-
PDF
- Split View
-
Views
-
Cite
Cite
Nathalie A. Desrosiers, Johannes G. Ramaekers, Emeline Chauchard, David A. Gorelick, Marilyn A. Huestis, Smoked Cannabis' Psychomotor and Neurocognitive Effects in Occasional and Frequent Smokers, Journal of Analytical Toxicology, Volume 39, Issue 4, May 2015, Pages 251–261, https://doi.org/10.1093/jat/bkv012
Close - Share Icon Share
Abstract
Δ9-Tetrahydrocannabinol (THC), the primary psychoactive constituent in cannabis, impairs psychomotor performance, cognition and driving ability; thus, driving under the influence of cannabis is a public safety concern. We documented cannabis' psychomotor, neurocognitive, subjective and physiological effects in occasional and frequent smokers to investigate potential differences between these smokers. Fourteen frequent (≥4x/week) and 11 occasional (<2x/week) cannabis smokers entered a secure research unit ∼19 h prior to smoking one 6.8% THC cigarette. Cognitive and psychomotor performance was evaluated with the critical tracking (CTT), divided attention (DAT), n-back (working memory) and Balloon Analog Risk (BART) (risk-taking) tasks at −1.75, 1.5, 3.5, 5.5 and 22.5 h after starting smoking. GLM (General Linear Model) repeated measures ANOVA was utilized to compare scores. Occasional smokers had significantly more difficulty compensating for CTT tracking error compared with frequent smokers 1.5 h after smoking. Divided attention performance declined significantly especially in occasional smokers, with session × group effects for tracking error, hits, false alarms and reaction time. Cannabis smoking did not elicit session × group effects on the n-back or BART. Controlled cannabis smoking impaired psychomotor function, more so in occasional smokers, suggesting some tolerance to psychomotor impairment in frequent users. These data have implications for cannabis-associated impairment in driving under the influence of cannabis cases.
Introduction
Cannabis is the most commonly consumed illicit drug worldwide, with 2.7–4.9% of 15–64 year-olds consuming cannabis at least once in 2012 (1). Δ9-tetrahydrocannabinol (THC), cannabis' main psychoactive compound, was the most prevalent illicit drug detected in injured drivers in Victoria, Australia (2) and cannabinoids were identified in 8.6% of nighttime drivers' blood and/or oral fluid in the 2007 US Roadside Survey (3). Increased recreational and medicinal cannabis use (1, 4, 5), decreased perceived risk of consumption (3) and changes to cannabis' legal status enhanced the need for in-depth understanding of cannabis' acute neurocognitive and psychomotor effects in occasional and frequent cannabis smokers and how these effects relate to driving performance.
Although many psychopharmacological studies examined cognitive function in abstinent cannabis smokers, fewer evaluated psychomotor and cognitive effects during acute intoxication, as related to driving. Acute effects include impaired psychomotor performance, cognition and driving ability in simulators and on-the-road driving tests (6–14). Tracking nearby car position and working memory (ability to transiently hold and process reasoning, comprehension and learning information) are critical processes for safe driving that also may be impaired. In addition, several observational studies suggest relationships between past cannabis smoking and risk-taking, including criminal behavior (15–17) and driving under the influence of cannabis (DUIC) (18, 19). Therefore, cannabis' acute effects are an important public health and safety concern.
Published acute studies often do not distinguish between occasional and frequent cannabis smokers, or only examine one group. The few studies that directly compared these smokers documented behavioral tolerance to some of cannabis' effects (20–22), emphasizing the importance of evaluating these measures in occasional and frequent users following cannabis smoking.
We evaluated smoked cannabis' effects on psychomotor function, working memory, risk-taking, subjective and physiological effects in occasional and frequent smokers following controlled smoking of a 6.8% THC cigarette for up to 22.5 h.
Methods
Participants
Adult cannabis smokers provided written informed consent to participate in this National Institute on Drug Abuse (NIDA) Intramural Research Program Institutional Review Board-approved study. Inclusion criteria were ages 18–45 years and self-reported average smoked cannabis frequency of less than twice per week (occasional smoker) or equal to or more than four times per week (frequent smoker) in the past 3 months. A positive urine cannabinoid test confirmed cannabis smoking in frequent smokers (50 µg/L THCCOOH, as assessed by the iScreen). Exclusion criteria included breastfeeding or pregnant women; current medical condition or history of neurological illness; clinically significant adverse event following cannabis intoxication; >450 mL blood donation within 30 days; elevated systolic (>140 mmHg) or diastolic (>90 mmHg) blood pressure or heart rate >100 bpm after 5 min rest; clinically significant electrocardiogram abnormality; or interest or participation in drug abuse treatment within 60 days. Pregnancy tests were administered at screening and on admission to women with reproductive potential.
Study design
Participants resided on a secure research unit ∼19 h prior to smoking to preclude intoxication at the time of cannabis dosing. Cannabis cigarettes containing (mean ± SD) 6.8 ± 0.2% THC (54 mg) were obtained through NIDA. Participants smoked one cigarette ad libitum within 10 min. As required by the ethical committee, participants smoked to their desired comfort level. Blood was collected in green-topped tubes (containing sodium heparin) on admission, 1 h before, and 0.5, 1, 2, 3, 4, 5, 6, 8, 10.5, 13.5, 21, 24, 26, 28 and 30 h after smoking initiation.
Cannabinoid analysis
Cannabinoids were quantified by a previously validated liquid chromatography-tandem mass spectrometry (LC–MS/MS) method (23). Briefly, 0.5 mL blood was deproteinized with acetonitrile, supernatants diluted prior to solid-phase extraction, and LC–MS/MS analyzed. Linear ranges were 1–100 µg/L THC, 11-OH-THC, THCCOOH, CBD, and CBN, 0.5–50 µg/L THC-glucuronide and 5–500 µg/L THCCOOH-glucuronide. Inter-assay (N = 20) analytical bias and imprecision were 93.8–113.1 and 4.9–10.4%, respectively.
