-
PDF
- Split View
-
Views
-
Cite
Cite
Saskia Hendriks, Eline A.F. Dancet, Ans M.M. van Pelt, Geert Hamer, Sjoerd Repping, Artificial gametes: a systematic review of biological progress towards clinical application, Human Reproduction Update, Volume 21, Issue 3, May/June 2015, Pages 285–296, https://doi.org/10.1093/humupd/dmv001
Close - Share Icon Share
Abstract
Recent progress in the formation of artificial gametes, i.e. gametes generated by manipulation of their progenitors or of somatic cells, has led to scientific and societal discussion about their use in medically assisted reproduction (MAR). Artificial gametes could potentially help infertile men and women but also post-menopausal women and gay couples conceive genetically related children. This systematic review aimed to provide insight in the progress of biological research towards clinical application of artificial gametes.
The electronic database ‘Medline/Pubmed’ was systematically searched with medical subject heading (MesH) terms, and reference lists of eligible studies were hand searched. Studies in English between January 1970 and December 2013 were selected based on meeting a priori defined starting- and end-points of gamete development, including gamete formation, fertilization and the birth of offspring. For each biologically plausible method to form artificial gametes, data were extracted on the potential to generate artificial gametes that might be used to achieve fertilization and to result in the birth of offspring in animals and humans.
The systematic search yielded 2424 articles, and 70 studies were included after screening. In animals, artificial sperm and artificial oocytes generated from germline stem cells (GSCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have resulted in the birth of viable offspring. Also in animals, artificial sperm and artificial oocytes have been generated from somatic cells directly, i.e. without documentation of intermediate stages of stem- or germ cell development or (epi)genetic status. Finally, although the subsequent embryos showed hampered development, haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte has led to fertilized artificial oocytes. In humans, artificial sperm has been generated from ESCs and iPSCs. Artificial human oocytes have been generated from GSCs, ESCs and somatic cells (without documentation of intermediate stages of stem- or germ cell development). Fertilization of a human artificial oocyte after haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte was also reported. Normal developmental potential, epigenetic and genetic stability and birth of children has not been reported following the use of human artificial gametes. In animals, artificial oocytes from a male have been created and fertilized and artificial sperm from a female has been fertilized and has resulted in the birth of viable offspring. In humans, artificial sperm has been generated from female iPSCs. To date, no study has reported the birth of human offspring from artificial gametes.
Our systematic review of the literature indicated that in animals live births have already been achieved using artificial gametes of varying (cell type) sources. Although experimental biological research is progressing steadily towards future clinical application, data on functionality, safety and efficiency of (human) artificial gametes are still preliminary. Although defining artificial gametes by start- and end-points limited the number of included studies, the search resulted in a clear overview of the subject. Clinical use of artificial gametes would expand the treatment possibilities of MAR and would have implications for society. Before potential clinical use, the societal and ethical implications of artificial gametes should be reflected on.
Introduction
Estimates of the 12-month prevalence rate of infertility range from 3 to 17% in more developed nations, where more than half of the couples confronted with infertility seek medical help (Boivin et al., 2007). Reproductive medicine is currently able to help only ∼70% of the couples to deliver a child within 5 years after visiting a fertility clinic while relying on either patients' own or donor gametes (Pinborg et al., 2008). Donor gametes are used in the case of failed homologue treatment and are the only current treatment option for patients without functional gametes, which includes men with non-obstructive azoospermia (0.63% of the general population; Jarow et al., 1989) and women with premature ovarian insufficiency (0.9% of the general population; Coulam et al., 1986). Moreover, gay couples are relying on donor gametes. Unfortunately, the current use of donor gametes does not lead to genetic parenthood, which is valued by most couples, although its objective importance is currently debated (Golomblok et al., 2006; Mertes and Pennings, 2008).
A potential future treatment alternative to using donor gametes is the use of so-called ‘artificial gametes’, i.e. gametes generated by manipulation of their progenitors or of somatic cells, which would allow children who are genetically related to both their parents to be conceived. The possibility of full genetic parenthood could also apply to gay couples, if reprogramming to the other sex germ line becomes an option, and to women of post-menopausal age.
Although the potential use of artificial gametes in medically assisted reproduction (MAR) has led to scientific and societal discussion, a systematic overview of the biological progress towards clinical application is lacking. This systematic review aimed to provide insight into the biological progress on all biological plausible routes to create artificial gametes in animals and humans, without evaluating quality, quantity and safety of the different approaches, which are currently unknown.
Methods
Search strategy
The electronic database MEDLINE was systematically searched with the search engine PubMed using the following medical subject heading terms (MeSH-terms): ‘cell differentiation’, ‘cells, cultured’, ‘cell culture techniques’, ‘cytoplasm/transplantation’, ‘diploidy’, ‘DNA, mitochondrial’, ‘embryonic stem cells’, ‘embryo transfer’, ‘female’, ‘fertilization’, ‘genotype’, ‘germ cells’, ‘germ cells/cytology’, ‘haploidy’, ‘human reproduction, assisted’, ‘meiosis’, ‘microinjections’, ‘micromanipulaton’, ‘nuclear transfer techniques’, ‘oocytes’, ‘oocytes/cytology’, ‘oocytes/physiology’, ‘oocytes/transplantation’, ‘oogenesis’, ‘oogonia/cytology’, ‘pluripotent stem cells’, ‘pregnancy’, ‘pregnancy outcome’, ‘reproductive techniques, assisted/ethics’, ‘reproductive techniques/ethics’, ‘spermatogenesis’, ‘spermatogonia/cytology’, ‘spermatozoa/cytology’, ‘stem cells/cytology’ and ‘stem cells/metabolism’. The reference lists of the eligible articles were subsequently hand searched (i.e. snowball strategy).
