89 (1.41 to 10.73) |
| Occasionally/often | 78 (4.4) | 16 (10.0) | 2.66 | 3.24 (1.29 to 8.13) |
| Unknown | 15 | 0 | ||
| Ptrend | .003 | .003 | ||
| History of STD | ||||
| Never | 1434 (81.8) | 102 (68.5) | 1.00 | 1.00 (Referent) |
| Ever | 318 (18.1) | 47 (31.5) | 1.85 | 2.05 (1.14 to 3.66) |
| Unknown | 129 | 18 | ||
| STD in regular partner ∥ | ||||
| Never | 1133 (82.3) | 84 (70.0) | 1.00 | 1.00 (Referent) |
| Ever | 244 (17.7) | 36 (30.0) | 1.89 | 2.01 (1.10 to 3.69) |
| Unknown | 190 | 28 | ||
| Tobacco smoking | ||||
| Never | 1604 (85.7) | 134 (81.2) | 1.00 | 1.00 (Referent) |
| Ever | 267 (14.3) | 31 (18.8) | 1.17 | 1.39 (0.76 to 2.55) |
| Unknown | 10 | 2 | ||
| Lifetime No. of pregnancies | ||||
| Never pregnant | 86 (4.6) | 3 (1.8) | 1.00 | 1.00 (Referent) |
| Ever pregnant | 1795 (95.4) | 164 (98.2) | 1.32 | 1.43 (0.38 to 5.42) |
| 1–2 | 336 (18.9) | 23 (14.0) | 1.00 | 1.00 (Referent) |
| 3–5 | 751 (42.2) | 48 (29.3) | 0.83 | 0.70 (0.38 to 1.28) |
| 6–7 | 313 (17.6) | 39 (23.8) | 1.37 | 1.40 (0.74 to 2.68) |
| ≥8 | 379 (21.3) | 54 (32.9) | 1.47 | 1.42 (0.74 to 2.73) |
| Unknown | 16 | 3 | ||
| Ptrend | .04 | .14 | ||
| Use of hormonal contraception | ||||
| Never use | 753 (55.0) | 63 (51.2) | 1.00 | 1.00 (Referent) |
| Ever use | 615 (45.0) | 60 (48.8) | 1.31 | 1.41 (0.80 to 2.48) |
| Unknown | 513 | 44 | ||
| Years of use of hormonal contraception | ||||
| <2 | 203 (41.4) | 18 (33.3) | 1.00 | 1.00 (Referent) |
| 2–4 | 148 (30.2) | 15 (27.8) | 1.08 | 0.95 (0.36 to 2.50) |
| ≥5 | 139 (28.4) | 21 (38.9) | 1.56 | 3.06 (1.13 to 8.29) |
| Unknown | 125 | 6 | ||
| Ptrend | .22 | .03 | ||
| Ever use of IUD ¶ | ||||
| Never | 1011 (78.6) | 128 (90.1) | 1.00 | 1.00 (Referent) |
| Ever | 275 (21.4) | 14 (9.9) | 0.41 | 0.44 (0.21 to 0.93) |
| Unknown | 269 | 0 | ||
| Lifetime No. of Pap smears until 12 mo before study entry | ||||
| None | 1018 (57.3) | 115 (72.8) | 1.00 | 1.00 (Referent) |
| 1 | 331 (18.6) | 17 (10.8) | 0.40 | 0.46 (0.24 to 0.88) |
| 2–5 | 289 (16.3) | 17 (10.8) | 0.46 | 0.55 (0.27 to 1.12) |
| ≥6 | 137 (7.7) | 9 (5.7) | 0.53 | 0.59 (0.24 to 1.45) |
| Unknown | 106 | 9 | ||
| Ptrend | .002 | .05 | ||
| No. of baths or showers per week § | ||||
| 1–5 | 676 (38.0) | 76 (47.5) | 1.00 | 1.00 (Referent) |
| 6–8 | 714 (40.2) | 42 (26.2) | 0.36 | 0.20 (0.10 to 0.41) |
| ≥9 | 388 (21.8) | 42 (26.2) | 0.37 | 0.11 (0.02 to 0.47) |
| Unknown | 2 | 0 | ||
| Ptrend | .002 | <.001 | ||
| HSV-2 serostatus # | ||||
| Negative | 728 (74.7) | 59 (55.7) | 1.00 | 1.00 (Referent) |
| Positive | 242 (24.8) | 46 (43.4) | 2.01 | 2.27 (1.23 to 4.18) |
| Inadequate ** | 4 (0.4) | 1 (0.94) | ||
| Unknown | 391 | 28 | ||
| Chlamydia trachomatis serostatus # | ||||
| Negative | 676 (70.6) | 60 (60.6) | 1.00 | 1.00 (Referent) |
| Positive | 282 (29.4) | 39 (39.4) | 1.29 | 1.05 (0.58 to 1.91) |
| Unknown | 407 | 35 |
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR1 † . | OR2 ‡ (95% CI) . |
|---|---|---|---|---|
| Total | 1881 (100) | 167 (100) | ||
| Country and world region | ||||
| North Africa | 405 (21.5) | 30 (18.0) | ||
| Algeria | 202 (10.7) | 14 (8.4) | ||
| Morocco | 203 (10.8) | 16 (9.6) | ||
| South America | 522 (27.7) | 50 (29.9) | ||
| Brazil | 225 (12.0) | 18 (10.8) | ||
| Paraguay | 101 (5.4) | 7 (4.2) | ||
| Peru | 196 (10.4) | 25 (15.0) | ||
| Southeast Asia | 954 (50.7) | 87 (52.1) | ||
| The Philippines | 387 (20.6) | 34 (20.4) | ||
| Thailand | 354 (18.8) | 41 (24.5) | ||
| India | 213 (11.3) | 12 (7.2) | ||
| Age group, y | ||||
| 18–42 | 640 (34.0) | 49 (29.3) | ||
| 43–53 | 617 (32.8) | 63 (37.7) | ||
| ≥54 | 624 (33.2) | 55 (32.9) | ||
| Years of schooling | ||||
| None | 460 (24.5) | 62 (37.1) | 1.00 | 1.00 (Referent) |
| 1–4 | 499 (26.6) | 43 (25.7) | 0.45 | 0.57 (0.31 to 1.05) |
| 5–9 | 452 (24.1) | 38 (22.7) | 0.49 | 0.56 (0.29 to 1.09) |
| ≥10 | 468 (24.9) | 24 (14.4) | 0.34 | 0.42 (0.19 to 0.92) |
| Unknown | 2 | 0 | ||
| Ptrend | <.001 | .03 | ||
| Age at first sexual intercourse, y | ||||
| ≥23 | 464 (25.0) | 19 (11.4) | 1.00 | 1.00 (Referent) |
| 20–22 | 405 (21.8) | 30 (18.1) | 1.69 | 1.49 (0.73 to 3.04) |
| 18–19 | 379 (20.4) | 40 (24.1) | 2.46 | 2.68 (1.32 to 5.42) |
| ≤17 | 611 (32.9) | 77 (46.4) | 2.88 | 2.08 (1.05 to 4.12) |
| Unknown | 22 | 1 | ||
| Ptrend | <.001 | .03 | ||
| Lifetime No. of sexual partners | ||||
| 1 | 1444 (78.0) | 100 (60.2) | 1.00 | 1.00 (Referent) |
| 2–3 | 352 (19.0) | 54 (32.5) | 1.85 | 1.56 (0.95 to 2.57) |
| ≥ 4 | 54 (2.9) | 12 (7.2) | 2.86 | 2.18 (0.80 to 5.94) |
| Unknown | 31 | 1 | ||
| Ptrend | <.001 | .04 | ||
| Anal intercourse § | ||||
| Never | 1611 (91.3) | 132 (82.5) | 1.00 | 1.00 (Referent) |
| Rarely | 76 (4.3) | 12 (7.5) | 2.14 | 3.89 (1.41 to 10.73) |
| Occasionally/often | 78 (4.4) | 16 (10.0) | 2.66 | 3.24 (1.29 to 8.13) |
| Unknown | 15 | 0 | ||
| Ptrend | .003 | .003 | ||
| History of STD | ||||
| Never | 1434 (81.8) | 102 (68.5) | 1.00 | 1.00 (Referent) |
| Ever | 318 (18.1) | 47 (31.5) | 1.85 | 2.05 (1.14 to 3.66) |
| Unknown | 129 | 18 | ||
| STD in regular partner ∥ | ||||
| Never | 1133 (82.3) | 84 (70.0) | 1.00 | 1.00 (Referent) |
| Ever | 244 (17.7) | 36 (30.0) | 1.89 | 2.01 (1.10 to 3.69) |
| Unknown | 190 | 28 | ||
| Tobacco smoking | ||||
| Never | 1604 (85.7) | 134 (81.2) | 1.00 | 1.00 (Referent) |
| Ever | 267 (14.3) | 31 (18.8) | 1.17 | 1.39 (0.76 to 2.55) |
| Unknown | 10 | 2 | ||
| Lifetime No. of pregnancies | ||||
| Never pregnant | 86 (4.6) | 3 (1.8) | 1.00 | 1.00 (Referent) |
| Ever pregnant | 1795 (95.4) | 164 (98.2) | 1.32 | 1.43 (0.38 to 5.42) |
| 1–2 | 336 (18.9) | 23 (14.0) | 1.00 | 1.00 (Referent) |
| 3–5 | 751 (42.2) | 48 (29.3) | 0.83 | 0.70 (0.38 to 1.28) |
| 6–7 | 313 (17.6) | 39 (23.8) | 1.37 | 1.40 (0.74 to 2.68) |
| ≥8 | 379 (21.3) | 54 (32.9) | 1.47 | 1.42 (0.74 to 2.73) |
| Unknown | 16 | 3 | ||
| Ptrend | .04 | .14 | ||
| Use of hormonal contraception | ||||
| Never use | 753 (55.0) | 63 (51.2) | 1.00 | 1.00 (Referent) |
| Ever use | 615 (45.0) | 60 (48.8) | 1.31 | 1.41 (0.80 to 2.48) |
| Unknown | 513 | 44 | ||
| Years of use of hormonal contraception | ||||
| <2 | 203 (41.4) | 18 (33.3) | 1.00 | 1.00 (Referent) |
| 2–4 | 148 (30.2) | 15 (27.8) | 1.08 | 0.95 (0.36 to 2.50) |
| ≥5 | 139 (28.4) | 21 (38.9) | 1.56 | 3.06 (1.13 to 8.29) |
| Unknown | 125 | 6 | ||
| Ptrend | .22 | .03 | ||
| Ever use of IUD ¶ | ||||
| Never | 1011 (78.6) | 128 (90.1) | 1.00 | 1.00 (Referent) |
| Ever | 275 (21.4) | 14 (9.9) | 0.41 | 0.44 (0.21 to 0.93) |
| Unknown | 269 | 0 | ||
| Lifetime No. of Pap smears until 12 mo before study entry | ||||
| None | 1018 (57.3) | 115 (72.8) | 1.00 | 1.00 (Referent) |
| 1 | 331 (18.6) | 17 (10.8) | 0.40 | 0.46 (0.24 to 0.88) |
| 2–5 | 289 (16.3) | 17 (10.8) | 0.46 | 0.55 (0.27 to 1.12) |
| ≥6 | 137 (7.7) | 9 (5.7) | 0.53 | 0.59 (0.24 to 1.45) |
| Unknown | 106 | 9 | ||
| Ptrend | .002 | .05 | ||
| No. of baths or showers per week § | ||||
| 1–5 | 676 (38.0) | 76 (47.5) | 1.00 | 1.00 (Referent) |
| 6–8 | 714 (40.2) | 42 (26.2) | 0.36 | 0.20 (0.10 to 0.41) |
| ≥9 | 388 (21.8) | 42 (26.2) | 0.37 | 0.11 (0.02 to 0.47) |
| Unknown | 2 | 0 | ||
| Ptrend | .002 | <.001 | ||
| HSV-2 serostatus # | ||||
| Negative | 728 (74.7) | 59 (55.7) | 1.00 | 1.00 (Referent) |
| Positive | 242 (24.8) | 46 (43.4) | 2.01 | 2.27 (1.23 to 4.18) |
| Inadequate ** | 4 (0.4) | 1 (0.94) | ||
| Unknown | 391 | 28 | ||
| Chlamydia trachomatis serostatus # | ||||
| Negative | 676 (70.6) | 60 (60.6) | 1.00 | 1.00 (Referent) |
| Positive | 282 (29.4) | 39 (39.4) | 1.29 | 1.05 (0.58 to 1.91) |
| Unknown | 407 | 35 |
OR = odds ratio; CI = confidence interval; STD = sexually transmitted disease; IUD = intrauterine device.