Task practice and training
All participants rigorously practiced tasks and achieve stable performance prior to admission to reduce learning and practice effects during the study. Detailed practice procedures are outlined for each task below.
Psychomotor control and divided attention
The ‘critical tracking task’ (CTT) (24) measures ability to control a displayed error signal in a first-order compensatory tracking task. Error is displayed as a horizontal cursor deviation from the linear scale's midpoint. The frequency of computer-generated cursor deviations and, therefore, the velocity, increases as a stochastic, linear function of time. Compensatory joystick movements correct the error by returning the cursor to the midpoint. Critical frequency or lambda-c (λc) occurs when control is lost (compensatory response lags the cursor's last movement by 180°). There are five trials; mean of the middle three scores is the final performance score. This task was performed at −1.75, 1.5, 3.5, 5.5 and 22.5 h after starting smoking.
The ‘divided attention task’ (DAT) (25) measures ability to divide attention between two simultaneous tasks. The primary task is the critical tracking task, with the exception that the error signal's velocity is kept at 50% of the participant's CTT performance score (λc/2). Tracking error is absolute distance (mm) between cursor's position and the center. The secondary task involves monitoring 24 single-digit numbers (0–9), displayed in the four corners of a central screen that change asynchronously every 5 s. The participant must remove his/her foot from a pedal-switch any time the numeral ‘2’ appears. Number of control losses, mean absolute tracking error (mm), number of correct ‘2’ detections (hits), false alarms and reaction time are the primary performance measures at each session. This task was performed at −1.65, 1.6, 3.6, 5.6 and 22.6 h after starting smoking.
Participants were trained before admission to achieve stable task performance and minimize practice effects. Critical tracking task was performed until participants performed with <15% difference from the average over three consecutive sets of five trials. The divided attention task was practiced for 12 min at the participant's λc/2, regardless of final performance.
Spatial working memory
The n-back parametric working memory task assesses effects of manipulating cognitive load on working memory performance. In this delayed match-to-sample task, participants are asked to determine whether a given stimulus matches a stimulus presented in either the previous trial (1-back), two trials previously (2-back) or three trials previously (3-back), thus simultaneously encoding and retrieving information. Low load (1-back) and high load tasks (2- and 3-back) were evaluated. Training consisted of five tests for each n-back trial. Accuracy, reaction time and errors of commission were the primary performance measures. Working memory was assessed at −1.5, 1.75, 3.75, 5.75 and 22.75 h after smoking.
Risk-taking and impulsivity
The Balloon Analog Risk Task (BART) (26–28) assesses risk-taking and impulsivity. BART scores correlate with self-reported risk-taking, sensation seeking behavior, impulsivity and drug use (26) and assessed risk-taking behavior with other drugs (28–30). The BART demonstrated acceptable test-retest reliability and stability over time within individual subjects (27).
Participants viewed a computer screen displaying a small balloon and balloon pump. Each click on the pump slightly inflated the balloon. Each click earned participants 1 cent, which was deposited into a temporary cache visible on the screen. Participants inflated the balloon as much as possible without popping; balloons popped between 1 and 128 pumps, with an average of 64 pumps. Each balloon's breakpoint was randomized; popping emptied the cache of money. Participants could stop balloon inflation at any time, which moved money to a permanent bank. Participants trained on 20 balloons before admission.
Each session had 20 trials. Adjusted pump number (average number of pumps on balloons that did not pop) was the primary performance measure, as it minimized between-subject variability (26). To increase task relevancy, participants received money remaining in the permanent bank at the end of each session (maximum $64 USD). Participant's risk-taking was assessed at −1.1, 1.9, 3.9, 5.9 and 22.9 h after smoking.
Self-reported risk-taking and impulsivity were assessed at task training with the Melbourne Decision Making (MDM) scales (31), Self-Assessments of Risk Perception and Sensation Seeking (32), Barratt Impulsiveness Scale version 11 (BIS-11) (33), Zuckerman-Kuhlman Personality Questionnaire (ZKPQ) (34) and Risk Perception Questionnaire (RPQ) (35) (Supplementary Data 1).
Subjective measures
Visual analog scales (VAS) were presented on a computer screen at −1, 0.25, 0.5, 1, 2, 3, 4 and 6 h after the start of smoking. Participants marked the magnitude of ‘Good Drug Effect’, ‘High’, ‘Stoned’, ‘Stimulated’, ‘Sedated’, ‘Anxious’ and ‘Restless’ on a 100-mm line anchored with ‘Not at all’ and ‘Most ever.’
5-point Likert scales for ‘Difficulty concentrating’, ‘Altered sense of time’, ‘Slowed or slurred speech’, ‘Body feels sluggish or heavy’, ‘Feel hungry’, ‘Feel thirsty’,‘Shakiness/tremulousness’, ‘Nausea’, ‘Headache’, ‘Palpitations’, ‘Dizzy’ and ‘Dry mouth or throat’ were presented immediately following the VAS. Participants selected the response best describing their condition: 1 (none), 2 (slight), 3 (mild), 4 (moderate) or 5 (severe).
Physiological measures
Heart rate, blood pressure (systolic and diastolic), and respiratory rate were measured before and after smoking at −0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5 and 6 h.