Article selection
Articles reporting on artificial gametes, published in English between January 1970 and December 2013, were considered for inclusion by screening their titles, abstracts and if necessary full-text reports.
Only studies reporting on originally collected biological data were considered, while reviews, opinion studies and other non-original work were excluded. Furthermore, studies needed to report on specific biological starting and end-points, which were defined a priori.
As starting points, i.e. cells that in a clinical application would be derived from the patient, adult GSCs and differentiated somatic cells were defined.
Adult GSCs with the reported capacity of migration to the stem cell niche in vivo after (xeno)transplantation (male GSCs) or proof of survival in vivo after (xeno)transplantation (female GSCs) were included as starting points. For the purpose of this review, we exclude the use of primordial germ cells, which, although they too are GSCs, cannot be retrieved from an adult commissioning parent and are thus not clinically relevant as starting point of treatment.
Differentiated somatic cells could be a starting point for artificial gamete formation in three ways.
First, artificial gametes could be formed from differentiated somatic cells via embryonic stem cells (ESCs). For clinical application, this would require somatic cell nuclear transfer of a patient nucleus into a donor ESC (Tachibana et al., 2013a, b); in this way, the ESCs contain the commissioning parent's genetic material. For the purpose of this review, however, ESCs as starting point of preclinical research were accepted for inclusion.
Second, artificial gametes could be formed from differentiated somatic cells via induced pluripotent stem cells (iPSCs). Articles that have iPSCs as starting point or use somatic cell reprogramming by ectopic expression were also included.
Third, artificial gametes could be formed from differentiated somatic cells by ‘direct’ differentiation into gametes without the occurrence or documentation of our defined intermediate stages of stem- and germ cell development [i.e. ESCs, iPSCs and germline stem cells (GSC)]; in this review referred to as ‘without documented intermediate stages’.
The following end-points were defined: artificial gamete formation, fertilization and birth of offspring.
For males, the formation of artificial gametes was defined as: the presence of haploid male sperm cells, as proven by DNA content analyses or similar techniques and/or the presence of post-meiotic markers. This does not include sperm morphology, motility and fertilization potential without the use of ICSI. For females, artificial gamete formation was defined as: the presence of metaphase II secondary oocytes proven by DNA content analyses, meiotic markers and/or polar body extrusion. This does not include epigenetic normality and developmental potential, for example, to, or past, the blastocyst stage. These conditions were chosen for the review as they describe gametes which could be used for MAR, yet they are not necessarily genetically and epigenetically equal to normal gametes with respect to quality and developmental potential. They will therefore all be referred to as artificial sperm or artificial oocytes.
Fertilization was defined as the presence of a zygote with two pronuclei or a cleavage-stage embryo in which donor-derived DNA is detected.
Birth of offspring was defined as the birth of one or more viable offspring, in which donor-derived DNA is detected.
Therefore, for example, studies that reported on spermatogenesis until the stage of the first meiotic division were excluded, as they did not achieve one of the three predefined end-points.
Meta-synthesis
Data were extracted using standardized data extraction sheets. Given the nature of the collected data, meta-synthesis, rather than meta-analyses was performed. The meta-synthesis was performed by two reviewers, independently, who discussed until consensus was met. In this way, the chance of a human error in classification or interpretation was reduced.
To structure the abundance of biological data, three strategies were used.
First, data were organized by the sex of the animal or commissioning parent whose genetic material was used (male or female) and by the type of artificial gamete that was formed (sperm or oocyte). More specifically, besides artificial generation of sperm from a male and of oocytes from a female, theoretically, it could be possible to create artificial sperm from a female and artificial oocytes from a male. Clinically, the latter could be relevant for gay couples wishing to have a child that is genetically related to both of them, instead of having to rely on donor gametes.
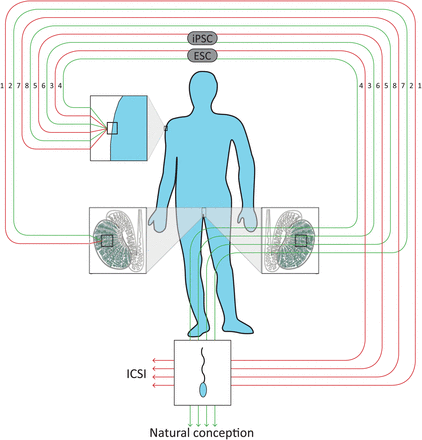
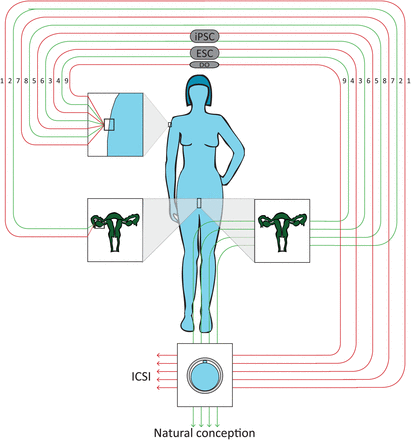
Second, nine biologically plausible routes of artificial gamete generation were defined based on insight in the literature and clinically available cell types, as described in Figs 1 and 2. Of the nine routes, two routes (Routes 1 and 2; the nature of Route 2 is exemplified in Figs 1 and 2) start from adult GSCs of the animal or commissioning parent whose genetic material will be used. Adult GSCs are spermatogonial stem cells (SSCs) or oogonial stem cells (OSCs), which would have to be retrieved from, respectively, the testis or ovary of the animal or commissioning parent whose genetic material will be used. The other seven routes (Routes 3–9) start from a somatic cell of the animal or commissioning parent.