Models adjusted by country, age group, years of schooling, and age at first sexual intercourse. The model fitted to compute ORs for a given adjusting covariate is not adjusted for that same covariate.
Models adjusted by country, age group, years of schooling, age at first sexual intercourse, cervical HPV DNA status, and lifetime number of Pap smears until 12 months before enrollment. The model fitted to compute ORs for a given adjusting covariate is not adjusted for that same covariate.
Excludes subjects from Paraguay.
Excludes subjects from India and Paraguay.
Excludes subjects from Paraguay and Brazil.
Excludes subjects from Algeria, Paraguay, and India, in whom serologic testing was not performed.
Serum was tested, but assay results were inconclusive.
Distribution of case patients and control subjects by selected sociodemographic, reproductive, and lifestyle characteristics and odds ratios for cervical adenocarcinoma *
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR1 † . | OR2 ‡ (95% CI) . |
|---|---|---|---|---|
| Total | 1881 (100) | 167 (100) | ||
| Country and world region | ||||
| North Africa | 405 (21.5) | 30 (18.0) | ||
| Algeria | 202 (10.7) | 14 (8.4) | ||
| Morocco | 203 (10.8) | 16 (9.6) | ||
| South America | 522 (27.7) | 50 (29.9) | ||
| Brazil | 225 (12.0) | 18 (10.8) | ||
| Paraguay | 101 (5.4) | 7 (4.2) | ||
| Peru | 196 (10.4) | 25 (15.0) | ||
| Southeast Asia | 954 (50.7) | 87 (52.1) | ||
| The Philippines | 387 (20.6) | 34 (20.4) | ||
| Thailand | 354 (18.8) | 41 (24.5) | ||
| India | 213 (11.3) | 12 (7.2) | ||
| Age group, y | ||||
| 18–42 | 640 (34.0) | 49 (29.3) | ||
| 43–53 | 617 (32.8) | 63 (37.7) | ||
| ≥54 | 624 (33.2) | 55 (32.9) | ||
| Years of schooling | ||||
| None | 460 (24.5) | 62 (37.1) | 1.00 | 1.00 (Referent) |
| 1–4 | 499 (26.6) | 43 (25.7) | 0.45 | 0.57 (0.31 to 1.05) |
| 5–9 | 452 (24.1) | 38 (22.7) | 0.49 | 0.56 (0.29 to 1.09) |
| ≥10 | 468 (24.9) | 24 (14.4) | 0.34 | 0.42 (0.19 to 0.92) |
| Unknown | 2 | 0 | ||
| Ptrend | <.001 | .03 | ||
| Age at first sexual intercourse, y | ||||
| ≥23 | 464 (25.0) | 19 (11.4) | 1.00 | 1.00 (Referent) |
| 20–22 | 405 (21.8) | 30 (18.1) | 1.69 | 1.49 (0.73 to 3.04) |
| 18–19 | 379 (20.4) | 40 (24.1) | 2.46 | 2.68 (1.32 to 5.42) |
| ≤17 | 611 (32.9) | 77 (46.4) | 2.88 | 2.08 (1.05 to 4.12) |
| Unknown | 22 | 1 | ||
| Ptrend | <.001 | .03 | ||
| Lifetime No. of sexual partners | ||||
| 1 | 1444 (78.0) | 100 (60.2) | 1.00 | 1.00 (Referent) |
| 2–3 | 352 (19.0) | 54 (32.5) | 1.85 | 1.56 (0.95 to 2.57) |
| ≥ 4 | 54 (2.9) | 12 (7.2) | 2.86 | 2.18 (0.80 to 5.94) |
| Unknown | 31 | 1 | ||
| Ptrend | <.001 | .04 | ||
| Anal intercourse § | ||||
| Never | 1611 (91.3) | 132 (82.5) | 1.00 | 1.00 (Referent) |
| Rarely | 76 (4.3) | 12 (7.5) | 2.14 | 3.89 (1.41 to 10.73) |
| Occasionally/often | 78 (4.4) | 16 (10.0) | 2.66 | 3.24 (1.29 to 8.13) |
| Unknown | 15 | 0 | ||
| Ptrend | .003 | .003 | ||
| History of STD | ||||
| Never | 1434 (81.8) | 102 (68.5) | 1.00 | 1.00 (Referent) |
| Ever | 318 (18.1) | 47 (31.5) | 1.85 | 2.05 (1.14 to 3.66) |
| Unknown | 129 | 18 | ||
| STD in regular partner ∥ | ||||
| Never | 1133 (82.3) | 84 (70.0) | 1.00 | 1.00 (Referent) |
| Ever | 244 (17.7) | 36 (30.0) | 1.89 | 2.01 (1.10 to 3.69) |
| Unknown | 190 | 28 | ||
| Tobacco smoking | ||||
| Never | 1604 (85.7) | 134 (81.2) | 1.00 | 1.00 (Referent) |
| Ever | 267 (14.3) | 31 (18.8) | 1.17 | 1.39 (0.76 to 2.55) |
| Unknown | 10 | 2 | ||
| Lifetime No. of pregnancies | ||||
| Never pregnant | 86 (4.6) | 3 (1.8) | 1.00 | 1.00 (Referent) |
| Ever pregnant | 1795 (95.4) | 164 (98.2) | 1.32 | 1.43 (0.38 to 5.42) |
| 1–2 | 336 (18.9) | 23 (14.0) | 1.00 | 1.00 (Referent) |
| 3–5 | 751 (42.2) | 48 (29.3) | 0.83 | 0.70 (0.38 to 1.28) |
| 6–7 | 313 (17.6) | 39 (23.8) | 1.37 | 1.40 (0.74 to 2.68) |
| ≥8 | 379 (21.3) | 54 (32.9) | 1.47 | 1.42 (0.74 to 2.73) |
| Unknown | 16 | 3 | ||
| Ptrend | .04 | .14 | ||
| Use of hormonal contraception | ||||
| Never use | 753 (55.0) | 63 (51.2) | 1.00 | 1.00 (Referent) |
| Ever use | 615 (45.0) | 60 (48.8) | 1.31 | 1.41 (0.80 to 2.48) |
| Unknown | 513 | 44 | ||
| Years of use of hormonal contraception | ||||
| <2 | 203 (41.4) | 18 (33.3) | 1.00 | 1.00 (Referent) |
| 2–4 | 148 (30.2) | 15 (27.8) | 1.08 | 0.95 (0.36 to 2.50) |
| ≥5 | 139 (28.4) | 21 (38.9) | 1.56 | 3.06 (1.13 to 8.29) |
| Unknown | 125 | 6 | ||
| Ptrend | .22 | .03 | ||
| Ever use of IUD ¶ | ||||
| Never | 1011 (78.6) | 128 (90.1) | 1.00 | 1.00 (Referent) |
| Ever | 275 (21.4) | 14 (9.9) | 0.41 | 0.44 (0.21 to 0.93) |
| Unknown | 269 | 0 | ||
| Lifetime No. of Pap smears until 12 mo before study entry | ||||
| None | 1018 (57.3) | 115 (72.8) | 1.00 | 1.00 (Referent) |
| 1 | 331 (18.6) | 17 (10.8) | 0.40 | 0.46 (0.24 to 0.88) |
| 2–5 | 289 (16.3) | 17 (10.8) | 0.46 | 0.55 (0.27 to 1.12) |
| ≥6 | 137 (7.7) | 9 (5.7) | 0.53 | 0.59 (0.24 to 1.45) |
| Unknown | 106 | 9 | ||
| Ptrend | .002 | .05 | ||
| No. of baths or showers per week § | ||||
| 1–5 | 676 (38.0) | 76 (47.5) | 1.00 | 1.00 (Referent) |
| 6–8 | 714 (40.2) | 42 (26.2) | 0.36 | 0.20 (0.10 to 0.41) |
| ≥9 | 388 (21.8) | 42 (26.2) | 0.37 | 0.11 (0.02 to 0.47) |
| Unknown | 2 | 0 | ||
| Ptrend | .002 | <.001 | ||
| HSV-2 serostatus # | ||||
| Negative | 728 (74.7) | 59 (55.7) | 1.00 | 1.00 (Referent) |
| Positive | 242 (24.8) | 46 (43.4) | 2.01 | 2.27 (1.23 to 4.18) |
| Inadequate ** | 4 (0.4) | 1 (0.94) | ||
| Unknown | 391 | 28 | ||
| Chlamydia trachomatis serostatus # | ||||
| Negative | 676 (70.6) | 60 (60.6) | 1.00 | 1.00 (Referent) |
| Positive | 282 (29.4) | 39 (39.4) | 1.29 | 1.05 (0.58 to 1.91) |
| Unknown | 407 | 35 |
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR1 † . | OR2 ‡ (95% CI) . |
|---|---|---|---|---|
| Total | 1881 (100) | 167 (100) | ||
| Country and world region | ||||
| North Africa | 405 (21.5) | 30 (18.0) | ||
| Algeria | 202 (10.7) | 14 (8.4) | ||
| Morocco | 203 (10.8) | 16 (9.6) | ||
| South America | 522 (27.7) | 50 (29.9) | ||
| Brazil | 225 (12.0) | 18 (10.8) | ||
| Paraguay | 101 (5.4) | 7 (4.2) | ||
| Peru | 196 (10.4) | 25 (15.0) | ||
| Southeast Asia | 954 (50.7) | 87 (52.1) | ||
| The Philippines | 387 (20.6) | 34 (20.4) | ||
| Thailand | 354 (18.8) | 41 (24.5) | ||
| India | 213 (11.3) | 12 (7.2) | ||
| Age group, y | ||||
| 18–42 | 640 (34.0) | 49 (29.3) | ||
| 43–53 | 617 (32.8) | 63 (37.7) | ||
| ≥54 | 624 (33.2) | 55 (32.9) | ||
| Years of schooling | ||||
| None | 460 (24.5) | 62 (37.1) | 1.00 | 1.00 (Referent) |
| 1–4 | 499 (26.6) | 43 (25.7) | 0.45 | 0.57 (0.31 to 1.05) |
| 5–9 | 452 (24.1) | 38 (22.7) | 0.49 | 0.56 (0.29 to 1.09) |
| ≥10 | 468 (24.9) | 24 (14.4) | 0.34 | 0.42 (0.19 to 0.92) |
| Unknown | 2 | 0 | ||
| Ptrend | <.001 | .03 | ||
| Age at first sexual intercourse, y | ||||
| ≥23 | 464 (25.0) | 19 (11.4) | 1.00 | 1.00 (Referent) |
| 20–22 | 405 (21.8) | 30 (18.1) | 1.69 | 1.49 (0.73 to 3.04) |
| 18–19 | 379 (20.4) | 40 (24.1) | 2.46 | 2.68 (1.32 to 5.42) |
| ≤17 | 611 (32.9) | 77 (46.4) | 2.88 | 2.08 (1.05 to 4.12) |
| Unknown | 22 | 1 | ||
| Ptrend | <.001 | .03 | ||
| Lifetime No. of sexual partners | ||||
| 1 | 1444 (78.0) | 100 (60.2) | 1.00 | 1.00 (Referent) |
| 2–3 | 352 (19.0) | 54 (32.5) | 1.85 | 1.56 (0.95 to 2.57) |
| ≥ 4 | 54 (2.9) | 12 (7.2) | 2.86 | 2.18 (0.80 to 5.94) |
| Unknown | 31 | 1 | ||
| Ptrend | <.001 | .04 | ||
| Anal intercourse § | ||||
| Never | 1611 (91.3) | 132 (82.5) | 1.00 | 1.00 (Referent) |
| Rarely | 76 (4.3) | 12 (7.5) | 2.14 | 3.89 (1.41 to 10.73) |
| Occasionally/often | 78 (4.4) | 16 (10.0) | 2.66 | 3.24 (1.29 to 8.13) |
| Unknown | 15 | 0 | ||
| Ptrend | .003 | .003 | ||
| History of STD | ||||
| Never | 1434 (81.8) | 102 (68.5) | 1.00 | 1.00 (Referent) |
| Ever | 318 (18.1) | 47 (31.5) | 1.85 | 2.05 (1.14 to 3.66) |
| Unknown | 129 | 18 | ||
| STD in regular partner ∥ | ||||
| Never | 1133 (82.3) | 84 (70.0) | 1.00 | 1.00 (Referent) |
| Ever | 244 (17.7) | 36 (30.0) | 1.89 | 2.01 (1.10 to 3.69) |
| Unknown | 190 | 28 | ||
| Tobacco smoking | ||||
| Never | 1604 (85.7) | 134 (81.2) | 1.00 | 1.00 (Referent) |
| Ever | 267 (14.3) | 31 (18.8) | 1.17 | 1.39 (0.76 to 2.55) |
| Unknown | 10 | 2 | ||
| Lifetime No. of pregnancies | ||||
| Never pregnant | 86 (4.6) | 3 (1.8) | 1.00 | 1.00 (Referent) |
| Ever pregnant | 1795 (95.4) | 164 (98.2) | 1.32 | 1.43 (0.38 to 5.42) |