Statistical analysis
Occasional and frequent smokers' baseline performance was compared with 2-tailed t-tests. Two-way, mixed [5, 9 or 10 time points (session) × 2 (condition: occasional vs. frequent smoker)] GLM (General Linear Model) ANOVA evaluated VAS, physiological, cognitive and psychomotor scores after cannabis administration (session refers to a given time point and all sessions were compared with their baseline.) Session effects document an observed change from baseline regardless of group; group effects reflect differences between occasional and frequent smokers regardless of time; and session by group interaction effects reveal differences between occasional and frequent smokers at a given time, relative to their respective baseline. Missing values (due to participant withdrawal or adverse event) were imputed with the group mean for that time point; data from poor performance from participant noncompliance was removed from the data set. The Huynh–Feldt correction adjusted for sphericity violations. Contrast evaluated differences between each post-smoking time point and baseline. Contrasts were performed independently of overall effects to reduce type II errors, as impairment was expected in the first 2 h post-dose and inclusion of 4–22 h in overall GLM could reduce power for overall effects. Likert scales were compared with Mann–Whitney exact test. Statistical significance was attributed at two-tailed P<0.05, with trends attributed at P < 0.1. All statistical tests were performed with SPSS® for Windows version 20 (IBM, Armonk, NY).
Results
Participants
Fourteen frequent and 11 occasional cannabis smokers (18 men, 7 women), ages 18–45 years participated (Table I). Frequent smokers were younger, smoked for fewer lifetime years and smoked more recently, on more days of the 14 prior to study session, and more frequently compared with occasional smokers. Two participants (M and N) self-reported occasional use, but were retrospectively reclassified as frequent smokers based on baseline and post-smoking cannabinoid concentrations, consistent with published blood (36), oral fluid (37) and urine (38) cannabinoid concentrations. Participants J and Y withdrew after 10.5 and 6 h, respectively, yielding incomplete data for the last time point (22–23 h after smoking).
Demographic Characteristics and Cannabis Smoking Histories for 14 Frequent (A-N) and 11 Occasional (O-Y) Smokers
| Participant . | Race and ethnicity . | Gender . | Age at admission . | BMIa . | Age 1st usea . | Lifetime years smokeda . | Time between last use and admission in h or d . | Number of days used in last 14b . | Average joint or joint equivalents /day or /monthb . |
|---|---|---|---|---|---|---|---|---|---|
| A | B | M | 29.6 | 27.6 | 12 | 17.6 | 7.4 h | 11 | 4/d |
| B | B | M | 19.4 | 22.6 | 15 | 4.4 | 4.3 h | 13 | 5/d |
| C | B | M | 22.6 | 31.4 | 14 | 8.6 | 5.1 h | 12 | 3/d |
| D | W | M | 25.5 | 23.0 | 13 | 12.5 | 3.9 h | 14 | 20/d |
| E | B | F | 19.9 | 32.4 | 11 | 8.9 | 2.6 h | 14 | 3.5/d |
| F | B | M | 24.2 | 27.4 | 13 | 11.2 | 23.2 h | 12 | 1.5/d |
| G | W | F | 22.9 | 24.8 | 16 | 6.9 | 17.2 h | 14 | 6/d |
| H | B | M | 37.3 | 23.0 | 25 | 12.3 | 1.6 h | 14 | 3/d |
| I | B | F | 27.6 | 35.4 | 18 | 9.6 | 2.4 h | 14 | 4/d |
| J | B | F | 26.9 | 20.4 | 14 | 12.9 | 3.8 h | 14 | 21/d |
| K | B | M | 23.4 | 24.3 | 19 | 4.4 | 1.2 h | 14 | 6/d |
| L | B | M | 28.7 | 28.1 | 14 | 14.7 | 9.5 h | 14 | 6/d |
| M | B | M | 28.0 | 19.4 | 14 | 14.0 | 67.4 hc | 2c | 2/mc |
| N | B | M | 23.8 | 30.7 | 14 | 9.8 | 273 hc | 1c | 4/mc |
| Frequent | Mean | 25.7 | 26.4 | 15.1 | 10.6 | – | 13.3 | – | |
| StdDev | 4.6 | 4.8 | 3.5 | 3.8 | – | 1.1 | – | ||
| Median | 24.8 | 26.1 | 14.0 | 10.5 | 4.1 h | 14.0 | 4.5/d | ||
| O | W | M | 25.6 | 29.4 | 16 | 9.6 | 16 d | 0 | 2/m |
| P | W | M | 25.4 | 23.7 | 13 | 12.4 | 31 d | 0 | 2/m |
| Q | W | M | 23.7 | 24.1 | 16 | 7.7 | 10 d | 2 | 7/m |
| R | B | M | 38.2 | 21.0 | 19 | 19.2 | 2 d | 2 | 2/m |