Roadmap to the eight biologically plausible routes of artificial sperm formation in men. (1) In vitro differentiation of GSCs. (2) In vitro proliferation of GSCs followed by autotransplantation. (3) In vitro differentiation of ESCs. (4) In vitro differentiation of ESCs followed by autotransplantation. (5) In vitro differentiation of iPSCs. (6) In vitro differentiation of iPSCs followed by autotransplantation. (7) In vitro somatic cell transformation into gametes without documented transitional cell types. (8) In vivo somatic cell transformation into gametes without documented transitional cell types.
Roadmap to the nine biologically plausible routes of artificial oocyte formation in women. (1) In vitro differentiation of GSCs. (2) In vitro proliferation of GSCs followed by autotransplantation. (3) In vitro differentiation of ESCs. (4) In vitro differentiation of ESCs followed by autotransplantation. (5) In vitro differentiation of iPSCs. (6) In vitro differentiation of iPSCs followed by autotransplantation. (7) In vitro somatic cell transformation into gametes without documented transitional cell types. (8) In vivo somatic cell transformation into gametes without documented transitional cell types. (9) Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte (DO).
Four routes starting from a somatic cell (Routes 3–6) require either ESCs (Routes 3 and 4) or iPSCs (Routes 5 and 6; the nature of Route 5 is exemplified in Figs 1 and 2) as transitional cell types. To generate transitional ESCs, the nucleus of an ESC of a donor embryo has to be replaced by the nucleus of a somatic cell from the animal or commissioning parent (Tachibana et al., 2013a, b), while iPSCs result directly from reprogramming somatic cells from the animal or commissioning parent (Takahashi et al., 2007a, b).
In the remaining three routes starting from somatic cells, artificial gametes are formed without a documented intermediate stage: somatic cell transformation into gametes without transitional cell types (Routes 7 and 8) and haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte (Route 9), in which the somatic cell nucleus of the commissioning mother is injected into an enucleated (donor) oocyte, which by division induces haploidization of the somatic nucleus.
Third, for each potential route of artificial gamete generation, clinical applications are specified, as shown in Table I. For the treatment of heterosexual couples (or infertile singles relying on material from a healthy donor instead of a partner), eight routes are biologically plausible to result in artificial sperm from a male, whereas nine routes are biologically plausible to result in artificial oocytes from a female. The routes for which GSCs are the starting point obviously cannot be used for patients whose gonads do not contain GSCs.
Studies demonstrating possible routes to create artificial gametes.
| Route creating artificial gamete . | Most advanced outcomes reacheda . | |||||
|---|---|---|---|---|---|---|
| Animal model . | Human . | |||||
| Gamete . | Fertilization . | Offspring . | Gamete . | Fertilization . | Offspring . | |
| Artificial sperm from male | ||||||
| (1) In vitro differentiation of germline stem cells (GSCs) | 1 | — | 2 | — | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | 3, 4, 5 | 6 | 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 | — | — | — |
| (3) In vitro differentiation of embryonic stem cells (ESCs) | — | 24, 25, 26 | 27 | 28, 29, 30, 31, 32 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | 33 | — | 34, 35, 36, 37 | |||
| (5) In vitro differentiation of induced pluripotent stem cell (iPSCs) | 38 | — | — | 29, 39, 31 | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | 40 | — | 35 | — | — | — |
| (7) In vitro somatic cell transformation into sperm without documented transitional cell types | — | — | — | — | — | — |
| (8) In vivo somatic cell transformation into sperm without documented transitional cell types | — | — | 41 | — | — | — |
| Artificial oocyte from female | ||||||
| (1) In vitro differentiation of GSCs | 42 | — | — | 42 | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | — | 42 | 43 | 42 | — | — |
| (3) In vitro differentiation of ESCs | 26, 44, 45, 46 | — | 47, 48 | 28 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | — | — | — | — | — | — |
| (5) In vitro differentiation of iPSCs | — | — | 47, 48 | — | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | — | — | — | — | — | — |
| (7) In vitro somatic cell transformation into oocytes without documented transitional cell types | 49, 50 | — | — | 51, 52 | — | — |
| (8) In vivo somatic cell transformation into oocytes without documented transitional cell types | 53, 54 | — | — | — | — | — |
| (9) Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte | 55, 56, 57, 58, 59, 60, 61 | 62 | — | 58, 63 | 59, 64, 65 | — |
| Artificial oocytes from a male | 44 | 24 | — | — | — | — |
| Artificial sperm from a female | — | 66, 67 | 68, 69, 70 | 39 | — | — |
| Route creating artificial gamete . | Most advanced outcomes reacheda . | |||||
|---|---|---|---|---|---|---|
| Animal model . | Human . | |||||
| Gamete . | Fertilization . | Offspring . | Gamete . | Fertilization . | Offspring . | |
| Artificial sperm from male | ||||||
| (1) In vitro differentiation of germline stem cells (GSCs) | 1 | — | 2 | — | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | 3, 4, 5 | 6 | 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 | — | — | — |
| (3) In vitro differentiation of embryonic stem cells (ESCs) | — | 24, 25, 26 | 27 | 28, 29, 30, 31, 32 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | 33 | — | 34, 35, 36, 37 | |||
| (5) In vitro differentiation of induced pluripotent stem cell (iPSCs) | 38 | — | — | 29, 39, 31 | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | 40 | — | 35 | — | — | — |
| (7) In vitro somatic cell transformation into sperm without documented transitional cell types | — | — | — | — | — | — |
| (8) In vivo somatic cell transformation into sperm without documented transitional cell types | — | — | 41 | — | — | — |
| Artificial oocyte from female | ||||||
| (1) In vitro differentiation of GSCs | 42 | — | — | 42 | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | — | 42 | 43 | 42 | — | — |
| (3) In vitro differentiation of ESCs | 26, 44, 45, 46 | — | 47, 48 | 28 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | — | — | — | — | — | — |
| (5) In vitro differentiation of iPSCs | — | — | 47, 48 | — | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | — | — | — | — | — | — |
| (7) In vitro somatic cell transformation into oocytes without documented transitional cell types | 49, 50 | — | — | 51, 52 | — | — |
| (8) In vivo somatic cell transformation into oocytes without documented transitional cell types | 53, 54 | — | — | — | — | — |
| (9) Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte | 55, 56, 57, 58, 59, 60, 61 | 62 | — | 58, 63 | 59, 64, 65 | — |
| Artificial oocytes from a male | 44 | 24 | — | — | — | — |
| Artificial sperm from a female | — | 66, 67 | 68, 69, 70 | 39 | — | — |
ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; GSC, germline stem cell; —, refers to no publication reporting on the respective outcome as a furthest end-point.