| 1–2 | 336 (18.9) | 23 (14.0) | 1.00 | 1.00 (Referent) |
| 3–5 | 751 (42.2) | 48 (29.3) | 0.83 | 0.70 (0.38 to 1.28) |
| 6–7 | 313 (17.6) | 39 (23.8) | 1.37 | 1.40 (0.74 to 2.68) |
| ≥8 | 379 (21.3) | 54 (32.9) | 1.47 | 1.42 (0.74 to 2.73) |
| Unknown | 16 | 3 | ||
| Ptrend | .04 | .14 | ||
| Use of hormonal contraception | ||||
| Never use | 753 (55.0) | 63 (51.2) | 1.00 | 1.00 (Referent) |
| Ever use | 615 (45.0) | 60 (48.8) | 1.31 | 1.41 (0.80 to 2.48) |
| Unknown | 513 | 44 | ||
| Years of use of hormonal contraception | ||||
| <2 | 203 (41.4) | 18 (33.3) | 1.00 | 1.00 (Referent) |
| 2–4 | 148 (30.2) | 15 (27.8) | 1.08 | 0.95 (0.36 to 2.50) |
| ≥5 | 139 (28.4) | 21 (38.9) | 1.56 | 3.06 (1.13 to 8.29) |
| Unknown | 125 | 6 | ||
| Ptrend | .22 | .03 | ||
| Ever use of IUD ¶ | ||||
| Never | 1011 (78.6) | 128 (90.1) | 1.00 | 1.00 (Referent) |
| Ever | 275 (21.4) | 14 (9.9) | 0.41 | 0.44 (0.21 to 0.93) |
| Unknown | 269 | 0 | ||
| Lifetime No. of Pap smears until 12 mo before study entry | ||||
| None | 1018 (57.3) | 115 (72.8) | 1.00 | 1.00 (Referent) |
| 1 | 331 (18.6) | 17 (10.8) | 0.40 | 0.46 (0.24 to 0.88) |
| 2–5 | 289 (16.3) | 17 (10.8) | 0.46 | 0.55 (0.27 to 1.12) |
| ≥6 | 137 (7.7) | 9 (5.7) | 0.53 | 0.59 (0.24 to 1.45) |
| Unknown | 106 | 9 | ||
| Ptrend | .002 | .05 | ||
| No. of baths or showers per week § | ||||
| 1–5 | 676 (38.0) | 76 (47.5) | 1.00 | 1.00 (Referent) |
| 6–8 | 714 (40.2) | 42 (26.2) | 0.36 | 0.20 (0.10 to 0.41) |
| ≥9 | 388 (21.8) | 42 (26.2) | 0.37 | 0.11 (0.02 to 0.47) |
| Unknown | 2 | 0 | ||
| Ptrend | .002 | <.001 | ||
| HSV-2 serostatus # | ||||
| Negative | 728 (74.7) | 59 (55.7) | 1.00 | 1.00 (Referent) |
| Positive | 242 (24.8) | 46 (43.4) | 2.01 | 2.27 (1.23 to 4.18) |
| Inadequate ** | 4 (0.4) | 1 (0.94) | ||
| Unknown | 391 | 28 | ||
| Chlamydia trachomatis serostatus # | ||||
| Negative | 676 (70.6) | 60 (60.6) | 1.00 | 1.00 (Referent) |
| Positive | 282 (29.4) | 39 (39.4) | 1.29 | 1.05 (0.58 to 1.91) |
| Unknown | 407 | 35 |
OR = odds ratio; CI = confidence interval; STD = sexually transmitted disease; IUD = intrauterine device.
Models adjusted by country, age group, years of schooling, and age at first sexual intercourse. The model fitted to compute ORs for a given adjusting covariate is not adjusted for that same covariate.
Models adjusted by country, age group, years of schooling, age at first sexual intercourse, cervical HPV DNA status, and lifetime number of Pap smears until 12 months before enrollment. The model fitted to compute ORs for a given adjusting covariate is not adjusted for that same covariate.
Excludes subjects from Paraguay.
Excludes subjects from India and Paraguay.
Excludes subjects from Paraguay and Brazil.
Excludes subjects from Algeria, Paraguay, and India, in whom serologic testing was not performed.
Serum was tested, but assay results were inconclusive.
Prevalence and Distribution of HPV Types
Of the 167 case patients and 1881 control subjects included in the initial analysis, valid PCR results were obtained for 157 case patients and 1609 control subjects ( Table 2 ). Tumor samples for DNA amplification were unavailable for two case patients and 104 control subjects. For eight case patients and 168 control subjects, the PCR technique amplified neither HPV nor β-globin, and the results were labeled as “inadequate.” Of the 157 case patients with a valid PCR result, specimens from 146 (93%) were positive for HPV DNA. Of the 146 HPV-positive specimens, a single HPV type was detected in 130 (89%). Most of the HPV-positive specimens were infected with a high-risk HPV type or types. None of the adenocarcinoma specimens was infected exclusively with a low-risk type or types. Among the 1609 control women with a valid PCR result, specimens from 266 (16.5%) tested positive for HPV DNA. Of these, 185 (69.5%) were infected with high-risk types, 47 (17.7%) were infected with only low-risk types, and 34 (12.8%) were infected with HPV X.
Human papillomavirus (HPV) DNA prevalence and type distribution by case–control status and odds ratios for cervical adenocarcinoma *
| HPV status and type . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 1881 (100) | 167 (100) | |
| HPV DNA detection status | |||
| HPV DNA negative | 1343 (71.4) | 11 (6.6) | 1.0 (Referent) |
| HPV DNA positive | 266 (14.1) | 146 (87.4) | 81.27 (42.04 to 57.11) |
| Inadequate sample or invalid PCR | 168 (8.9) | 8 (4.8) | 4.44 (1.47 to 13.41) |
| Not tested † | 104 (5.5) | 2 (1.2) | 2.46 (0.51 to 11.76) |
| Infection with a single HPV type ‡ | 223 (13.9) | 133 (84.7) | 87.72 (45.13 to 170.5) |
| 16 | 76 (4.7) | 67 (42.7) | 164.12 (76.09 to 354.0) |
| 18 | 19 (1.2) | 50 (31.8) | 410.32 (167.44 to ∞) |
| 33 | 1 (0.1) | 1 (0.6) | 117.42 (5.89 to ∞) |
| 35 | 6 (0.4) | 2 (1.3) | 47.14 (6.92 to 321.10) |
| 45 | 11 (0.7) | 6 (3.8) | 47.06 (12.79 to 173.2) |
| 51 | 4 (0.2) | 1 (0.6) | 22.36 (2.10 to 238.6) |
| 58 | 7 (0.4) | 1 (0.6) | 18.09 (1.86 to 175.86) |
| 59 | 2 (0.1) | 2 (1.3) | 162.58 (17.70 to ∞) |
| Other single infections § | 63 (3.9) | 0 (0) | 0.01 |
| Infection by HPV X ∥ | 34 (2.1) | 3 (1.9) | 11.83 (2.99 to 46.73) |
| Infection with multiple HPV types | 43 (2.7) | 13 (8.3) | 44.72 (18.02 to 111.0) |
| 16 and 18 | 7 (0.4) | 5 (3.2) | 99.41 (22.03 to 448.6) |
| 16 and other ¶ | 8 (0.5) | 4 (2.5) | 96.75 (20.08 to 466.1) |
| 18 and other # | 3 (0.2) | 2 (1.3) | 99.47 (11.59 to 853.6) |
| Other double infections ** | 17 (1.1) | 2 (1.3) | 14.77 (2.83 to 77.05) |
| >2 infections †† | 8 (0.5) | 0 (0) | 0.02 |
| Infection by any high-risk HPV type | 185 (11.5) | 143 (91.1) | 112.98 (57.09 to 223.6) |
| Infection by low-risk types only | 47 (2.9) | 0 (0) | 0.04 |
| HPV status and type . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 1881 (100) | 167 (100) | |
| HPV DNA detection status | |||
| HPV DNA negative | 1343 (71.4) | 11 (6.6) | 1.0 (Referent) |
| HPV DNA positive | 266 (14.1) | 146 (87.4) | 81.27 (42.04 to 57.11) |
| Inadequate sample or invalid PCR | 168 (8.9) | 8 (4.8) | 4.44 (1.47 to 13.41) |
| Not tested † | 104 (5.5) | 2 (1.2) | 2.46 (0.51 to 11.76) |
| Infection with a single HPV type ‡ | 223 (13.9) | 133 (84.7) | 87.72 (45.13 to 170.5) |
| 16 | 76 (4.7) | 67 (42.7) | 164.12 (76.09 to 354.0) |
| 18 | 19 (1.2) | 50 (31.8) | 410.32 (167.44 to ∞) |
| 33 | 1 (0.1) | 1 (0.6) | 117.42 (5.89 to ∞) |
| 35 | 6 (0.4) | 2 (1.3) | 47.14 (6.92 to 321.10) |
| 45 | 11 (0.7) | 6 (3.8) | 47.06 (12.79 to 173.2) |
| 51 | 4 (0.2) | 1 (0.6) | 22.36 (2.10 to 238.6) |
| 58 | 7 (0.4) | 1 (0.6) | 18.09 (1.86 to 175.86) |
| 59 | 2 (0.1) | 2 (1.3) | 162.58 (17.70 to ∞) |
| Other single infections § | 63 (3.9) | 0 (0) | 0.01 |
| Infection by HPV X ∥ | 34 (2.1) | 3 (1.9) | 11.83 (2.99 to 46.73) |
| Infection with multiple HPV types | 43 (2.7) | 13 (8.3) | 44.72 (18.02 to 111.0) |
| 16 and 18 | 7 (0.4) | 5 (3.2) | 99.41 (22.03 to 448.6) |
| 16 and other ¶ | 8 (0.5) | 4 (2.5) | 96.75 (20.08 to 466.1) |
| 18 and other # | 3 (0.2) | 2 (1.3) | 99.47 (11.59 to 853.6) |
| Other double infections ** | 17 (1.1) | 2 (1.3) | 14.77 (2.83 to 77.05) |
| >2 infections †† | 8 (0.5) | 0 (0) | 0.02 |
| Infection by any high-risk HPV type | 185 (11.5) | 143 (91.1) | 112.98 (57.09 to 223.6) |
| Infection by low-risk types only | 47 (2.9) | 0 (0) | 0.04 |
OR = odds ratio; CI = confidence interval. ORs were computed from logistic regression models that were adjusted for country, age group, years of schooling, age at first sexual intercourse, and lifetime number of Pap smears until 12 months before study entry. For all ORs, the reference group is HPV-negative women.
Samples were not tested because they were not provided or were unsuitable for testing.
All specified HPV types are high risk.
HPV types (No. in control subjects/No. in case patients) are as follows. Low-risk (LR) types: 81 (7/0), 6 (6/0), 70 (6/0), 42 (4/0), 72 (4/0), 11 (3/0), 40 (3/0), 43 (3/0), 54 (3/0), 44 (1/0), 61 (1/0), CP6108 (1/0), 84 (1/0); high-risk (HR) types: 31 (8/0), 56 (6/0), 52 (4/0), 68 (1/0), 73 (1/0).
HPV X denotes unknown HPV type—that is, sample tested positive for HPV DNA by the GP5+/6+ general primer PCR but negative by any of the 33 specific probes considered in the assay.
“Other” HPV types (No. in control subjects/No. in case patients) are as follows: LR types: 42 (3/0); HR types: 33 (1/2), 45 (0/2), 39 (2/0), 31 (1/0), 35 (1/0).