| S | M | M | 41.3 | 22.0 | 16 | 25.3 | 7 d | 5 | 10/md |
| T | U | F | 34.9 | 31.7 | 13 | 21.9 | 9 d | 1 | 2/m |
| U | B | F | 36.5 | 47.8 | 18 | 18.5 | 2 d | 2 | 4/m |
| V | M & H | M | 22.5 | 25.2 | 13 | 9.5 | 86 d | 0 | 6/m |
| W | W | F | 34.2 | 26.6 | 14 | 20.2 | 3 d | 1 | 0.25/m |
| X | B & U | M | 31.7 | 21.8 | 16 | 15.7 | 18 d | 0 | 8/m |
| Y | B | M | 31.9 | 22.6 | 15 | 16.9 | 68 d | 0 | 2/m |
| Occasional | Mean | 31.4 | 26.9 | 15.4 | 16.1 | – | 1.2 | – | |
| StdDev | 6.3 | 7.7 | 2.0 | 5.7 | – | 1.5 | – | ||
| Median | 31.9* | 24.1 | 16.0 | 16.9* | 10 d* | 1.0* | 2/m* |
| Participant . | Race and ethnicity . | Gender . | Age at admission . | BMIa . | Age 1st usea . | Lifetime years smokeda . | Time between last use and admission in h or d . | Number of days used in last 14b . | Average joint or joint equivalents /day or /monthb . |
|---|---|---|---|---|---|---|---|---|---|
| A | B | M | 29.6 | 27.6 | 12 | 17.6 | 7.4 h | 11 | 4/d |
| B | B | M | 19.4 | 22.6 | 15 | 4.4 | 4.3 h | 13 | 5/d |
| C | B | M | 22.6 | 31.4 | 14 | 8.6 | 5.1 h | 12 | 3/d |
| D | W | M | 25.5 | 23.0 | 13 | 12.5 | 3.9 h | 14 | 20/d |
| E | B | F | 19.9 | 32.4 | 11 | 8.9 | 2.6 h | 14 | 3.5/d |
| F | B | M | 24.2 | 27.4 | 13 | 11.2 | 23.2 h | 12 | 1.5/d |
| G | W | F | 22.9 | 24.8 | 16 | 6.9 | 17.2 h | 14 | 6/d |
| H | B | M | 37.3 | 23.0 | 25 | 12.3 | 1.6 h | 14 | 3/d |
| I | B | F | 27.6 | 35.4 | 18 | 9.6 | 2.4 h | 14 | 4/d |
| J | B | F | 26.9 | 20.4 | 14 | 12.9 | 3.8 h | 14 | 21/d |
| K | B | M | 23.4 | 24.3 | 19 | 4.4 | 1.2 h | 14 | 6/d |
| L | B | M | 28.7 | 28.1 | 14 | 14.7 | 9.5 h | 14 | 6/d |
| M | B | M | 28.0 | 19.4 | 14 | 14.0 | 67.4 hc | 2c | 2/mc |
| N | B | M | 23.8 | 30.7 | 14 | 9.8 | 273 hc | 1c | 4/mc |
| Frequent | Mean | 25.7 | 26.4 | 15.1 | 10.6 | – | 13.3 | – | |
| StdDev | 4.6 | 4.8 | 3.5 | 3.8 | – | 1.1 | – | ||
| Median | 24.8 | 26.1 | 14.0 | 10.5 | 4.1 h | 14.0 | 4.5/d | ||
| O | W | M | 25.6 | 29.4 | 16 | 9.6 | 16 d | 0 | 2/m |
| P | W | M | 25.4 | 23.7 | 13 | 12.4 | 31 d | 0 | 2/m |
| Q | W | M | 23.7 | 24.1 | 16 | 7.7 | 10 d | 2 | 7/m |
| R | B | M | 38.2 | 21.0 | 19 | 19.2 | 2 d | 2 | 2/m |
| S | M | M | 41.3 | 22.0 | 16 | 25.3 | 7 d | 5 | 10/md |
| T | U | F | 34.9 | 31.7 | 13 | 21.9 | 9 d | 1 | 2/m |
| U | B | F | 36.5 | 47.8 | 18 | 18.5 | 2 d | 2 | 4/m |
| V | M & H | M | 22.5 | 25.2 | 13 | 9.5 | 86 d | 0 | 6/m |
| W | W | F | 34.2 | 26.6 | 14 | 20.2 | 3 d | 1 | 0.25/m |
| X | B & U | M | 31.7 | 21.8 | 16 | 15.7 | 18 d | 0 | 8/m |
| Y | B | M | 31.9 | 22.6 | 15 | 16.9 | 68 d | 0 | 2/m |
| Occasional | Mean | 31.4 | 26.9 | 15.4 | 16.1 | – | 1.2 | – | |
| StdDev | 6.3 | 7.7 | 2.0 | 5.7 | – | 1.5 | – | ||
| Median | 31.9* | 24.1 | 16.0 | 16.9* | 10 d* | 1.0* | 2/m* |
F, Frequent smoker; O, Occasional smoker; W, White; B, Black or African American; M, Mixed; U, Unknown; H, Hispanic or Latino.
aData collected at admission.
bData collected prior to study smoking.
cSelf-reported data not consistent with biological sample concentrations. Data excluded from mean and median.
dSelf-reported average use at screening of 0.5 joints, 3–4 times per month.
*Significant difference between groups (P < 0.05).
Demographic Characteristics and Cannabis Smoking Histories for 14 Frequent (A-N) and 11 Occasional (O-Y) Smokers
| Participant . | Race and ethnicity . | Gender . | Age at admission . | BMIa . | Age 1st usea . | Lifetime years smokeda . | Time between last use and admission in h or d . | Number of days used in last 14b . | Average joint or joint equivalents /day or /monthb . |
|---|---|---|---|---|---|---|---|---|---|
| A | B | M | 29.6 | 27.6 | 12 | 17.6 | 7.4 h | 11 | 4/d |
| B | B | M | 19.4 | 22.6 | 15 | 4.4 | 4.3 h | 13 | 5/d |
| C | B | M | 22.6 | 31.4 | 14 | 8.6 | 5.1 h | 12 | 3/d |
| D | W | M | 25.5 | 23.0 | 13 | 12.5 | 3.9 h | 14 | 20/d |
| E | B | F | 19.9 | 32.4 | 11 | 8.9 | 2.6 h | 14 | 3.5/d |
| F | B | M | 24.2 | 27.4 | 13 | 11.2 | 23.2 h | 12 | 1.5/d |