aNumbers (Supplementary data) indicate the appropriate reference with the respective outcome as furthest end-point.
Studies demonstrating possible routes to create artificial gametes.
| Route creating artificial gamete . | Most advanced outcomes reacheda . | |||||
|---|---|---|---|---|---|---|
| Animal model . | Human . | |||||
| Gamete . | Fertilization . | Offspring . | Gamete . | Fertilization . | Offspring . | |
| Artificial sperm from male | ||||||
| (1) In vitro differentiation of germline stem cells (GSCs) | 1 | — | 2 | — | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | 3, 4, 5 | 6 | 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 | — | — | — |
| (3) In vitro differentiation of embryonic stem cells (ESCs) | — | 24, 25, 26 | 27 | 28, 29, 30, 31, 32 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | 33 | — | 34, 35, 36, 37 | |||
| (5) In vitro differentiation of induced pluripotent stem cell (iPSCs) | 38 | — | — | 29, 39, 31 | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | 40 | — | 35 | — | — | — |
| (7) In vitro somatic cell transformation into sperm without documented transitional cell types | — | — | — | — | — | — |
| (8) In vivo somatic cell transformation into sperm without documented transitional cell types | — | — | 41 | — | — | — |
| Artificial oocyte from female | ||||||
| (1) In vitro differentiation of GSCs | 42 | — | — | 42 | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | — | 42 | 43 | 42 | — | — |
| (3) In vitro differentiation of ESCs | 26, 44, 45, 46 | — | 47, 48 | 28 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | — | — | — | — | — | — |
| (5) In vitro differentiation of iPSCs | — | — | 47, 48 | — | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | — | — | — | — | — | — |
| (7) In vitro somatic cell transformation into oocytes without documented transitional cell types | 49, 50 | — | — | 51, 52 | — | — |
| (8) In vivo somatic cell transformation into oocytes without documented transitional cell types | 53, 54 | — | — | — | — | — |
| (9) Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte | 55, 56, 57, 58, 59, 60, 61 | 62 | — | 58, 63 | 59, 64, 65 | — |
| Artificial oocytes from a male | 44 | 24 | — | — | — | — |
| Artificial sperm from a female | — | 66, 67 | 68, 69, 70 | 39 | — | — |
| Route creating artificial gamete . | Most advanced outcomes reacheda . | |||||
|---|---|---|---|---|---|---|
| Animal model . | Human . | |||||
| Gamete . | Fertilization . | Offspring . | Gamete . | Fertilization . | Offspring . | |
| Artificial sperm from male | ||||||
| (1) In vitro differentiation of germline stem cells (GSCs) | 1 | — | 2 | — | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | 3, 4, 5 | 6 | 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 | — | — | — |
| (3) In vitro differentiation of embryonic stem cells (ESCs) | — | 24, 25, 26 | 27 | 28, 29, 30, 31, 32 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | 33 | — | 34, 35, 36, 37 | |||
| (5) In vitro differentiation of induced pluripotent stem cell (iPSCs) | 38 | — | — | 29, 39, 31 | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | 40 | — | 35 | — | — | — |
| (7) In vitro somatic cell transformation into sperm without documented transitional cell types | — | — | — | — | — | — |
| (8) In vivo somatic cell transformation into sperm without documented transitional cell types | — | — | 41 | — | — | — |
| Artificial oocyte from female | ||||||
| (1) In vitro differentiation of GSCs | 42 | — | — | 42 | — | — |
| (2) In vitro proliferation of GSCs followed by autotransplantation | — | 42 | 43 | 42 | — | — |
| (3) In vitro differentiation of ESCs | 26, 44, 45, 46 | — | 47, 48 | 28 | — | — |
| (4) In vitro differentiation of ESCs followed by autotransplantation | — | — | — | — | — | — |
| (5) In vitro differentiation of iPSCs | — | — | 47, 48 | — | — | — |
| (6) In vitro differentiation of iPSCs followed by autotransplantation | — | — | — | — | — | — |
| (7) In vitro somatic cell transformation into oocytes without documented transitional cell types | 49, 50 | — | — | 51, 52 | — | — |
| (8) In vivo somatic cell transformation into oocytes without documented transitional cell types | 53, 54 | — | — | — | — | — |
| (9) Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte | 55, 56, 57, 58, 59, 60, 61 | 62 | — | 58, 63 | 59, 64, 65 | — |
| Artificial oocytes from a male | 44 | 24 | — | — | — | — |
| Artificial sperm from a female | — | 66, 67 | 68, 69, 70 | 39 | — | — |
ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; GSC, germline stem cell; —, refers to no publication reporting on the respective outcome as a furthest end-point.
aNumbers (Supplementary data) indicate the appropriate reference with the respective outcome as furthest end-point.