“Other” HPV types (No. in control subjects/No. in case patients) are as follows: HR types: 45 (1/1), 31 (1/0), 52 (1/0), 59 (0/1).
Both HPV types HR (No. in control subjects/No. in case patients): 31 and 35 (1/0), 31 and 51 (1/0), 31 and 59 (0/1), 31 and 68 (1/0), 33 and 35 (1/0), 33 and 45 (1/0), 35 and 59 (0/1), 45 and 58 (1/0), 45 and 59 (1/0), 58 and 82 (1/0), 6 and 31 (1/0); HR and LR types or both types LR: 31 and 42 (1/0), 40 and CP6108 (1/0), 43 and 45 (1/0), 44 and CP6108 (1/0), 45 and 70 (1/0), 45 and 84 (1/0), 70 and 81 (1/0), 83 and 73 (1/0).
HPV types (No. in control subjects/No. in case patients) were: 16, 33, and 58 (1/0); 18, 33, and 35 (1/0); 52, 58, and 68 (1/0); 18, 33, 39, and 58 (1/0); 40, 84, and CP6108 (1/0); 45, 56, and 70 (1/0); 6, 33, and 58 (1/0); 40, 56, 82, 73, and 81(1/0).
Human papillomavirus (HPV) DNA prevalence and type distribution by case–control status and odds ratios for cervical adenocarcinoma *
| HPV status and type . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 1881 (100) | 167 (100) | |
| HPV DNA detection status | |||
| HPV DNA negative | 1343 (71.4) | 11 (6.6) | 1.0 (Referent) |
| HPV DNA positive | 266 (14.1) | 146 (87.4) | 81.27 (42.04 to 57.11) |
| Inadequate sample or invalid PCR | 168 (8.9) | 8 (4.8) | 4.44 (1.47 to 13.41) |
| Not tested † | 104 (5.5) | 2 (1.2) | 2.46 (0.51 to 11.76) |
| Infection with a single HPV type ‡ | 223 (13.9) | 133 (84.7) | 87.72 (45.13 to 170.5) |
| 16 | 76 (4.7) | 67 (42.7) | 164.12 (76.09 to 354.0) |
| 18 | 19 (1.2) | 50 (31.8) | 410.32 (167.44 to ∞) |
| 33 | 1 (0.1) | 1 (0.6) | 117.42 (5.89 to ∞) |
| 35 | 6 (0.4) | 2 (1.3) | 47.14 (6.92 to 321.10) |
| 45 | 11 (0.7) | 6 (3.8) | 47.06 (12.79 to 173.2) |
| 51 | 4 (0.2) | 1 (0.6) | 22.36 (2.10 to 238.6) |
| 58 | 7 (0.4) | 1 (0.6) | 18.09 (1.86 to 175.86) |
| 59 | 2 (0.1) | 2 (1.3) | 162.58 (17.70 to ∞) |
| Other single infections § | 63 (3.9) | 0 (0) | 0.01 |
| Infection by HPV X ∥ | 34 (2.1) | 3 (1.9) | 11.83 (2.99 to 46.73) |
| Infection with multiple HPV types | 43 (2.7) | 13 (8.3) | 44.72 (18.02 to 111.0) |
| 16 and 18 | 7 (0.4) | 5 (3.2) | 99.41 (22.03 to 448.6) |
| 16 and other ¶ | 8 (0.5) | 4 (2.5) | 96.75 (20.08 to 466.1) |
| 18 and other # | 3 (0.2) | 2 (1.3) | 99.47 (11.59 to 853.6) |
| Other double infections ** | 17 (1.1) | 2 (1.3) | 14.77 (2.83 to 77.05) |
| >2 infections †† | 8 (0.5) | 0 (0) | 0.02 |
| Infection by any high-risk HPV type | 185 (11.5) | 143 (91.1) | 112.98 (57.09 to 223.6) |
| Infection by low-risk types only | 47 (2.9) | 0 (0) | 0.04 |
| HPV status and type . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 1881 (100) | 167 (100) | |
| HPV DNA detection status | |||
| HPV DNA negative | 1343 (71.4) | 11 (6.6) | 1.0 (Referent) |
| HPV DNA positive | 266 (14.1) | 146 (87.4) | 81.27 (42.04 to 57.11) |
| Inadequate sample or invalid PCR | 168 (8.9) | 8 (4.8) | 4.44 (1.47 to 13.41) |
| Not tested † | 104 (5.5) | 2 (1.2) | 2.46 (0.51 to 11.76) |
| Infection with a single HPV type ‡ | 223 (13.9) | 133 (84.7) | 87.72 (45.13 to 170.5) |
| 16 | 76 (4.7) | 67 (42.7) | 164.12 (76.09 to 354.0) |
| 18 | 19 (1.2) | 50 (31.8) | 410.32 (167.44 to ∞) |
| 33 | 1 (0.1) | 1 (0.6) | 117.42 (5.89 to ∞) |
| 35 | 6 (0.4) | 2 (1.3) | 47.14 (6.92 to 321.10) |
| 45 | 11 (0.7) | 6 (3.8) | 47.06 (12.79 to 173.2) |
| 51 | 4 (0.2) | 1 (0.6) | 22.36 (2.10 to 238.6) |
| 58 | 7 (0.4) | 1 (0.6) | 18.09 (1.86 to 175.86) |
| 59 | 2 (0.1) | 2 (1.3) | 162.58 (17.70 to ∞) |
| Other single infections § | 63 (3.9) | 0 (0) | 0.01 |
| Infection by HPV X ∥ | 34 (2.1) | 3 (1.9) | 11.83 (2.99 to 46.73) |
| Infection with multiple HPV types | 43 (2.7) | 13 (8.3) | 44.72 (18.02 to 111.0) |
| 16 and 18 | 7 (0.4) | 5 (3.2) | 99.41 (22.03 to 448.6) |
| 16 and other ¶ | 8 (0.5) | 4 (2.5) | 96.75 (20.08 to 466.1) |
| 18 and other # | 3 (0.2) | 2 (1.3) | 99.47 (11.59 to 853.6) |
| Other double infections ** | 17 (1.1) | 2 (1.3) | 14.77 (2.83 to 77.05) |
| >2 infections †† | 8 (0.5) | 0 (0) | 0.02 |
| Infection by any high-risk HPV type | 185 (11.5) | 143 (91.1) | 112.98 (57.09 to 223.6) |
| Infection by low-risk types only | 47 (2.9) | 0 (0) | 0.04 |
OR = odds ratio; CI = confidence interval. ORs were computed from logistic regression models that were adjusted for country, age group, years of schooling, age at first sexual intercourse, and lifetime number of Pap smears until 12 months before study entry. For all ORs, the reference group is HPV-negative women.
Samples were not tested because they were not provided or were unsuitable for testing.
All specified HPV types are high risk.
HPV types (No. in control subjects/No. in case patients) are as follows. Low-risk (LR) types: 81 (7/0), 6 (6/0), 70 (6/0), 42 (4/0), 72 (4/0), 11 (3/0), 40 (3/0), 43 (3/0), 54 (3/0), 44 (1/0), 61 (1/0), CP6108 (1/0), 84 (1/0); high-risk (HR) types: 31 (8/0), 56 (6/0), 52 (4/0), 68 (1/0), 73 (1/0).
HPV X denotes unknown HPV type—that is, sample tested positive for HPV DNA by the GP5+/6+ general primer PCR but negative by any of the 33 specific probes considered in the assay.
“Other” HPV types (No. in control subjects/No. in case patients) are as follows: LR types: 42 (3/0); HR types: 33 (1/2), 45 (0/2), 39 (2/0), 31 (1/0), 35 (1/0).
“Other” HPV types (No. in control subjects/No. in case patients) are as follows: HR types: 45 (1/1), 31 (1/0), 52 (1/0), 59 (0/1).
Both HPV types HR (No. in control subjects/No. in case patients): 31 and 35 (1/0), 31 and 51 (1/0), 31 and 59 (0/1), 31 and 68 (1/0), 33 and 35 (1/0), 33 and 45 (1/0), 35 and 59 (0/1), 45 and 58 (1/0), 45 and 59 (1/0), 58 and 82 (1/0), 6 and 31 (1/0); HR and LR types or both types LR: 31 and 42 (1/0), 40 and CP6108 (1/0), 43 and 45 (1/0), 44 and CP6108 (1/0), 45 and 70 (1/0), 45 and 84 (1/0), 70 and 81 (1/0), 83 and 73 (1/0).
HPV types (No. in control subjects/No. in case patients) were: 16, 33, and 58 (1/0); 18, 33, and 35 (1/0); 52, 58, and 68 (1/0); 18, 33, 39, and 58 (1/0); 40, 84, and CP6108 (1/0); 45, 56, and 70 (1/0); 6, 33, and 58 (1/0); 40, 56, 82, 73, and 81(1/0).
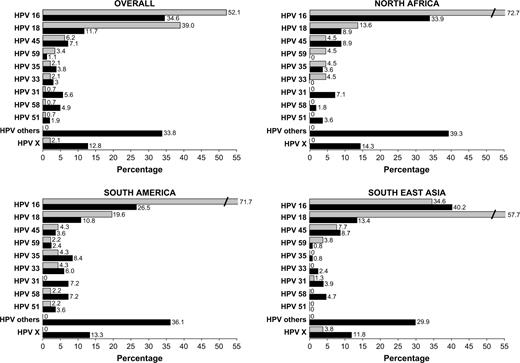
Analysis of the HPV type–specific distribution among HPV-positive participants ( Fig. 1 ) showed that HPV 16 and 18 were by far the most frequently detected HPV types in both case patients and control subjects, followed, in descending order of frequency, by HPV 45, 59, 35, and 33 (among case patients) and by HPV 45, 31, 58, 35 and 33 (among control subjects). When the distribution of HPV types was analyzed by the three world regions represented by the countries in the study, the HPV type distributions in North Africa and South America were similar to one another. By contrast, in Southeast Asia HPV 18 was the predominant HPV type among case patients, although HPV 16 was again the most frequently detected HPV type among control subjects. In every world region, a high percentage of control subjects but not case patients were infected by several other HPV types ( Fig. 1 ). For example, HPV 31 was detected in 5.6% of HPV-positive control women overall but was extremely rare among HPV-positive case patients (in only one patient in Southeast Asia). Similarly, HPV 58 was detected in 4.9% of HPV-positive control subjects overall but in only one case patient, from South America. HPV 51 was detected in no case patients or control subjects from Southeast Asia.
Human papillomavirus (HPV) genotype distribution among HPV-positive case patients and control subjects, overall and by world region. For each region, percentages were computed by dividing the number of women infected with a given HPV type (singly or simultaneously with other types) by the total number of HPV-positive women. Because women infected with multiple types contribute multiple times in the numerator but only once in the denominator, the percentage totals exceed 100. Shaded bars = HPV genotype distribution in case patients with cervical adenocarcinoma; solid bars = HPV genotype distribution in control subjects. “HPV others” are as follows, with HPV genotype (No. of control subjects/No. of case patients) in descending order of frequency: Overall: HPV 70 (9/0), 81 (9/0), 6 (8/0), 42 (8/0), 56 (8/0), 40 (6/0), 52 (6/0), 43 (4/0), 72 (4/0), CP6108 (4/0), 11 (3/0), 39 (3/0), 54 (3/0), 68 (3/0), 73 (3/0), 84 (3/0), 44 (2/0), 82 (2/0), 61 (1/0), 83 (1/0); North Africa: 42 (3/0), 72 (3/0), 6 (2/0), 43 (2/0), 56 (2/0), 70 (2/0), 73 (2/0), 39 (1/0), 52 (1/0), 68 (1/0), 81 (1/0), 83 (1/0), 84 (1/0); South America: HPV 56 (4/0), 6 (3/0), 52 (3/0), 70 (3/0), 40 (2/0), 42 (2/0), 54 (2/0), 68 (2/0), 81 (2/0), 11 (1/0), 39 (1/0), 43 (1/0), 44 (1/0), 61 (1/0), 84 (1/0), CP6108 (1/0); Southeast Asia: HPV 81 (6/0), 40 (4/0), 70 (4/0), 6 (3/0), 42 (3/0), CP6108 (3/0), 11 (2/0), 52 (2/0), 56 (2/0), 82 (2/0), 39 (1/0), 43 (1/0), 44 (1/0), 54 (1/0), 72 (1/0), 73 (1/0), 84 (1/0). HPV X denotes unknown HPV type—that is, sample tested positive for HPV DNA by the GP5+/6+ general primer PCR but negative by any of the 33 specific probes considered in the assay.