| G | W | F | 22.9 | 24.8 | 16 | 6.9 | 17.2 h | 14 | 6/d |
| H | B | M | 37.3 | 23.0 | 25 | 12.3 | 1.6 h | 14 | 3/d |
| I | B | F | 27.6 | 35.4 | 18 | 9.6 | 2.4 h | 14 | 4/d |
| J | B | F | 26.9 | 20.4 | 14 | 12.9 | 3.8 h | 14 | 21/d |
| K | B | M | 23.4 | 24.3 | 19 | 4.4 | 1.2 h | 14 | 6/d |
| L | B | M | 28.7 | 28.1 | 14 | 14.7 | 9.5 h | 14 | 6/d |
| M | B | M | 28.0 | 19.4 | 14 | 14.0 | 67.4 hc | 2c | 2/mc |
| N | B | M | 23.8 | 30.7 | 14 | 9.8 | 273 hc | 1c | 4/mc |
| Frequent | Mean | 25.7 | 26.4 | 15.1 | 10.6 | – | 13.3 | – | |
| StdDev | 4.6 | 4.8 | 3.5 | 3.8 | – | 1.1 | – | ||
| Median | 24.8 | 26.1 | 14.0 | 10.5 | 4.1 h | 14.0 | 4.5/d | ||
| O | W | M | 25.6 | 29.4 | 16 | 9.6 | 16 d | 0 | 2/m |
| P | W | M | 25.4 | 23.7 | 13 | 12.4 | 31 d | 0 | 2/m |
| Q | W | M | 23.7 | 24.1 | 16 | 7.7 | 10 d | 2 | 7/m |
| R | B | M | 38.2 | 21.0 | 19 | 19.2 | 2 d | 2 | 2/m |
| S | M | M | 41.3 | 22.0 | 16 | 25.3 | 7 d | 5 | 10/md |
| T | U | F | 34.9 | 31.7 | 13 | 21.9 | 9 d | 1 | 2/m |
| U | B | F | 36.5 | 47.8 | 18 | 18.5 | 2 d | 2 | 4/m |
| V | M & H | M | 22.5 | 25.2 | 13 | 9.5 | 86 d | 0 | 6/m |
| W | W | F | 34.2 | 26.6 | 14 | 20.2 | 3 d | 1 | 0.25/m |
| X | B & U | M | 31.7 | 21.8 | 16 | 15.7 | 18 d | 0 | 8/m |
| Y | B | M | 31.9 | 22.6 | 15 | 16.9 | 68 d | 0 | 2/m |
| Occasional | Mean | 31.4 | 26.9 | 15.4 | 16.1 | – | 1.2 | – | |
| StdDev | 6.3 | 7.7 | 2.0 | 5.7 | – | 1.5 | – | ||
| Median | 31.9* | 24.1 | 16.0 | 16.9* | 10 d* | 1.0* | 2/m* |
| Participant . | Race and ethnicity . | Gender . | Age at admission . | BMIa . | Age 1st usea . | Lifetime years smokeda . | Time between last use and admission in h or d . | Number of days used in last 14b . | Average joint or joint equivalents /day or /monthb . |
|---|---|---|---|---|---|---|---|---|---|
| A | B | M | 29.6 | 27.6 | 12 | 17.6 | 7.4 h | 11 | 4/d |
| B | B | M | 19.4 | 22.6 | 15 | 4.4 | 4.3 h | 13 | 5/d |
| C | B | M | 22.6 | 31.4 | 14 | 8.6 | 5.1 h | 12 | 3/d |
| D | W | M | 25.5 | 23.0 | 13 | 12.5 | 3.9 h | 14 | 20/d |
| E | B | F | 19.9 | 32.4 | 11 | 8.9 | 2.6 h | 14 | 3.5/d |
| F | B | M | 24.2 | 27.4 | 13 | 11.2 | 23.2 h | 12 | 1.5/d |
| G | W | F | 22.9 | 24.8 | 16 | 6.9 | 17.2 h | 14 | 6/d |
| H | B | M | 37.3 | 23.0 | 25 | 12.3 | 1.6 h | 14 | 3/d |
| I | B | F | 27.6 | 35.4 | 18 | 9.6 | 2.4 h | 14 | 4/d |
| J | B | F | 26.9 | 20.4 | 14 | 12.9 | 3.8 h | 14 | 21/d |
| K | B | M | 23.4 | 24.3 | 19 | 4.4 | 1.2 h | 14 | 6/d |
| L | B | M | 28.7 | 28.1 | 14 | 14.7 | 9.5 h | 14 | 6/d |
| M | B | M | 28.0 | 19.4 | 14 | 14.0 | 67.4 hc | 2c | 2/mc |
| N | B | M | 23.8 | 30.7 | 14 | 9.8 | 273 hc | 1c | 4/mc |
| Frequent | Mean | 25.7 | 26.4 | 15.1 | 10.6 | – | 13.3 | – | |
| StdDev | 4.6 | 4.8 | 3.5 | 3.8 | – | 1.1 | – | ||
| Median | 24.8 | 26.1 | 14.0 | 10.5 | 4.1 h | 14.0 | 4.5/d | ||
| O | W | M | 25.6 | 29.4 | 16 | 9.6 | 16 d | 0 | 2/m |
| P | W | M | 25.4 | 23.7 | 13 | 12.4 | 31 d | 0 | 2/m |
| Q | W | M | 23.7 | 24.1 | 16 | 7.7 | 10 d | 2 | 7/m |
| R | B | M | 38.2 | 21.0 | 19 | 19.2 | 2 d | 2 | 2/m |
| S | M | M | 41.3 | 22.0 | 16 | 25.3 | 7 d | 5 | 10/md |
| T | U | F | 34.9 | 31.7 | 13 | 21.9 | 9 d | 1 | 2/m |
| U | B | F | 36.5 | 47.8 | 18 | 18.5 | 2 d | 2 | 4/m |
| V | M & H | M | 22.5 | 25.2 | 13 | 9.5 | 86 d | 0 | 6/m |
| W | W | F | 34.2 | 26.6 | 14 | 20.2 | 3 d | 1 | 0.25/m |
| X | B & U | M | 31.7 | 21.8 | 16 | 15.7 | 18 d | 0 | 8/m |
| Y | B | M | 31.9 | 22.6 | 15 | 16.9 | 68 d | 0 | 2/m |
| Occasional | Mean | 31.4 | 26.9 | 15.4 | 16.1 | – | 1.2 | – | |
| StdDev | 6.3 | 7.7 | 2.0 | 5.7 | – | 1.5 | – | ||
| Median | 31.9* | 24.1 | 16.0 | 16.9* | 10 d* | 1.0* | 2/m* |
F, Frequent smoker; O, Occasional smoker; W, White; B, Black or African American; M, Mixed; U, Unknown; H, Hispanic or Latino.
aData collected at admission.
bData collected prior to study smoking.
cSelf-reported data not consistent with biological sample concentrations. Data excluded from mean and median.
dSelf-reported average use at screening of 0.5 joints, 3–4 times per month.