For the treatment of gay couples, five routes might theoretically result in artificial oocytes from males, and six routes may result in artificial sperm from females.
Data were extracted for each of the nine plausible routes of artificial gamete formation based on the achievement of the farthest of the following end-points: artificial gamete formation, fertilization and birth of offspring. This review reports only on the farthest end-point reached, because of clinical relevance and because not all studies documented the achievement of intermediate end-points (e.g. a study reporting birth of offspring might not have investigated or documented fertilization). Achievements from animal and human research were differentiated and the type of animals examined was specified.
Results
Search strategy
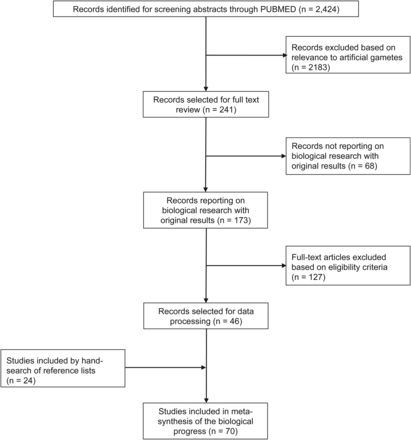
The systematic search yielded 2424 articles (Fig. 3). Based on eligibility, 46 studies were included. Hand searches of the reference lists of these studies, resulted in the inclusion of 24 additional studies. Thus, in total, 70 studies were included.
Meta-synthesis
Table I reports on the most advanced end-points achieved in animals and humans of all nine biologically plausible routes for the formation of artificial sperm.
Artificial sperm from a male
Of the eight biologically plausible routes leading to the formation of artificial sperm from a male (Fig. 1), seven were reported in literature as achieving at least one of the specified end-points in animals and/or humans (Table I). In animal research, for one route (Route 5), the formation of artificial sperm was achieved as the most advanced end-point, while for six routes (Routes 1, 2, 3, 4, 6 and 8), one or more studies reported the birth of viable offspring. In humans, only two of the biologically plausible routes to artificial gamete formation (Routes 3 and 5) actually led to the generation of artificial sperm. In humans, no studies reported on fertilization or the birth of offspring.
In vitro differentiation of SSCs
In vitro culture and differentiation of SSCs resulted in artificial sperm in cattle (Izadyar et al., 2002). Birth of offspring was achieved in mice (Sato et al., 2011).
In vitro proliferation of SSCs followed by (auto)transplantation
In vivo haploidization after in vitro proliferation of SSCs resulted in the formation of artificial sperm in mice (Nagano et al., 2000; Lee et al., 2009) and rats (Hamra et al., 2005). Fertilization was reported in hamsters (Kanatsu-Shinohara et al., 2008). Birth of offspring was reported in mice (Kanatsu-Shinohara et al., 2003, 2005a, b, 2006, 2010, 2011; Takahashi and Yamanaks, 2006; Kubota et al., 2004, 2009; Kita et al., 2007; Nagano et al., 2001; Ohta et al., 2009; Shiura et al., 2013), rats (Hamra et al., 2002; Ryu et al., 2005; Wu et al., 2009) and zebrafish (Kawasaki et al., 2012).
In vitro differentiation of ESCs
In mice, this route resulted in fertilization (Geijsen et al., 2003; Kerkis et al., 2007; Yu et al., 2009). In one report, the birth of offspring was reported (Nayernia et al., 2006). Human artificial sperm, expressing post-meiotic markers and/or haploidization attested by RNA expression analyses and DNA content analysis, respectively, has been generated from ESCs (Aflatoonian et al., 2009; Kee et al., 2009; Panula et al., 2011; West et al., 2011; Easley et al., 2012).
In vitro differentiation of ESCs followed by autotransplantation
In mouse studies, in vitro differentiation of ESCs followed by autotransplantation resulted in the formation of artificial sperm (Toyooka et al., 2003) and the birth of offspring (Chuma et al., 2004; Ohinata et al., 2009; Hayashi et al., 2011; Nakaki et al., 2013).
In vitro differentiation of iPSCs
Artificial sperm cells were created by in vitro iPSC differentiation in mice (Yang et al., 2012). Human artificial sperm was also created by in vitro differentiation of iPSCs (Eguizabal et al., 2011; Panula et al., 2011; Easley et al., 2012), some of which revealed incomplete imprinting (Eguizabal et al., 2011).
In vitro differentiation of iPSCs followed by autotransplantation
Differentiating iPSCs in vitro followed by autotransplantation resulted in the formation of artificial sperm in mice (Zhu et al., 2012). Mouse offspring were born from iPSCs that were differentiated into primordial germ cell-like cells (PGCLCs) in vitro and subsequently formed artificial sperm in vivo after transplantation into the seminiferous tubules (Hayashi et al., 2011).