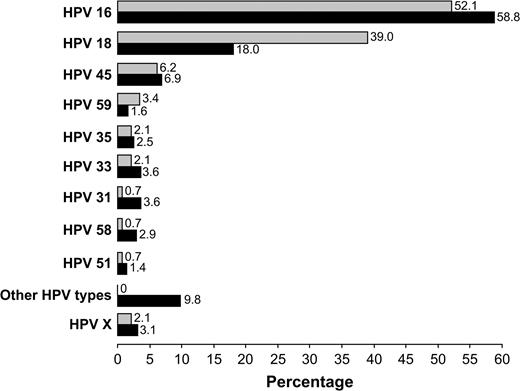
No statistically significant differences in HPV type–specific distributions were noted between adenocarcinoma and adenosquamous carcinoma (data not shown). HPV 16 was detected in specimens from 47% and 43% of adenocarcinoma and adenosquamous carcinoma patients, respectively. Corresponding percentages for HPV 18 were 34% and 35%.
We performed an analysis stratified by age to explore the age dependency of detection of the most frequent HPV types. Among HPV-positive case patients, HPV 16 was detected slightly more frequently in women older than 50 years than in women aged 50 years or younger (62% and 46%, respectively; P = .06). By contrast, HPV 18 was more frequently detected in younger case patients than in older case patients (46% and 26%, respectively; P = .02). No differences by age were noted for other HPV types or among control women (data not shown).
Risk of Adenocarcinoma Associated With HPV Types
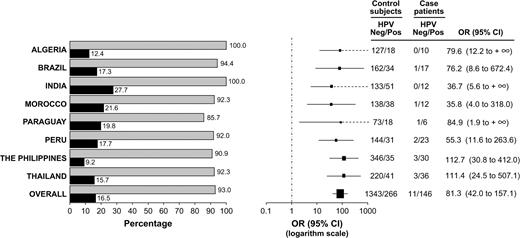
HPV prevalence and associated odds ratios for cervical adenocarcinoma were analyzed by country ( Fig. 2 ). As noted above, HPV DNA was detected in cervical samples of 93% of the case patients and 16.5% of the control subjects for whom valid PCR results were available. Among case patients, the prevalence of HPV DNA ranged from 86% in Paraguay to 100% in Algeria and India. Among control subjects, HPV prevalence ranged from 9% in the Philippines to 28% in India. The presence of HPV DNA was associated with an 81-fold increase in the risk of cervical adenocarcinoma in all countries combined. In analyses by country, the increase ranged from 36-fold in Morocco to 113-fold in the Philippines.
Prevalence of human papillomavirus (HPV) DNA by country and case–control status, with odds ratios for the association between HPV DNA status and cervical adenocarcinoma. Odds ratios were adjusted by age group, years of schooling, age at first sexual intercourse, and number of Pap smears before 12 months before enrollment. Left ) HPV DNA prevalence by country. Shaded bars = HPV prevalence among case patients with cervical adenocarcinoma; solid bars = HPV prevalence among control subjects. Right ) Odds ratios (OR; solid squares ) for cervical adenocarcinoma, with 95% confidence intervals (CIs; bars ). Size of each square is proportional to the number of subjects included in the estimation of that odds ratio. Dashed lines indicate that the upper limit of the CI is infinity and therefore cannot be represented graphically.
Analysis of the risk of developing adenocarcinoma associated with specific HPV types ( Table 2 ) showed that the highest risks were associated with HPV 18 (OR = 410), 16 (OR = 164), 59 (OR = 163), and 33 (OR = 117). Other HPV types that were statistically significantly and strongly associated with adenocarcinoma included HPV 35, 45, 51, and 58. The excess risk in women infected with multiple HPV types (OR = 45) was somewhat lower than that among those infected with single HPV types (OR = 88), but the difference was not statistically significant.
The risk of adenocarcinoma associated with any HPV infection did not vary with histologic subtype (for adenocarcinoma, OR = 71 [95% CI = 34 to 151], and for adenosquamous carcinoma, OR = 89 [95% CI = 27 to 292]). Similarly, the risk of adenocarcinoma associated with single HPV types did not differ statistically significantly by histologic subtype. Fully adjusted odds ratios for adenocarcinoma and adenosquamous carcinoma were as follows: 149 [95% CI = 65 to 346] and 177 [95% CI = 49 to 644] for HPV 16; 334 [95% CI = 129 to 867] and 585 [95% CI = 145 to infinity] for HPV 18; 28 [95% CI = 3 to 279] and 52 [95% CI = 4 to 669] for HPV 35; and 76 [95% CI = 20 to 293] and 34 [95% CI = 3 to 380] for HPV 45.
Associations With Cofactors
Multivariable analyses were carried out among HPV DNA–positive women (any type) to investigate the associations between selected cofactors and cervical adenocarcinoma risk ( Table 3 ). The risk of cervical adenocarcinoma among HPV-positive women was statistically significantly increased in women with no schooling, younger age at first intercourse, a large number of sexual partners, a history of sexually transmitted disease, a regular partner with a history of sexually transmitted disease, and a history of practicing anal intercourse. The risk of adenocarcinoma increased with increasing number of lifetime pregnancies ( Ptrend = .02) and with increasing years of having used hormonal contraception ( Ptrend = .009). Both IUD use and a high frequency of baths or showers showed a statistically significant inverse association with adenocarcinoma risk. There was marginal evidence of a reduced risk of adenocarcinoma with increasing number of previous Pap smears ( Ptrend = .07), but for all categories the odds ratios were not statistically significantly different from that of the reference group (no previous Pap smears). No statistically significant associations were found with status, intensity, or duration of cigarette smoking (data not shown). Finally, HSV-2 seropositivity was associated with a more than twofold increase in the risk of cervical adenocarcinoma ( Table 3 ), but C. trachomatis seropositivity was not associated with adenocarcinoma risk. Further simultaneous adjustment in the multivariable model for other covariates, including number of pregnancies, history of sexually transmitted disease, number of baths or showers per week, and ever IUD use, did not substantially alter the magnitude of the point estimates of the odds ratios (data not shown). No statistically significant differences were noted on the effect of the main hypothesized cofactors (i.e., history of sexually transmitted disease, history of tobacco smoking, number of pregnancies, history of oral contraceptive use, ever use of the IUD, number of baths or showers per week, HSV-2 or C. trachomatis seropositivity, and history of Pap smear testing) by histologic subtype (data not shown).
Odds ratios for cervical adenocarcinoma associated with selected cofactors among human papillomavirus (HPV)–positive case patients and control subjects *
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 266 (100) | 146 (100) | |
| Years of schooling | |||
| None | 72 (27.2) | 53 (36.3) | 1.00 (Referent) |
| 1–4 | 66 (24.9) | 40 (27.4) | 0.65 (0.32 to 1.30) |
| 5–9 | 62 (23.4) | 33 (22.6) | 0.43 (0.20 to 0.92) |
| ≥10 | 65 (27.5) | 20 (13.7) | 0.30 (0.12 to 0.72) |
| Unknown | 1 | 0 | |
| Ptrend | 0.005 | ||
| Age at first sexual intercourse, y | |||
| ≥23 | 61 (23.1) | 14 (9.7) | 1.00 (Referent) |
| 20–22 | 53 (20 1) | 28 (19.3) | 2.20 (0.99 to 4.93) |
| 18–19 | 47 (17.8) | 36 (24.8) | 3.80 (1.69 to 8.57) |
| ≤17 | 103 (39.0) | 67 (46.2) | 2.94 (1.36 to 6.37) |
| Unknown | 2 | 1 | |
| Ptrend | 0.01 | ||
| Lifetime No. of sexual partners | |||
| 1 | 195 (74.7) | 85 (58.6) | 1.00 (Referent) |
| 2–3 | 54 (20.7) | 50 (34.5) | 1.66 (0.96 to 2.88) |
| ≥4 | 12 (4.6) | 10 (6.9) | 1.59 (0.53 to 4.76) |
| Unknown | 5 | 1 | |
| Ptrend | 0.1 | ||
| Anal intercourse † | |||
| Never | 227 (93.0) | 116 (82.9) | 1.00 (Referent) |
| Rarely | 7 (2.9) | 10 (7.1) | 3.96 (1.23 to 12.77) |
| Occasionally/often | 10 (4.1) | 14 (10.0) | 3.59 (1.26 to 10.20) |
| Unknown | 4 | 0 | |
| Ptrend | 0.005 | ||
| History of STD | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 2.32 (1.16 to 4.61) |
| Unknown | 23 | 16 | |
| STD in regular partner ‡ | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 1.94 (0.95 to 3.93) |
| Unknown | 25 | 25 | |
| Lifetime No. of pregnancies | |||
| 1–2 | 55 (22.0) | 19 (13.2) | 1.00 (Referent) |
| 3–5 | 109 (43.6) | 46 (31.9) | 1.25 (0.62 to 2.52) |
| 6–7 | 43 (17.2) | 32 (22.2) | 1.77 (0.78 to 4.02) |
| ≥8 | 43 (17.2) | 47 (32.6) | 2.44 (1.06 to 5.62) |
| Unknown | 2 | 0 | |
| Ptrend | 0.02 | ||
| Years of use of hormonal contraception | |||
| <2 | 30 (40.0) | 15 (30.6) | 1.00 (Referent) |
| 2–4 | 33 (44.0) | 15 (30.6) | 1.30 (0.46 to 3.68) |
| ≥5 | 12 (16.0) | 19 (38.8) | 4.71 (1.47 to 15.07) |
| Unknown | 11 | 4 | |
| Ptrend | 0.009 | ||
| Use of IUD § | |||
| Never | 124 (77.5) | 111 (90.2) | 1.00 (Referent) |
| Ever | 36 (22.5) | 12 (9.76) | 0.41 (0.18 to 0.93) |
| Unknown | 54 | 0 | |
| Lifetime No. of Pap smears before last 12 mo | |||
| None | 153 (61.0) | 102 (72.3) | 1.00 (Referent) |
| 1 | 39 (15.5) | 17 (12.1) | 0.58 (0.29 to 1.18) |
| 2–5 | 38 (15.1) | 14 (9.9) | 0.49 (0.22 to 1.10) |
| ≥6 | 21 (8.4) | 8 (5.7) | 0.58 (0.22 to 1.55) |
| Unknown | 15 | 5 | |
| Ptrend | 0.07 | ||
| No. of baths or showers per week † | |||
| 1–5 | 86 (35.0) | 65 (46.4) | 1.00 (Referent) |
| 6–8 | 111 (45.1) | 39 (27.9) | 0.15 (0.06 to 0.36) |
| ≥9 | 49 (19.9) | 36 (25.7) | 0.05 (0.01 to 0.35) |
| Unknown | 2 | 0 | |
| Ptrend | <0.001 | ||
| HSV-2 serostatus ∥ | |||
| Negative | 100 (70.9) | 52 (54.2) | 1.00 (Referent) |
| Positive | 39 (27.7) | 43 (44.8) | 2.63 (1.30 to 5.29) |
| Inadequate ¶ | 2 (1.4) | 1 (1.0) | |
| Not tested | 38 | 22 |
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 266 (100) | 146 (100) | |
| Years of schooling | |||
| None | 72 (27.2) | 53 (36.3) | 1.00 (Referent) |
| 1–4 | 66 (24.9) | 40 (27.4) | 0.65 (0.32 to 1.30) |
| 5–9 | 62 (23.4) | 33 (22.6) | 0.43 (0.20 to 0.92) |
| ≥10 | 65 (27.5) | 20 (13.7) | 0.30 (0.12 to 0.72) |
| Unknown | 1 | 0 | |
| Ptrend | 0.005 | ||
| Age at first sexual intercourse, y | |||
| ≥23 | 61 (23.1) | 14 (9.7) | 1.00 (Referent) |