*Significant difference between groups (P < 0.05).
Blood concentrations
Full pharmacokinetic profiles and blood concentrations are presented elsewhere (39). Mean ± SD baseline blood THC concentrations were none detected in occasional smokers and 3.3 ± 2.1 µg/L in frequent smokers. Blood THC concentrations at 0.5 h were 32.3 ± 11.2 µg/L in frequent and 17.4 ± 12.7 µg/L in occasional smokers. At 6 h, frequent smokers' THC concentrations were 4.1 ± 2.2 µg/L and only two occasional smokers remained positive at 1.3 and 1.0 µg/L. At 24 h, all occasional smokers' THC concentrations were below the limit of quantification, while frequent smokers' mean THC concentration was 2.9 ± 2.1 µg/L.
Psychomotor control and divided attention
Subjects U, V and X (occasional smokers) CTTs' data were excluded because of poor baseline performance, precluding decreases in λc after smoking. For the DAT, data from participants V and X (5.6 h) and participants J (frequent smoker) and Y (occasional smoker) (22.6 h) were missing and replaced with mean group values.
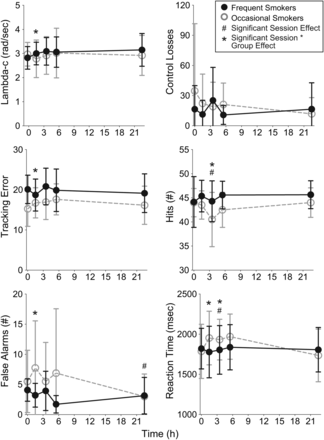
There were no significant differences in CTT baseline between occasional and frequent smokers, nor were there significant overall session, group or session by group interaction effects on the CTT (Figure 1, Supplementary Data 2 and 3). Contrasts indicated that occasional smokers' CTT performance decreased 1.5 h after smoking compared with frequent smokers' performance, relative to their respective baselines (F(1,20) = 5.22, P < 0.05) (Figure 1, Supplementary Data 4).
Mean (standard deviation) for critical tracking task (lambda-c) and divided attention (Control losses, tracking error, hits, false alarms and reaction time) for 14 frequent and 8 (CTT) or 11 (DAT) occasional cannabis smokers following controlled smoking of a 6.8% THC (54 mg) cannabis cigarette.
Occasional smokers had significantly fewer baseline DAT tracking errors than frequent smokers (Supplementary Data 2). There was a significant main session effect on false alarms (F(4,92) = 2.67, P < 0.05) and reaction time (F(3.3,75.7) = 2.65, P < 0.05), as well as a trend for a session effect on hits (Figure 1, Supplementary Data 3). Occasional smokers had significantly fewer hits (F(1,23) = 5.67, P < 0.05) and trended towards fewer tracking errors but more false alarms, relative to frequent users (Figure 1, Supplementary Data 3). There was a significant overall session by group interaction effect for false alarms (F(4,92) = 2.55, P < 0.05) and reaction time (F(3.3,75.7) = 3.05, P < 0.05) (Figure 1, Supplementary Data 3). Relative to their baselines, occasional smokers had more tracking errors and false alarms, and longer reaction times at 1.6 h, and fewer hits and longer reaction times at 3.6 h compared with frequent smokers. Trends were noted for fewer hits at 1.6 and 5.6 h, and control losses at 22.6 h in occasional smokers (Figure 1, Supplementary Data 4).
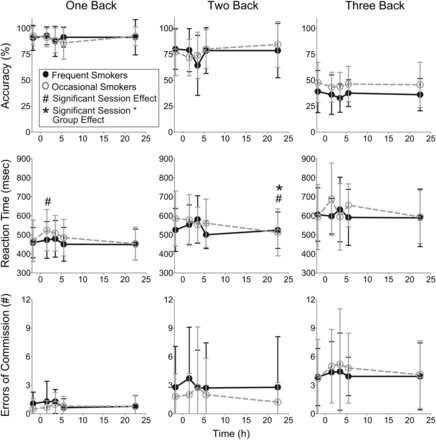
Spatial working memory
All occasional smoker V's n-back scores were excluded because he button-pressed repeatedly rather than identifying target n-back responses. There were no baseline differences between occasional and frequent smokers for any n-back measure (Supplementary Data 2). There was an overall significant session effect (independent of group) on 2-back accuracy (F(3.5,76.5) = 3.06, P < 0.05), 1-back reaction time (F(4,88) = 3.34, P < 0.05) and 2-back reaction time (F(4,88) = 2.61, P < 0.05), a trend towards an interaction effect between session by group for 2-back reaction time, but no overall group effect (Figure 2, Supplementary Data 3). Compared with baseline, participants had significantly increased mean 1-back reaction time at 1.75 h, trended towards longer 1-back mean reaction time at 3.75 h and had significantly shorter reaction time at 22.75 h (Figure 2, Supplementary Data 4). Relative to baseline, occasional smokers had greater decreases in 2-back reaction time compared with frequent smokers at 22.75 h; there was a trend toward decreased reaction time in occasional smokers and increased reaction times in frequent smokers on the 2-back at 3.75 h (Figure 2, Supplementary Data 4).
Mean (standard deviation) for accuracy, reaction time and errors of commission on the n-back for 14 frequent and 10 occasional cannabis smokers following controlled smoking of a 6.8% THC (54 mg) cannabis cigarette.
Risk-taking and impulsivity
Missing data from participants V and X (occasional smokers) (5.9 h) and participant J (frequent smoker) (22.9 h) were replaced with mean group values; data from participant Y (occasional smoker) were not replaced because mean group values were substantially higher than his adjusted pumps, potentially skewing results towards more risk-taking behavior after smoking. There were no BART baseline differences between occasional and frequent smokers (Supplementary Data 2). There was an overall increase over sessions for number of adjusted pumps on the BART (F(4,88) = 3.72, P < 0.01), but no significant group or session by group interaction (Supplementary Data 3). Contrast indicated no session or session by group interaction different from baseline, although there was a trend towards higher adjusted pumps at 22.9 h compared with baseline (Supplementary Data 4).