In vitro somatic cell transformation into sperm without documented intermediate cell types
No studies achieving artificial sperm formation, fertilization or birth of offspring via this route were identified.
In vivo somatic cell transformation into sperm without documented intermediate cell types
Injection of mesenchymal stem cells into rats, previously sterilized by busulfan treatment, led to fertility and live births (Cakici et al., 2013).
Artificial oocytes from a female
Of the nine biologically plausible routes to artificial oocyte formation from a female (Fig. 2), seven achieved at least one of the specified end-points in animals and/or humans (Table I). In animal research, as the most advanced end-point, formation of animal artificial oocytes was achieved via three routes (Routes 1, 7 and 8), fertilization via one route (Route 9) and the birth of offspring was achieved via three routes (Routes 2, 3 and 5). In humans, four routes (Routes 1, 2, 3 and 7) achieved the formation of artificial oocytes as farthest end-point and one route (Route 9) resulted in fertilization.
In vitro differentiation of OSCs
Both mouse and human female germ stem cells have undergone meiosis in vitro, resulting in artificial oocytes (White et al., 2012).
In vitro proliferation of OSCs followed by (auto)transplantation
After proliferation in vitro, mouse OSCs were transplanted to ovaries of recipient mice and the resulting artificial oocytes could be fertilized (White et al., 2012) and, in another study, contributed to the birth of offspring (Zou et al., 2009). Human OSCs were proliferated in vitro and transplantated to human ovary tissue xenografted into mice, resulting in the formation of artificial oocytes (White et al., 2012).
In vitro differentiation of ESCs
Starting with ESCs, in vitro differentiation led to the formation of artificial oocytes in mice but did not result in viable embryos or offspring so far (Hübner et al., 2003; Qing et al., 2007; Salvador et al., 2008; Yu et al., 2009). Offspring were born in mice by differentiating iPSCs into PGCLCs that formed aggregates in culture that, after being grafted under the ovary bursa, formed mature artificial oocytes under in vivo-like conditions (Hayashi et al., 2012; Hayashi and Saitou, 2013). In humans, follicle-like structures were formed, but no oocytes with zona pellucida were identified (Aflatoonian et al., 2009).
In vitro differentiation of ESCs followed by testicular transplantation
No studies achieving artificial oocyte formation, fertilization or birth of offspring via this route were identified.
In vitro differentiation of iPSCs
Offspring were born in mice by differentiating iPSCs into PGCLCs that formed aggregates in culture that, after being grafted under the ovary bursa, formed mature artificial oocytes under in vivo-like conditions (Hayashi et al., 2012; Hayashi and Saitou, 2013).
In vitro differentiation of iPSCs followed by autotransplantation
No studies achieving artificial oocyte formation, fertilization or birth of offspring via this route were identified.
In vitro somatic cell transformation into oocytes without documented intermediate cell types
Somatic cells were shown to develop into artificial oocytes starting from rat pancreatic stem cells (Danner et al., 2006) and porcine skin stem cells (Dyce et al., 2006) with uncharacterized genomic integrity and epigenetic status. In humans, amniotic stem cells differentiated in vitro into artificial oocytes with unknown ploidy status and a diameter of 50–60 μm (Cheng et al., 2012) and a hepatic cell line differentiated in vitro into artificial oocytes, of which some spontaneously activated and developed germ cell/embryonic tumors in vivo (Ma et al., 2013).
In vivo somatic cell transformation into oocytes without documented intermediate cell types
Mouse bone marrow transplants were shown to lead to the formation of artificial oocytes containing the genetic material of the donor. These oocytes, however, failed to lead to offspring (Johnson et al., 2005; Lee et al., 2007). No studies described the achievement of the specified end-points using human cells.
Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte
Artificial oocytes were formed by injecting somatic cell nuclei into enucleated (donor) oocytes. Although usually with low efficiency and unknown imprinting patterns, this induced haploidization of the somatic nucleus in mice (Fulka et al., 2002; Nagy et al., 2002; Palermo et al., 2002a, b; Tateno et al., 2003; Chang et al., 2003) and rabbits (Zhang et al., 2005). Fertilization achieved after haploidization was infrequent and the development of embryos into 2-cell and blastocyst stages was reported without further knowledge about their developmental potential (Heindryckx et al., 2004). Haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte also resulted in human artificial oocytes (Palermo et al., 2002a; Galat et al., 2005) that could be fertilized by ICSI (Tesarik et al., 2001; Palermo et al., 2002b; Takeuchi et al., 2005).
Artificial oocytes from a male
Of the nine biologically plausible routes that could theoretically lead to the formation of artificial oocytes from a male (Fig. 1), one (Route 3) was reported in literature as achieving at least one of the specified end-points in animals and/or humans (Table I). Artificial mouse oocytes were derived from male ESCs in vitro (Hübner et al., 2003). Male mouse ESCs differentiated in vitro into both artificial sperm and artificial oocytes. While being cultured in the same Petri dish, these artificial oocytes were fertilized (parthenogenetically) by the artificial sperm cells from the same ESC donor (Kerkis et al., 2007). No studies described the achievement of the specified end-points using human cells.