| 20–22 | 53 (20 1) | 28 (19.3) | 2.20 (0.99 to 4.93) |
| 18–19 | 47 (17.8) | 36 (24.8) | 3.80 (1.69 to 8.57) |
| ≤17 | 103 (39.0) | 67 (46.2) | 2.94 (1.36 to 6.37) |
| Unknown | 2 | 1 | |
| Ptrend | 0.01 | ||
| Lifetime No. of sexual partners | |||
| 1 | 195 (74.7) | 85 (58.6) | 1.00 (Referent) |
| 2–3 | 54 (20.7) | 50 (34.5) | 1.66 (0.96 to 2.88) |
| ≥4 | 12 (4.6) | 10 (6.9) | 1.59 (0.53 to 4.76) |
| Unknown | 5 | 1 | |
| Ptrend | 0.1 | ||
| Anal intercourse † | |||
| Never | 227 (93.0) | 116 (82.9) | 1.00 (Referent) |
| Rarely | 7 (2.9) | 10 (7.1) | 3.96 (1.23 to 12.77) |
| Occasionally/often | 10 (4.1) | 14 (10.0) | 3.59 (1.26 to 10.20) |
| Unknown | 4 | 0 | |
| Ptrend | 0.005 | ||
| History of STD | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 2.32 (1.16 to 4.61) |
| Unknown | 23 | 16 | |
| STD in regular partner ‡ | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 1.94 (0.95 to 3.93) |
| Unknown | 25 | 25 | |
| Lifetime No. of pregnancies | |||
| 1–2 | 55 (22.0) | 19 (13.2) | 1.00 (Referent) |
| 3–5 | 109 (43.6) | 46 (31.9) | 1.25 (0.62 to 2.52) |
| 6–7 | 43 (17.2) | 32 (22.2) | 1.77 (0.78 to 4.02) |
| ≥8 | 43 (17.2) | 47 (32.6) | 2.44 (1.06 to 5.62) |
| Unknown | 2 | 0 | |
| Ptrend | 0.02 | ||
| Years of use of hormonal contraception | |||
| <2 | 30 (40.0) | 15 (30.6) | 1.00 (Referent) |
| 2–4 | 33 (44.0) | 15 (30.6) | 1.30 (0.46 to 3.68) |
| ≥5 | 12 (16.0) | 19 (38.8) | 4.71 (1.47 to 15.07) |
| Unknown | 11 | 4 | |
| Ptrend | 0.009 | ||
| Use of IUD § | |||
| Never | 124 (77.5) | 111 (90.2) | 1.00 (Referent) |
| Ever | 36 (22.5) | 12 (9.76) | 0.41 (0.18 to 0.93) |
| Unknown | 54 | 0 | |
| Lifetime No. of Pap smears before last 12 mo | |||
| None | 153 (61.0) | 102 (72.3) | 1.00 (Referent) |
| 1 | 39 (15.5) | 17 (12.1) | 0.58 (0.29 to 1.18) |
| 2–5 | 38 (15.1) | 14 (9.9) | 0.49 (0.22 to 1.10) |
| ≥6 | 21 (8.4) | 8 (5.7) | 0.58 (0.22 to 1.55) |
| Unknown | 15 | 5 | |
| Ptrend | 0.07 | ||
| No. of baths or showers per week † | |||
| 1–5 | 86 (35.0) | 65 (46.4) | 1.00 (Referent) |
| 6–8 | 111 (45.1) | 39 (27.9) | 0.15 (0.06 to 0.36) |
| ≥9 | 49 (19.9) | 36 (25.7) | 0.05 (0.01 to 0.35) |
| Unknown | 2 | 0 | |
| Ptrend | <0.001 | ||
| HSV-2 serostatus ∥ | |||
| Negative | 100 (70.9) | 52 (54.2) | 1.00 (Referent) |
| Positive | 39 (27.7) | 43 (44.8) | 2.63 (1.30 to 5.29) |
| Inadequate ¶ | 2 (1.4) | 1 (1.0) | |
| Not tested | 38 | 22 |
OR = odds ratio; CI = confidence interval; STD = sexually transmitted disease; IUD = intrauterine device; HSV = herpes simplex virus. ORs are from logistic regression models adjusted four country, age group, years of schooling, age at first sexual intercourse, and lifetime number of Pap smears until 12 months before enrollment. The model fitted to compute ORs for a given adjusting covariate is not adjusted for that same covariate.
Excludes subjects from Paraguay.
Excludes subjects from India and Paraguay.
Excludes subjects from Paraguay and Brazil.
Excludes subjects from Algeria, Paraguay, and India in whom serological testing was not performed.
Serum was tested but assay results were inconclusive.
Odds ratios for cervical adenocarcinoma associated with selected cofactors among human papillomavirus (HPV)–positive case patients and control subjects *
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 266 (100) | 146 (100) | |
| Years of schooling | |||
| None | 72 (27.2) | 53 (36.3) | 1.00 (Referent) |
| 1–4 | 66 (24.9) | 40 (27.4) | 0.65 (0.32 to 1.30) |
| 5–9 | 62 (23.4) | 33 (22.6) | 0.43 (0.20 to 0.92) |
| ≥10 | 65 (27.5) | 20 (13.7) | 0.30 (0.12 to 0.72) |
| Unknown | 1 | 0 | |
| Ptrend | 0.005 | ||
| Age at first sexual intercourse, y | |||
| ≥23 | 61 (23.1) | 14 (9.7) | 1.00 (Referent) |
| 20–22 | 53 (20 1) | 28 (19.3) | 2.20 (0.99 to 4.93) |
| 18–19 | 47 (17.8) | 36 (24.8) | 3.80 (1.69 to 8.57) |
| ≤17 | 103 (39.0) | 67 (46.2) | 2.94 (1.36 to 6.37) |
| Unknown | 2 | 1 | |
| Ptrend | 0.01 | ||
| Lifetime No. of sexual partners | |||
| 1 | 195 (74.7) | 85 (58.6) | 1.00 (Referent) |
| 2–3 | 54 (20.7) | 50 (34.5) | 1.66 (0.96 to 2.88) |
| ≥4 | 12 (4.6) | 10 (6.9) | 1.59 (0.53 to 4.76) |
| Unknown | 5 | 1 | |
| Ptrend | 0.1 | ||
| Anal intercourse † | |||
| Never | 227 (93.0) | 116 (82.9) | 1.00 (Referent) |
| Rarely | 7 (2.9) | 10 (7.1) | 3.96 (1.23 to 12.77) |
| Occasionally/often | 10 (4.1) | 14 (10.0) | 3.59 (1.26 to 10.20) |
| Unknown | 4 | 0 | |
| Ptrend | 0.005 | ||
| History of STD | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 2.32 (1.16 to 4.61) |
| Unknown | 23 | 16 | |
| STD in regular partner ‡ | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 1.94 (0.95 to 3.93) |
| Unknown | 25 | 25 | |
| Lifetime No. of pregnancies | |||
| 1–2 | 55 (22.0) | 19 (13.2) | 1.00 (Referent) |
| 3–5 | 109 (43.6) | 46 (31.9) | 1.25 (0.62 to 2.52) |
| 6–7 | 43 (17.2) | 32 (22.2) | 1.77 (0.78 to 4.02) |
| ≥8 | 43 (17.2) | 47 (32.6) | 2.44 (1.06 to 5.62) |
| Unknown | 2 | 0 | |
| Ptrend | 0.02 | ||
| Years of use of hormonal contraception | |||
| <2 | 30 (40.0) | 15 (30.6) | 1.00 (Referent) |
| 2–4 | 33 (44.0) | 15 (30.6) | 1.30 (0.46 to 3.68) |
| ≥5 | 12 (16.0) | 19 (38.8) | 4.71 (1.47 to 15.07) |
| Unknown | 11 | 4 | |
| Ptrend | 0.009 | ||
| Use of IUD § | |||
| Never | 124 (77.5) | 111 (90.2) | 1.00 (Referent) |
| Ever | 36 (22.5) | 12 (9.76) | 0.41 (0.18 to 0.93) |
| Unknown | 54 | 0 | |
| Lifetime No. of Pap smears before last 12 mo | |||
| None | 153 (61.0) | 102 (72.3) | 1.00 (Referent) |
| 1 | 39 (15.5) | 17 (12.1) | 0.58 (0.29 to 1.18) |
| 2–5 | 38 (15.1) | 14 (9.9) | 0.49 (0.22 to 1.10) |
| ≥6 | 21 (8.4) | 8 (5.7) | 0.58 (0.22 to 1.55) |
| Unknown | 15 | 5 | |
| Ptrend | 0.07 | ||
| No. of baths or showers per week † | |||
| 1–5 | 86 (35.0) | 65 (46.4) | 1.00 (Referent) |
| 6–8 | 111 (45.1) | 39 (27.9) | 0.15 (0.06 to 0.36) |
| ≥9 | 49 (19.9) | 36 (25.7) | 0.05 (0.01 to 0.35) |
| Unknown | 2 | 0 | |
| Ptrend | <0.001 | ||
| HSV-2 serostatus ∥ | |||
| Negative | 100 (70.9) | 52 (54.2) | 1.00 (Referent) |
| Positive | 39 (27.7) | 43 (44.8) | 2.63 (1.30 to 5.29) |
| Inadequate ¶ | 2 (1.4) | 1 (1.0) | |
| Not tested | 38 | 22 |
| Characteristic . | Control subjects, No. (%) . | Case patients, No. (%) . | OR (95% CI) . |
|---|---|---|---|
| Total | 266 (100) | 146 (100) | |
| Years of schooling | |||
| None | 72 (27.2) | 53 (36.3) | 1.00 (Referent) |
| 1–4 | 66 (24.9) | 40 (27.4) | 0.65 (0.32 to 1.30) |
| 5–9 | 62 (23.4) | 33 (22.6) | 0.43 (0.20 to 0.92) |
| ≥10 | 65 (27.5) | 20 (13.7) | 0.30 (0.12 to 0.72) |
| Unknown | 1 | 0 | |
| Ptrend | 0.005 | ||
| Age at first sexual intercourse, y | |||
| ≥23 | 61 (23.1) | 14 (9.7) | 1.00 (Referent) |
| 20–22 | 53 (20 1) | 28 (19.3) | 2.20 (0.99 to 4.93) |
| 18–19 | 47 (17.8) | 36 (24.8) | 3.80 (1.69 to 8.57) |
| ≤17 | 103 (39.0) | 67 (46.2) | 2.94 (1.36 to 6.37) |
| Unknown | 2 | 1 | |
| Ptrend | 0.01 | ||
| Lifetime No. of sexual partners | |||
| 1 | 195 (74.7) | 85 (58.6) | 1.00 (Referent) |
| 2–3 | 54 (20.7) | 50 (34.5) | 1.66 (0.96 to 2.88) |
| ≥4 | 12 (4.6) | 10 (6.9) | 1.59 (0.53 to 4.76) |
| Unknown | 5 | 1 | |
| Ptrend | 0.1 | ||
| Anal intercourse † | |||
| Never | 227 (93.0) | 116 (82.9) | 1.00 (Referent) |
| Rarely | 7 (2.9) | 10 (7.1) | 3.96 (1.23 to 12.77) |
| Occasionally/often | 10 (4.1) | 14 (10.0) | 3.59 (1.26 to 10.20) |
| Unknown | 4 | 0 | |
| Ptrend | 0.005 | ||
| History of STD | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 2.32 (1.16 to 4.61) |
| Unknown | 23 | 16 | |
| STD in regular partner ‡ | |||
| Never | 136 (79.1) | 71 (68.9) | 1.00 (Referent) |
| Ever | 36 (20.9) | 32 (31.1) | 1.94 (0.95 to 3.93) |
| Unknown | 25 | 25 | |
| Lifetime No. of pregnancies | |||
| 1–2 | 55 (22.0) | 19 (13.2) | 1.00 (Referent) |
| 3–5 | 109 (43.6) | 46 (31.9) | 1.25 (0.62 to 2.52) |
| 6–7 | 43 (17.2) | 32 (22.2) | 1.77 (0.78 to 4.02) |
| ≥8 | 43 (17.2) | 47 (32.6) | 2.44 (1.06 to 5.62) |
| Unknown | 2 | 0 | |
| Ptrend | 0.02 | ||
| Years of use of hormonal contraception | |||
| <2 | 30 (40.0) | 15 (30.6) | 1.00 (Referent) |
| 2–4 | 33 (44.0) | 15 (30.6) | 1.30 (0.46 to 3.68) |
| ≥5 | 12 (16.0) | 19 (38.8) | 4.71 (1.47 to 15.07) |
| Unknown | 11 | 4 | |
| Ptrend | 0.009 | ||
| Use of IUD § | |||
| Never | 124 (77.5) | 111 (90.2) | 1.00 (Referent) |
| Ever | 36 (22.5) | 12 (9.76) | 0.41 (0.18 to 0.93) |
| Unknown | 54 | 0 | |
| Lifetime No. of Pap smears before last 12 mo | |||
| None | 153 (61.0) | 102 (72.3) | 1.00 (Referent) |
| 1 | 39 (15.5) | 17 (12.1) | 0.58 (0.29 to 1.18) |
| 2–5 | 38 (15.1) | 14 (9.9) | 0.49 (0.22 to 1.10) |
| ≥6 | 21 (8.4) | 8 (5.7) | 0.58 (0.22 to 1.55) |
| Unknown | 15 | 5 | |
| Ptrend | 0.07 | ||
| No. of baths or showers per week † | |||
| 1–5 | 86 (35.0) | 65 (46.4) | 1.00 (Referent) |
| 6–8 | 111 (45.1) | 39 (27.9) | 0.15 (0.06 to 0.36) |
| ≥9 | 49 (19.9) | 36 (25.7) | 0.05 (0.01 to 0.35) |
| Unknown | 2 | 0 | |
| Ptrend | <0.001 | ||
| HSV-2 serostatus ∥ | |||
| Negative | 100 (70.9) | 52 (54.2) | 1.00 (Referent) |
| Positive | 39 (27.7) | 43 (44.8) | 2.63 (1.30 to 5.29) |
| Inadequate ¶ | 2 (1.4) | 1 (1.0) | |
| Not tested | 38 | 22 |
OR = odds ratio; CI = confidence interval; STD = sexually transmitted disease; IUD = intrauterine device; HSV = herpes simplex virus. ORs are from logistic regression models adjusted four country, age group, years of schooling, age at first sexual intercourse, and lifetime number of Pap smears until 12 months before enrollment. The model fitted to compute ORs for a given adjusting covariate is not adjusted for that same covariate.