In occasional smokers, positive correlations between baseline adjusted pump number and BIS total and Risk perception scores were noted, and trends for positive correlations between baseline adjusted pump number and BIS-11 attentional impulse, BIS total non-planning impulsiveness, ZKPQ impulsivity and RPQ neutral were documented (Supplementary Data 5). In frequent smokers, no subjective scales significantly correlated with baseline adjusted pump numbers, but there was a positive trend in MDM vigilance scale.
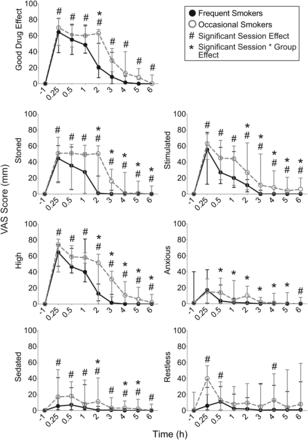
Subjective measures
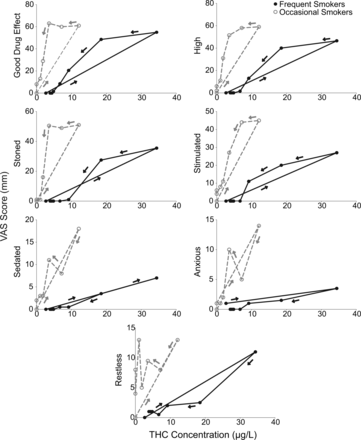
All VAS measures significantly increased after cannabis smoking, and later decreased over time (Figure 3). Occasional smokers had significantly higher scores than frequent smokers for ‘High’ (F(1,23) = 4.81, P < 0.05) and ‘Stimulated’ (F(1,23) = 6.19, P < 0.05), and more intense anxiety increases, as evidenced by session by group interaction (F(2.8,64.2) = 3.46, P < 0.05) (Supplementary Data 3). Session by group contrasts revealed more intense subjective effects 2 h after smoking in occasional smokers (Figure 3, Supplementary Data 4). Subjective VAS scores displayed clear counter-clockwise hysteresis for ‘Good Drug Effect’, ‘High’, ‘Stoned’ and ‘Stimulated’, and were displaced to the right in frequent smokers because of their higher THC body burden and possibly different smoking typography leading to higher THC concentrations (Figure 4).
Mean (standard deviation) Visual Analog Scale (VAS) score for 14 frequent and 11 occasional cannabis smokers following controlled smoking of a 6.8% THC (54 mg) cannabis cigarette.
Median VAS scores in function of THC concentrations for 14 frequent and 11 occasional cannabis smokers following controlled smoking of a 6.8% THC (54 mg) cannabis cigarette.
Significantly higher scores were reported by occasional than frequent smokers for ‘Difficulty Concentrating’ at 3 h, ‘Altered Sense of Time’ at 3 and 4 h, ‘Feel Hungry’ at 5 h, ‘Feel Thirsty’ at 4 and 6 h, ‘Shakiness/Tremulousness’ at 2 h and ‘Dry Mouth or Throat’ at 2–6 h (Supplementary Data 6).
Physiological measures
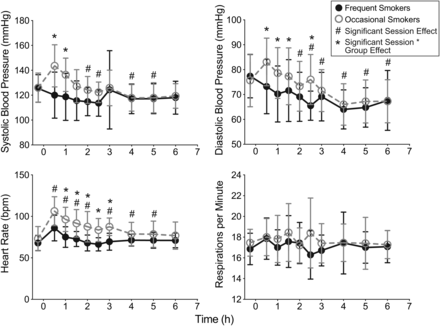
Session by group interactions were documented for heart rate, systolic and diastolic blood pressures (F(4.4,100.3) = 3.66, F(4.9,113.2) = 2.67 and F(9,207) = 2.31, respectively, P < 0.05 Supplementary Data 3). Compared with frequent smokers, occasional smokers had significantly increased heart rates (0.5–3 h post-dose), relative to baseline and higher systolic and diastolic blood pressure just after dosing (1 h) (Figure 5, Supplementary Data 4).
Mean (standard deviation) physiological measures for 14 frequent and 11 occasional cannabis smokers following controlled smoking of a 6.8% THC (54 mg) cannabis cigarette.
Discussion
We documented that cannabis smoking impaired psychomotor function (tracking error, hits, false alarms and reaction time), more so in occasional smokers, suggesting some tolerance to psychomotor impairment in frequent smokers despite higher blood THC concentrations. Furthermore, occasional smokers reported significantly longer and more intense subjective effects compared with frequent smokers who had higher THC concentrations. Occasional smokers had significantly higher heart rate increases than frequent smokers following cannabis smoking. Although both occasional and frequent smokers exhibited overall blood pressure decreases following smoking, occasional smokers also had blood pressure increases 30 min after smoking. Cannabis smoking did not elicit session × group effects on the n-back (working memory) or BART (risk-taking) performance.
Psychomotor control and divided attention
Cannabis significantly impaired psychomotor function up to 3.5 h after smoking, with more impairment in occasional smokers, suggesting some tolerance development in frequent smokers. These findings suggest that following cannabis smoking, people may have more difficulty controlling a car, dividing their attention and reacting at normal speeds.
Our data are consistent with prior data documenting impairment in the CTT and DAT within 1 h after cannabis smoking in occasional smokers (20). In that study, cannabis also increased tracking error, control losses and decreased hits; reaction time was not monitored. Other studies examining frequent smokers' performance report conflicting results for cannabis' psychomotor effects, with some documenting no effects (40), some reporting impairment after smoking a 17 mg THC cigarette (41) and others reporting improvement on a divided attention task following a 3.9% but not 1.8% THC cigarette (9). Differences in effects may be due to tolerance development, improvement by attenuation of withdrawal or sample size and power. A larger study (n = 61) documented psychomotor impairment in frequent smokers following smoking of a single cannabis dose (42). Others documented that ethanol at 0.05–0.07 mg/dL BAC also significantly reduced lambda-c in the critical tracking task and increased tracking error, control losses and reaction times (43, 44).