Artificial sperm from a female
Of the nine biologically plausible routes leading to the formation of artificial sperm from a female (Fig. 2), three were reported in literature as achieving at least one of the specified end-points in animals and/or humans (Table I). The injection of a mouse cumulus cell into a mouse oocyte resulted in fertilization and embryos with chromosomal abnormalities (Lacham-Kaplan et al., 2001; Route 8; Chen et al., 2004). A variation to method nine (i.e. haploidization by transplantation of somatic cell nucleus into an enucleated donor oocyte), namely serial nuclear transfer of an oocyte nucleus (of Mother 1) into enucleated donor oocytes, could generate an ‘imprint-free’ oocyte from genetically manipulated females, which once injected into a final (nucleated) oocyte (of Mother 2), resulted in the birth of viable mice (Kono et al., 2004; Kawahara et al., 2007; Kawahara and Kono, 2010). In the latter case, the offspring had a different phenotype (longevity) from the donor (Kawahara and Kono, 2010). Haploid artificial sperm was formed by differentiating female human iPSCs, although the authors specifically refer to their incorrect or unknown epigenetic status (Route 5; Eguizabal et al., 2011).
Discussion
This systematic review on the biological progress on artificial gametes towards clinical use is relevant to all MAR professionals. Previous reviews on artificial gametes (Roelen, 2011; Yao et al., 2011; West et al., 2013) were narrative rather than systematic and did not include all studies identified by our extensive search.
Our overview of biological progress documents important advances in the formation of artificial gametes in animals and in humans. The ultimate proof that artificial gametes were generated is the birth of normal healthy offspring. The birth of animal offspring has been achieved using several methods, and although long-term safety has not been unambiguously proven, animal models thus provide a proof of principle that artificial gametes can be generated. The birth of animal offspring has been proven for both the generation of artificial sperm from a male and the generation of artificial oocytes from a female starting from both ESC and iPSCs. Additionally, animal offspring have been born using artificial sperm from females starting from somatic cells (via iPSCs and without documented intermediate stages) and oocytes.
To date, no study has reported the birth of human offspring from artificial gametes. The creation of human sperm from males was reported for two of the eight biologically plausible routes, but fertilization was not reported. The creation of human oocytes from females was reported for four of the nine biologically plausible routes and one route even resulted in fertilization. Human and/or animal research more often focused on creating artificial sperm (n = 41) than on creating artificial oocytes (n = 27). Research less often focused on creating artificial gametes from the opposite sex of the animal or commissioning parent than on creating artificial gametes from the same sex (i.e. 8/70 versus 66/70, respectively; of note, three studies reported on both). In humans, artificial sperm from a female was created, but fertilization was not reported. The shortage of research on creating artificial gametes from the opposite sex might, amongst others, be due to the following factors: (i) technical challenges (e.g. it is more challenging to develop oocytes from males than spermatozoa from males; Hinxton group, 2008) that are imposed by the biological nature of male and female cells (e.g. sex-specific differences in the meiotic processes (Hunt and Hassold, 2002); (ii) less presumed demand from gay couples than from infertile heterosexual couples and (iii) less societal acceptance (Mertes and Pennings, 2010).
Clearly, the full range of findings, including both the results that have been reported (e.g. birth of mice offspring) and the results that have not (yet) been reported (e.g. birth of children), is crucial to understanding the level of progress of the field. The functionality of human artificial gametes, the chromosomal and epigenetic stability of animal and human artificial gametes, and the viability and long-term health of artificial gamete-derived offspring have not been unambiguously proven. Furthermore, many findings are yet to be validated, by different research groups repeating the experiments and enhancing the efficiency of the techniques. Accordingly, the findings presented here should be considered as preliminary data, opening up new avenues for research that may eventually lead to clinical applications of artificial gametes. The pace of scientific progress and, with that, the timeframe for any potential future clinical application of artificial gametes, is difficult to predict (Hinxton Group, 2008).
The systematic rather than narrative approach in this review has resulted in a complete overview of the current literature. The unconventional broad focus of our review, although relevant for MAR health care professionals, required overcoming several challenges.
First, the search strategy had to be very broad to identify articles covering different novel techniques, which are generally poorly indexed. We therefore used the snowball strategy to identify about one-third of our studies that were not in our initial search but were mentioned in the initially included studies.
Second, risk of bias in the included studies was not assessed, as no sets of quality criteria are available for biological proof-of-concept studies or opinion studies. Initially, we attempted to develop a set of quality criteria for biological proof-of-concept studies, but this resulted in a very limited number of quantifiable and reproducible quality criteria, which did not cover the entire quality of the studies. For example, specifying certain markers to be used or validation of gamete morphology assessments were not considered objective enough. Identifying quality criteria for biological proof-of-concept studies remains a challenge. We therefore did not exclude any of the studies meeting our predefined inclusion criteria and end-points for means of fairness. This review synthesizes all that has been reported, including methods that seem controversial at the moment. More specifically, (i) some groups (Oatley and Hunt, 2012; Zhang et al., 2012) doubt the presence of OSCs that have been reported by others (a.o. White et al., 2012); (ii) some groups doubt the biological plausibility of haploidization by transplantation of a somatic cell nucleus into an enucleated donor oocyte (Tateno et al., 2003) reported by others (Tesarik et al., 2001; Palermo et al., 2002b; Takeuchi et al., 2005) and there is controversy on whether bone marrow cells can contribute to the formation of female germ cells (Eggan et al., 2006. As we refrain from assessing the quality of the individual studies cited in the absence of objective bias assessment criteria, we need to consider alternatives. The number of references (ideally from different research groups) for each end-point mentioned in Table I reflects reproducibility, which is an important quality criterion in biological studies.