Excludes subjects from Paraguay.
Excludes subjects from India and Paraguay.
Excludes subjects from Paraguay and Brazil.
Excludes subjects from Algeria, Paraguay, and India in whom serological testing was not performed.
Serum was tested but assay results were inconclusive.
D ISCUSSION
In this report we have presented results of a pooled analysis of data on the association of HPV infection and potential cofactors with the risk of cervical adenocarcinoma from eight case–control studies conducted on three continents: North Africa, South America, and Southeast Asia. Our results strongly indicate that HPV appears to be the most important risk factor for cervical adenocarcinoma because infection of cervical cells with HPV was associated with an 80-fold increase in the risk of cervical adenocarcinoma. Our results also indicate that, in HPV-positive women, never schooling, poor hygiene, long-term use of hormonal contraceptives, indicators of sexual promiscuity, HSV-2 seropositivity, and, to a lessor extent, very high parity, were all associated with an increased risk of developing cervical adenocarcinoma, whereas IUD use was associated with a decreased risk.
HPV Prevalence and Type Distribution
Our detection rates of HPV DNA in patients support the hypothesis that HPV plays a central role in the etiology of cervical adenocarcinoma. In previous reports, HPV prevalence in cervical adenocarcinomas was more variable and generally lower than that reported for squamous cell carcinoma ( 37 – 42 ) . The differences between our findings and those of previous studies may reflect technical factors related to sampling and DNA detection or histologic misclassification of true endometrial adenocarcinomas in the earlier studies. Support for the latter possibility comes from a recent comprehensive analysis of a series of adenocarcinomas localized to the cervix ( 43 ) , in which adenocarcinomas of the cervix were differentiated from adenocarcinomas of the endometrium not only by histologic characteristics but also by immunohistochemical staining for p53 and p16INK4a, a potential marker of HPV E7 function, suggesting that incorporation of HPV testing increases diagnostic accuracy. Also, when sensitive HPV DNA detection assays are used, as in this study, HPV prevalence in adenocarcinomas and in squamous cell carcinomas overlaps, suggesting that HPV is central to the carcinogenesis of both histologic types of cervical cancer.
The HPV type distribution in cervical adenocarcinoma using data from the eight IARC case–control studies included in this analysis varies slightly from that in squamous cell cervical carcinoma cases from the same studies ( 44 ) ( Fig. 3 ). The two predominant HPV types in both histologies are HPV 16 and 18, followed by HPV 45 and distantly by HPV 59, 35, 33, 31, 58 and 51. This distribution is generally consistent with what has been published in the literature for both histologies, although in a few studies ( 40 , 43 ) , HPV 18 predominates over HPV 16. The comparison in Fig. 3 also shows that the prevalence of HPV 18 in adenocarcinomas (39%) is statistically significantly greater ( P <.001) than that in squamous cell carcinoma (18%). Nevertheless, the prevalence of HPV 18 exceeded that of HPV 16 only in case patients from Southeast Asia. HPV 16 was still the most frequent HPV type in case patients and control subjects from North Africa and South America and control subjects from Southeast Asia.
Comparison of human papillomavirus (HPV) type–specific distributions in adenocarcinomas and in squamous cell carcinomas in the eight combined IARC multicenter case–control studies of cervical cancer that include both cervical adenocarcinoma and squamous cell carcinoma. Percentages were computed by dividing the number of women infected with a given HPV type (singly or simultaneously with other types) by the total number of HPV-positive women. Because women infected with multiple types contribute multiple times in the numerator but only once in the denominator, percentage totals exceed 100. Shaded bars = HPV prevalence in patients with cervical adenocarcinoma; solid bars = HPV prevalence in patients with squamous cell carcinoma. Data for patients with squamous cell carcinoma are from a subset of the studies in Munoz et al. ( 44 ) . Overall HPV prevalence among patients with cervical adenocarcinoma was 93.0%. Overall HPV prevalence among patients with cervical squamous cell carcinoma was 96.2%. “Other HPV types” are HPV types other than HPV 16, 18, 45, 59, 35, 33, 31, 58 and 51. “HPV X” denotes unknown HPV type—that is, sample tested positive for HPV DNA by the GP5+/6+ general primer PCR but negative by any of the 33 specific probes considered in the assay.
The cumulative proportion of HPV types indicates that five types (HPV 16, 18, 45, 59, and 35) were present in 96% of the adenocarcinoma cases. An equivalent number of types (HPV 16, 18, 45, 31, and 33) accounted for a slightly smaller proportion (88%) of the HPV types in squamous cell cancers. From these eight IARC case–control studies we estimate that the attributable fraction for adenocarcinoma linked to HPV is 93%, close to that for squamous cell carcinoma (96%).The HPV type distribution of main types within each world region was largely consistent across studies. However, we cannot rule out small geographic differences for the other, less frequent genotypes, because the number of cases contributed by each participating center was small.
HPV and Adenocarcinoma Risk
This multicenter study demonstrates the existence of a consistent, strong, and robust increased association between infection by high-risk HPV types and risk of development of adenocarcinoma. These results confirm previous findings of strong associations from several smaller case–control studies of nonsquamous cervical cancer ( 10 , 40 , 42 , 43 ) . In addition to HPV types 16 and 18, we were able to estimate odds ratios for adenocarcinoma linked to six other HPV types, demonstrating very strong associations (OR > 100) for HPV 59 and 33, and strong associations (OR > 18) for HPV 35, 45, 51, and 58. Because no adenocarcinoma case patients were infected with types 39, 52, 56, 68, 73, or 82, and only one patient was infected with HPV 31, these types—although classified as high-risk types in our previous study of squamous cell carcinoma ( 30 ) —could not be confirmed as high-risk types for adenocarcinoma. The study also confirms the absence in adenocarcinoma of all the types classified as low risk, as was also found for squamous cell carcinoma. Indeed, all single infections with low-risk types (i.e., HPV 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, 84, and CP6108) detected in this study were observed in control women, and no single infections with low-risk types were detected among adenocarcinoma case patients.
Cofactors for Cervical Adenocarcinoma
This study indicates that hormonal factors, both endogenous (i.e., parity) and exogenous (i.e., use of hormonal contraceptives), are cofactors in the pathogenesis of cervical adenocarcinoma. Previous studies had shown the association of hormonal cofactors with squamous cell cervical carcinoma ( 9 – 11 , 13 , 15 , 16 , 45 ) , but the evidence for adenocarcinoma was limited. Our estimate of the relative risk for cervical adenocarcinoma linked to use of hormonal contraception is substantially higher than that previously reported in a recent systematic review ( 46 ) and adds further evidence to the view that prolonged hormonal contraceptive use in HPV-positive women may indeed also increase the risk of developing adenocarcinoma. We found an overall trend of increasing number of pregnancies with adenocarcinoma risk among HPV positive women, but only the odds ratio for eight or more pregnancies was actually statistically significant ( Table 3 ). These findings are consistent with a previous report ( 13 ) but not with another one ( 10 ) , which showed an inverse association. The evidence involving parity as a cofactor for cervical adenocarcinoma is thus weaker and less consistent than what is observed for squamous cell carcinoma. The reason for the difference is not clear. One possibility is that cervical adenocarcinomas may represent a histological entity that shares risk factors related to endometrial cancer, in which high parity is inversely associated with risk, and risk factors linked to cervical squamous cell carcinoma, in which parity is associated with an increased risk.
We found no association between smoking and adenocarcinoma risk. Because the prevalence of smoking in our study populations was relatively low, however, our power to detect an association was limited, and we cannot rule out the possibility of a moderate effect. Our previous pooled analysis of smoking and cervical cancer showed an increased risk of squamous cell carcinoma, but not of adenocarcinoma, in smokers ( 21 ) . Although several previous studies of adenocarcinomas that did not take HPV into account ( 47 – 51 ) found no association with smoking, a multicenter case–control study that controlled for HPV found that smoking was inversely related to cervical adenocarcinoma risk ( 12 ) . A recent meta-analysis of data from six studies ( 14 ) also showed no association between smoking and adenocarcinoma risk.
An interesting finding of our study was the inverse relationship between IUD use and risk of adenocarcinoma. IUD use has been consistently inversely associated with risk of endometrial cancer ( 52 – 59 ) , but only a few reports have investigated the association between IUD use and cervical cancer ( 60 – 63 ) . The inverse associations were much less consistent in these latter studies, and none of them took into account the central role of HPV in the development of cervical cancer. The inverse association that we observed between IUD use and risk of cervical adenocarcinoma is unlikely to be explained by differences in the Pap screening histories of IUD users and nonusers because the effect remained after adjusting for the number of previous screening Pap smears and because Pap screening itself was only weakly associated with a reduced risk of cervical adenocarcinoma. Nevertheless, residual confounding or potential selection bias could still explain the association. More evidence from other studies and populations is needed to determine whether the association reflects a true biologic mechanism or the fact that IUD use provides more opportunities for cervical cancer screening.
Implications for Screening and Vaccination
Clinical and epidemiologic studies have shown that Pap smears are less sensitive for detecting precursor lesions of adenocarcinoma of the cervix than they are for detecting precursor lesions for squamous cell carcinoma ( 5 – 7 ) . Consistent with this observation, our data provide only weak evidence that previous Pap smears may reduce somewhat the risk of cervical adenocarcinoma, because the risk reduction we found was moderate and did not reach statistical significance.
The confirmation from our findings that HPV is the central cause of cervical adenocarcinoma and that the same HPV types that are known to be involved in squamous cell cervical carcinomas are involved in cervical adenocarcinomas further implies that the introduction of HPV testing to primary screening programs for cervical cancer should improve the efficiency of these programs at detecting precancerous glandular lesions. From the type-specific HPV prevalence obtained in this study, we estimate that current HPV screening mixtures, such as that in the widely used Hybrid Capture II test—the only FDA-approved HPV test—have the potential to detect 97.9% of HPV-positive adenocarcinomas.
Our results indicate that HPV 16 and 18 are by far the most frequently detected HPV types in adenocarcinomas in the three world regions included in our analysis. On the basis of the data from this study, we estimate that the currently most widely tested vaccines ( 64 , 65 ) , which are designed to prevent infection by at least HPV 16 and 18, have the potential to prevent 85.6% of adenocarcinomas worldwide without large differences across regions (86.4% in North Africa, 83.7% in South America, and 86.5% in Southeast Asia). This preventive potential is larger than that for squamous cell carcinoma, which is estimated to be approximately 70% ( 44 ) . Nevertheless, it would be highly desirable in terms of overall cervical cancer prevention to develop HPV vaccines that cover up to the eight most common HPV types worldwide, namely HPV 16, 18, 45, 59, 35, 33, 31, and 58. Additional studies in some parts of the world, notably sub-Saharan Africa and Asia, are still warranted to obtain accurate estimates of the distribution of HPV types in these high-risk areas and to assess the preventive potential of HPV vaccination strategies in these regions.