Spatial working memory
We found minimal spatial working memory impairment following cannabis smoking (6.8% THC). Session effects were documented for 2-back accuracy, and 1- and 2-back reaction times but no overall session × group effects. Others documented significant deficits in working memory following acute cannabis intake in adults (45, 46) and following at least 12 h cannabis abstinence in adolescents (47). Other studies documented no effects on n-back accuracy, but increases in reaction time at 60 and 110 min after a 3.9% and at 60 min after 1.8 and 3.9% THC cigarettes in 24 frequent smokers (48).
Previous work documented decreased accuracy compared with placebo in occasional smokers at moderate, but not low and high loads; reaction time was longer at medium and only some of high loads (49, 50). This is similar to our results, documenting significantly lengthened reaction time on the 1- and 2-back but not the 3-back task. The authors postulated that impairment at lower loads may be explained by psychomotor rather than working memory impairment, as participants only needed to identify the target, rather than correctly memorizing which item lit up n times before (49). On the 2-back task, the significantly shorter reaction time at 22.75 h in occasional smokers might reflect the baseline differences between occasional and frequent smokers', rather than a drug effect. Others documented that although scores were similar between groups, cannabis smokers had significantly more brain activation during a simple spatial working memory task in areas typically associated with this function, and also required additional regions not associated with spatial working memory, possibly because cannabis smokers may compensate for neurophysiological deficits by ‘working harder’ and calling upon additional brain regions to meet task demands (51, 52). Hence, while participants in the present study may not have significant deficits during the n-back, neural functioning may have been altered. The lack of observed significant differences on the n-back also could have been influenced by practice effects countering potential drug effects. Furthermore, recent research suggested that the n-back task is not a pure measure of working memory, but may be better at identifying subtle differences in cognitive functioning such as fluid intelligence (53–55).
Risk-taking and impulsivity
Our finding of no acute effect of cannabis smoking on risk-taking behavior contrasts with previous research documenting significant THC-induced increases in risky choices (41, 42, 56). Others documented no increased risk-taking, but significantly slower decision-making time (57). Lane et al. (56) observed effects on risk-taking with a 3.58% THC cigarette, but not with cigarettes containing lower THC (1.77%) concentrations. McDonald et al. (58) found that 15 mg oral dronabinol (THC) yielded mixed results across four different impulsivity tasks. This difference may reflect decreased bioavailability and lower peak blood THC concentrations following oral dronabinol administration compared with smoked cannabis.
Subjective measures
Cannabis smoking produced expected increases in subjective ratings. Occasional smokers reported significantly longer and more intense subjective effects compared with frequent smokers. In particular, cannabis smoking significantly increased ratings of ‘Anxiety’ only in occasional smokers. This supports previous data documenting stronger subjective effectives in occasional compared with frequent cannabis smokers (20), and suggests that there can be substantial tolerance to subjective effects of cannabis.
Counter-clockwise hysteresis, a phenomenon in which stronger effects occur during the distribution and/or elimination phases of the time–concentration curve than during absorption, even at the same THC concentrations, was documented for ‘Good Drug Effect’, ‘High’, ‘Stoned’ and ‘Stimulated’. Although counter-clockwise hysteresis was present in both groups for ‘Good Drug Effect’, ‘High’, ‘Stoned’ and ‘Stimulated’, hysteresis profiles were substantially different due to significantly higher blood concentrations in frequent smokers (39) and higher subjective effects in occasional smokers.
Physiological measures
Cannabis smoking produced expected increases in heart rate and decreases in blood pressure; occasional smokers had significantly higher heart rate increases than frequent smokers and exhibited blood pressure increases prior to decreases, suggesting possible frequent smoker tolerance to cannabis' cardiovascular effects. This is only partially consistent with prior studies, which found significant tolerance to heart rate, but not blood pressure effects, in frequent smokers (20, 59).
The initial increase in blood pressure in occasional smokers, followed by the expected decrease, was not previously reported, to our knowledge. This initial increase was not observed in frequent smokers, potentially due to cannabis tolerance in this group.
Limitations
A placebo session and larger sample size may have improved power to identify significant cannabis effects. We also excluded some participants' data due to poor task performance. Our findings should be interpreted cautiously, as observed changes from baseline (session effects) include smoked cannabis' effects, but also potential practice, fatigue or boredom effects. Training to stable baseline performance prior to the smoked cannabis session should have minimized potential practice effects; however, except for risk-taking, practice effects would improve scores, rather than produce deficits, as observed in this study. Nevertheless group × session interaction effects (to which we focused our discussion) should reflect true drug effects, as learning, fatigue or boredom effects should be similar between groups.
Conclusions
We documented significant differences between occasional and frequent cannabis smokers in psychomotor, subjective and physiological effects following cannabis smoking, with weaker effects in frequent smokers suggesting tolerance development. Impairment domains included those that play a key role in a driver's ability to accurately control a car or to react to events on the road. These data help advance our understanding of cannabis' effects and will be valuable for interpretation of driving under the influence of cannabis and accident responsibility cases.
Funding
This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH.
Acknowledgments
We acknowledge the contributions of the clinical staffs of the National Institute on Drug Abuse, Intramural Research Program and Behavioral Pharmacology Research Unit and Clinical Research Unit, Johns Hopkins Bayview Medical Center; Drs David M. Schwope and Sandrine Pirard; the Graduate Partnership Program, NIH; and the ‘Fondation Baxter et Alma Ricard’ for NAD's graduate fellowship. This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH.
References