Third, some critical remarks on the three predefined end-points (artificial gamete formation, fertilization, the birth off offspring) chosen for this review should be made. These end-points do not merely reflect phases in the process of the use of artificial gametes, but also reflect different levels of proof of functionality, in which the birth of offspring is the only real proof while ‘artificial gamete formation’ and ‘fertilization’ can be considered pseudo-proof. However, it should be noted that functionality does not necessarily mean that artificial gametes are genetically and epigenetically equal to normal gametes. Within our end-point ‘artificial gamete formation’, several forms of proof were accepted (i.e. DNA content analyses, presence of markers and, for oocytes, polar body extrusion). It is important to note that in general, DNA content analyses are seen as more reliable than the presence of markers, and oocyte polar body extrusion (a form of morphological evidence) is seen as least reliable. Moreover, recent evidence suggests the production of a polar body is dissociable from the chromosomal events of meiosis (Dokshin et al., 2013), stressing the importance of discerning between the different levels of proof of gamete formation presented by the papers included in this review, varying between polar body extrusion and the birth of offspring. Furthermore, excluding intermediate end-points (e.g. the formation of primordial germ cells; Tilgner et al., 2008) resulted in the exclusion of interesting studies. Finally, the predefined end-points for artificial oocyte formation were less strict than those for artificial sperm formation in accordance with the natural differences between spermatogenesis and oogenesis, resulting in less robust evidence on artificial oocyte formation. For example, polar body extrusion was accepted as indication of artificial oocyte formation, whereas morphological evidence only was not accepted as indication of artificial sperm formation.
Fourth, frameworks had to be developed to structure the meta-synthesis of our findings to ensure understandability for professionals who are not specialists in artificial gametes. This resulted in the need to use a priori defined start- and end-points and to differentiate between all biologically plausible routes to create artificial gametes. However, excluding ‘patient gametes’ as a starting point prevented us from that addressing some interesting techniques (e.g. oocyte nuclear transfer, in which the nucleus of a patient's oocyte is transferred into an enucleated, younger, donor oocyte; Tachibana et al., 2013a, b). Moreover, excluding intermediate end-points (e.g. the formation of primordial germ cells; Tilgner et al., 2008) resulted in the exclusion of other interesting studies. Finally, the predefined end-points for artificial oocyte formation were less strict than those for artificial sperm formation, in accordance with the natural differences between spermatogenesis and oogenesis, resulting in less robust evidence on artificial oocyte formation. For example, polar body extrusion was accepted as indication of artificial oocyte formation, whereas only morphological evidence (i.e. an elongated cell with a ‘tail’) was not accepted for sperm formation.
Fifth, this review was limited to describing effectiveness (gamete formation, fertilization, the birth of offspring) rather than efficiency and/or long-term safety, as the included studies focus on effectiveness.
Future preclinical research could contribute to safeguarding the following three dimensions of quality of healthcare defined by the Institute of Medicine: ‘effectiveness’ (i.e. providing services based on scientific knowledge in order to result in benefit), ‘efficiency’ (i.e. avoiding waste) and ‘safety’ (i.e. avoiding injuries to patients and their offspring; Corrigan et al., 2001). Currently, the main preclinical biological research focus is on the proof of principle (i.e. effectiveness) proven by its final test: the birth of viable offspring. For some groups of beneficiaries (e.g. gay men requiring artificial oocytes from males), however, more research on effectiveness still needs to be performed than for others (e.g. heterosexual couples with male infertility requiring artificial sperm from males). In our opinion, the focus of preclinical biological research should be on healthy offspring, rather than viable offspring, which is a combined measure of effectiveness and safety. Only a minority of studies assessed safety in terms of genetic and epigenetic normality of artificial gametes, or the epigenetic status and (long-term) health of offspring derived from them. Some studies (e.g. Zou et al., 2009; Kawahara and Kono, 2010; Sato et al., 2011) did describe (ab)normal growth, the capability to reproduce and/or the life expectancy of offspring. Obviously, clinical application will require rigorous production of the appropriate safety data, for which we recommend setting up long-term follow-up of offspring, first in animals and later in children.
Regarding efficiency of the biologically plausible methods, little has been reported (e.g. how many attempts were required to end up with one artificial gamete or offspring). Before clinical application, efficiency requires extensive research. After all, for clinical purposes, it is crucial that treatment options fall within humanly reasonable scales relating to, for example, the number of GSCs needed or the number of donor oocytes required for one pregnancy.
Based on reviewing the biological evidence, we identified the different routes to generate artificial gametes, and their progress. However, there is insufficient evidence to recommend focusing on one or more superior route(s), as all routes are at the forefront of biology (i.e. they challenge our understanding and technical possibilities).
Conclusion
Although they are currently still in an experimental stage, and the time frame until possible clinical application is difficult to predict, studies on artificial gametes seem to be progressing steadily towards possible future clinical application. The increasing amount of biological studies will point us towards the safest and most efficient method to create artificial gametes. Deciding to introduce artificial gametes in clinical practice, however, requires a point of view that goes beyond biologic parameters. First, artificial gametes could change the field of MAR dramatically by discarding the entire concept of infertility, and potentially allowing new groups of patients (e.g. heterosexual couples without functional gametes, post-menopausal women and gay couples) to have genetically related children. Second, we are unable to acquire informed consent from the children that will be conceived. Therefore, to prevent premature implementation of artificial gametes, driven by profit and patients' demands, as with ICSI and PGD (Steele et al., 1999; Leese and Whittall, 2001; Schatten, 2002; Winston and Hardy, 2002; van Steirteghem, 2008; Harper et al., 2012), all stakeholders should be involved in deciding on the timing and conditions (including, but not limited to, safety and efficiency) of any future implementation into clinical practice.
References