Our study has several strengths. First, it was large enough to include a substantial number of HPV-positive control women, thus allowing relatively robust analyses restricted to HPV-positive women. Second, it was conducted in several different countries in which women are at high risk of cervical cancer, under strictly comparable protocols for field work and HPV DNA detection and genotyping procedures. Third, it paralleled a much larger study on cervical squamous cell cancer ( 30 ) , thus enabling a comparison of risk factors among these two histologic entities. Fourth, all specimens were collected before treatment was initiated, eliminating possible confounding by treatment. Fifth, it included only invasive cancer. Sixth, it included populations with high parity and little screening, making it possible to assess the role of parity as a cofactor for HPV in the pathogenesis of adenocarcinomas. Finally, serum samples were analyzed for chlamydial and HSV2 serology.
A weakness of this combined case–control study is, however, that our estimates of the relative risks for cervical adenocarcinoma linked to HPV are of limited value in providing absolute risk inferences. Ideally, in a deterministic causal model such as that of HPV and cervical cancer, estimated absolute risks would be more appropriate and informative than estimated relative risks because absolute risks provide information on a woman's risk of progression given her HPV infection status and type as well as her relevant profile of cofactors. Indeed, data analyzed with alternative statistical approaches and prospective study designs of associations with precancerous lesions such as cervical intraepithelial neoplasia (CIN) 2 and CIN3 are needed to provide estimates of meaningful probabilities of cervical cancer progression for a woman at a given age who is infected with a given HPV type and exposed to a given set of cofactors.
In summary, the pattern of associations found in this large pooled analysis indicates that the overall carcinogenesis model for the development of cervical adenocarcinoma does not differ greatly from the established model for cervical squamous cell carcinoma ( 8 ) . The few differences in the associations that we observed relate not to the involvement of HPV, which is clearly the etiological cause of cervical adenocarcinoma (although with some differences in the relative importance of HPV 18) but rather to the lack of association with tobacco smoking and C. trachomatis seropositivity. HPV testing in primary screening using current HPV type mixtures and HPV vaccination against the most common types have the potential to reduce the incidence of invasive adenocarcinoma worldwide.
The IARC Multi-center Cervical Cancer Study Group includes the following additional researchers: M. Plummer (IARC, Lyon), France ; V. Moreno (Institut Català d'Oncologia, Barcelona), Spain ; P. Alonso de Ruiz (General Hospital of Mexico, Mexico), Mexico ; S. Chichareon (Prince of Songkla University, Hat-Yai), Thailand ; C. Ngelangel (University of the Philippines, Manila), The Philippines ; J. Eluf-Neto (Universidade de Sao Paulo, Sao Paulo), Brazil ; P. A. Rolón (Laboratorio de Anatomia Patológica y Citologia, Asunción), Paraguay ; E. Caceres and C. Santos (Maes Heller Cancer Research Institute, Lima), Peru ; N. Chaouki and B. El Gueddari (Institut National d'Oncologie, Rabat), Morocco ; D. Hammouda (Institut National de Santé Publique, Département Contrôle des Maladies, Alger), Algeria ; T. Rajkumar (Cancer Institute, Adyar, Chennai), India .
Supported by the Fondo de Investigaciones Sanitarias, Spain, grant numbers FIS 01/1236 (PI: Xavier Castellsagué), FIS 01/1237 (PI: Xavier Bosch); by the Instituto de Salud Carlos III Network, Spain, grant numbers RCESP C03/09 & RCESP C03/10 (to Institut Català d'Oncologia); by the Programa Interministerial de Investigación y Desarrollo, Spain, grant number SAF 96/0323 (PI: Xavier Bosch); by the Ministerio de Educación, Cultura y Deporte. Spain, grant number SAB2000-0261 (PI: Xavier Bosch); and by Preventiefonds, The Netherlands., grant number 28-1502.1 (PI: Chris Meijer). Xavier Castellsagué was partly supported by grant BAE 01/5013. None of the funding agencies had a role in the design, conduct, analysis, or reporting of the results.
References
Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Borras J, et al. International trends in incidence of cervical cancer: II. Squamous-cell carcinoma.
Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Parkin DM. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas.
Bulk S, Visser O, Rozendaal L, Verheijen RH, Meijer CJ. Cervical cancer in the Netherlands 1989-1998: Decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women.
Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based study.
Krane JF, Granter SR, Trask CE, Hogan CL, Lee KR. Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix: a study of 49 cases.
Mitchell H, Medley G, Gordon I, Giles G. Cervical cytology reported as negative and risk of adenocarcinoma of the cervix: no strong evidence of benefit.
Raab SS. Can glandular lesions be diagnosed in pap smear cytology?
Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer.
Lacey JV Jr, Brinton LA, Abbas FM, Barnes WA, Gravitt PE, Greenberg MD, et al. Oral contraceptives as risk factors for cervical adenocarcinomas and squamous cell carcinomas.
Altekruse SF, Lacey JV Jr, Brinton LA, Gravitt PE, Silverberg SG, Barnes WA Jr, et al. Comparison of human papillomavirus genotypes, sexual, and reproductive risk factors of cervical adenocarcinoma and squamous cell carcinoma: Northeastern United States.
Hildesheim A, Herrero R, Castle PE, Wacholder S, Bratti MC, Sherman ME, et al. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica.
Lacey JV Jr, Frisch M, Brinton LA, Abbas FM, Barnes WA, Gravitt PE, et al. Associations between smoking and adenocarcinomas and squamous cell carcinomas of the uterine cervix (United States).
Munoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS, et al. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study.
Berrington DG, Sweetland S, Green J. Comparison of risk factors for squamous cell and adenocarcinomas of the cervix: a meta-analysis.
Lacey JV Jr, Swanson CA, Brinton LA, Altekruse SF, Barnes WA, Gravitt PE, et al. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix.
Lacey JV Jr, Brinton LA, Barnes WA, Gravitt PE, Greenberg MD, Hadjimichael OC, et al. Use of hormone replacement therapy and adenocarcinomas and squamous cell carcinomas of the uterine cervix.
Castellsague X, Bosch FX, Munoz N. Environmental co-factors in HPV carcinogenesis.
Castellsague X, Munoz N. Cofactors in human papillomavirus carcinogenesis—role of parity, oral contraceptives, and tobacco smoking.
Smith JS, Herrero R, Bosetti C, Munoz N, Bosch FX, Eluf-Neto J, et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer.
Smith JS, Bosetti C, Munoz N, Herrero R, Bosch FX, Eluf-Neto J, et al. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study.
Plummer M, Herrero R, Franceschi S, Meijer CJ, Snijders P, Bosch FX, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case—control study.
Hammouda D, Munoz N, Herrero R, Arslan A, Bouhadef A, Oublil M, et al. Cervical carcinoma in Algiers, Algeria: human papillomavirus and lifestyle risk factors.
Chaouki N, Bosch FX, Munoz N, Meijer CJ, El Gueddari B, El Ghazi A, et al. The viral origin of cervical cancer in Rabat, Morocco.
Eluf-Neto J, Booth M, Munoz N, Bosch FX, Meijer CJ, Walboomers JM. Human papillomavirus and invasive cervical cancer in Brazil.
Rolon PA, Smith JS, Munoz N, Klug SJ, Herrero R, Bosch X, et al. Human papillomavirus infection and invasive cervical cancer in Paraguay.
Santos C, Munoz N, Klug S, Almonte M, Guerrero I, Alvarez M, et al. HPV types and cofactors causing cervical cancer in Peru.
Franceschi S, Rajkumar T, Vaccarella S, Gajalakshmi V, Sharmila A, Snijders PJ, et al. Human papillomavirus and risk factors for cervical cancer in Chennai, India: a case-control study.
Chichareon S, Herrero R, Munoz N, Bosch FX, Jacobs MV, Deacon J, et al. Risk factors for cervical cancer in Thailand: a case-control study.
Ngelangel C, Munoz N, Bosch FX, Limson GM, Festin MR, Deacon J, et al. Causes of cervical cancer in the Philippines: a case-control study.
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer.
Roda Husman AM, Snijders PJ, Stel HV, Van den Brule AJ, Meijer CJ, Walboomers JM. Processing of long-stored archival cervical smears for human papillomavirus detection by the polymerase chain reaction.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide.
Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera.
Smith JS, Herrero R, Munoz N, Eluf-Neto J, Ngelangel C, Bosch FX, et al. Prevalence and risk factors for herpes simplex virus type 2 infection among middle-age women in Brazil and the Philippines.
Smith JS, Munoz N, Herrero R, Eluf-Neto J, Ngelangel C, Franceschi S, et al. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines.
Wang SP, Grayston JT. Micro immunofluorescence antibody responses in Chlamydia trachomatis infection. A review. In: Mardh PA, Holmes KK, Oriel JD, Piot P, Schachter J, editors. Chlamydial infections. Amsterdam (The Netherlands): Elsevier–Biomedical Press;
Anciaux D, Lawrence WD, Gregoire L. Glandular lesions of the uterine cervix: prognostic implications of human papillomavirus status.
Andersson S, Rylander E, Larsson B, Strand A, Silfversvard C, Wilander E. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis.
Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group.
Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis.
Lee MF, Chang MC, Wu CH. Detection of human papillomavirus types in cervical adenocarcinoma by the polymerase chain reaction.
Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, et al. Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma.
Zielinski GD, Snijders PJ, Rozendaal L, Daalmeijer NF, Risse EK, Voorhorst FJ, et al. The presence of high-risk HPV combined with specific p53 and p16INK4a expression patterns points to high-risk HPV as the main causative agent for adenocarcinoma in situ and adenocarcinoma of the cervix.
Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective.
Moreno V, Bosch FX, Munoz N, Meijer CJ, Shah KV, Walboomers JM, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study.
Smith JS, Green J, Berrington DG, Appleby P, Peto J, Plummer M, et al. Cervical cancer and use of hormonal contraceptives: a systematic review.
Brinton LA, Tashima KT, Lehman HF, Levine RS, Mallin K, Savitz DA, et al. Epidemiology of cervical cancer by cell type.
Brinton LA, Herrero R, Reeves WC, de Britton RC, Gaitan E, Tenorio F. Risk factors for cervical cancer by histology.
Parazzini F, La Vecchia C, Negri E, Fasoli M, Cecchetti G. Risk factors for adenocarcinoma of the cervix: a case-control study.
Thomas DB, Ray RM. Oral contraceptives and invasive adenocarcinomas and adenosquamous carcinomas of the uterine cervix. The World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives.
Ursin G, Pike MC, Preston-Martin S, d'Ablaing G III, Peters RK. Sexual, reproductive, and other risk factors for adenocarcinoma of the cervix: results from a population-based case-control study (California, United States).
Guleria K, Agarwal N, Mishra K, Gulati R, Mehendiratta A. Evaluation of endometrial steroid receptors and cell mitotic activity in women using copper intrauterine device: Can Cu-T prevent endometrial cancer?
Benshushan A, Paltiel O, Rojansky N, Brzezinski A, Laufer N. IUD use and the risk of endometrial cancer.
Hubacher D, Grimes DA. Noncontraceptive health benefits of intrauterine devices: a systematic review.
Hill DA, Weiss NS, Voigt LF, Beresford SA. Endometrial cancer in relation to intra-uterine device use.
Sturgeon SR, Brinton LA, Berman ML, Mortel R, Twiggs LB, Barrett RJ, et al. Intrauterine device use and endometrial cancer risk.
Rosenblatt KA, Thomas DB. Intrauterine devices and endometrial cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives.
Parazzini F, La Vecchia C, Moroni S. Intrauterine device use and risk of endometrial cancer.
Castellsague X, Thompson WD, Dubrow R. Intra-uterine contraception and the risk of endometrial cancer.
Li HQ, Thomas DB, Jin SK, Wu F. Tubal sterilization and use of an IUD and risk of cervical cancer.
Kohler U, Wuttke P. [Results of a case-control study of the current effect of various factors of cervical cancer risk. 2. Contraceptive behavior and the smoking factor].
Lassise DL, Savitz DA, Hamman RF, Baron AE, Brinton LA, Levines RS. Invasive cervical cancer and intrauterine device use.
Batar I. [The Szontagh IUD and cervix carcinoma (results of a 10-year follow up study)].
Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial.
Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial.