-
PDF
- Split View
-
Views
-
Cite
Cite
Loredana Gandini, Paolo Sgrò, Francesco Lombardo, Donatella Paoli, Franco Culasso, Lucia Toselli, Petros Tsamatropoulos, Andrea Lenzi, Effect of chemo- or radiotherapy on sperm parameters of testicular cancer patients, Human Reproduction, Volume 21, Issue 11, 1 November 2006, Pages 2882–2889, https://doi.org/10.1093/humrep/del167
Close - Share Icon Share
Abstract
BACKGROUND: The aims of our study were to investigate the short- and long-term effects of chemo- or radiotherapy on spermatogenesis in patients with testicular cancer and to establish any correlation between pre-therapy sperm parameters, histotype and treatment type/intensity and the progress of spermatogenesis during the post-therapy period. METHODS: We evaluated 166 patients affected by testicular cancer, who cryobanked about 1 month after the removal of the cancerous testis and before beginning chemo- (CH group; n = 71) or radiotherapy (RT group; n = 95). RESULTS: For the CH group, there was a statistically significant decrease in sperm parameters, which was most significant 3 months after the end of chemotherapy. For the RT group, this decrease was most relevant 6 months after the end of radiotherapy. Two years after therapy, 3% of the CH group and 6% of the RT group remained azoospermic. To evaluate whether spermatogenesis recovery is a function of baseline semen quality, we divided each group into two subgroups by pre-therapy total sperm count (A, <40 × 106/ejaculate; B, ≥40 × 106/ejaculate). At t24, subgroup A of both the CH and RT groups showed improved sperm parameters over the baseline, whereas subgroup B for both CH and RT groups showed a return of sperm parameters to those of baseline values. CONCLUSIONS: In conclusion, the recovery of spermatogenesis after chemo- or radiotherapy in our group of testicular cancer patients was not a function of pre-therapy sperm parameter quality. Cryopreservation of sperm before performing such therapy is therefore imperative.
Introduction
Testicular cancer represents about 1% of all cancers in men but is the most frequent tumour in men aged 15–39 years. Since the 1990s, the use of chemo- and radiotherapy combined with surgical techniques has enabled the cure of 90% of the cases (Fossa et al., 1986), to the extent that testicular cancer therapy is today one of the outstanding successes of medical science (Presti et al., 1993; Dearnaley et al., 2001; Laguna et al., 2001).
However, these treatments can cause serious alterations to spermatogenesis—with possible transient or permanent azoospermia (Cullen et al., 1996; Pont et al., 1996; Bahadur, 2000)—and sperm chromatin structure (Martin et al., 1999; De Mas et al., 2001; Morris, 2002). Given the young age of these patients, their frequent lack of children and their improved long-term prognosis, it is essential that they are given the opportunity to cryopreserve their sperm before undergoing a therapy which may have an irreversible effect on their fertilizing ability.
Numerous papers in the literature have evaluated the effect of cancer therapy on sperm parameters. However, they are sometimes limited by the low number of patients evaluated (Fossa et al., 1985, 1986; Reiter et al., 1998), different semen evaluation methods, diversity of cancer pathologies taken into consideration (Bahadur et al., 2005), varying therapy intensity and the variety of cancer agents used (Petersen et al., 1994; Lampe et al., 1997; Huyghe et al., 2004).
The aims of our study were to (i) study the short- and long-term effects of chemo- or radiotherapy on spermatogenesis in a significant number of patients with testicular cancer and (ii) establish any correlation between pre-therapy sperm parameters, histotype and treatment type/intensity and the progress of spermatogenesis during the post-therapy observation period, to identify any factor predictive of patients’ response to the therapy in terms of semen quality.
Materials and methods
The study was approved by our University Hospital Ethics Committee. The sperm parameters of 166 testicular cancer patients attending the Laboratory of Seminology and Immunology of Reproduction, Department of Medical Pathophysiology, University of Rome ‘La Sapienza’ to undergo semen cryopreservation were examined. All patients underwent semen collection about 1 month after the removal of the cancerous testis and before beginning treatment (t0); none had previously undergone a semen analysis. Patients were divided into two groups on the basis of their therapeutic treatment according to their histotype. The chemotherapy (CH) group, consisting of 71 patients with embryonal carcinoma or mixed tumours (variable association of seminoma, teratocarcinoma, choriocarcinoma and yolk sac tumours), underwent chemotherapy under the PEB regimen (cisplatin, etoposide and bleomycin). Chemotherapy dose and administration regimen were as follows: days 1, 2, 3, 4 and 5 cisplatin 20 mg/m2 i.v. and etoposide 100 mg/m2 i.v.; days 2, 9 and 16 bleomycin 18 mg/m2 i.v. every 3 weeks for a maximum of four cycles. A total of 23 patients were exposed to two chemotherapy cycles, 24 to three cycles and 23 to four cycles.
The radiotherapy (RT) group included 95 seminoma patients, who underwent irradiation of the lumbar–aortic lymph nodes (with screening of remaining testicle). The protocol involved a daily dose of 180 cGy for 15–20 days at a mean dose of 2600 rad (range 1460–4200 rad). Sperm parameters were evaluated at 3 (t3), 6 (t6), 9 (t9), 12 (t12) and 24 (t24) months after the end of therapy; some patients missed one or more follow-ups before returning for a later control. Semen samples were collected by masturbation directly into a sterile plastic container after 3–5 days of sexual abstinence. They were allowed to liquefy for 60 min at 37°C and then examined by light microscope according to World Health Organization (WHO) criteria (WHO, 1992, 1999). The following variables were taken into consideration: ejaculate volume (ml), sperm concentration per ml (n × 106/ml), total sperm count (n × 106), forward motility (%) and morphology (% abnormal forms). Owing to the urgent need for patients to begin therapy, seminal fluid was collected and analysed once only for each patient. All seminal fluid examinations were carried out by the same biologist (L.G.).
All patients signed their informed consent to both cryopreservation and follow-up. To evaluate whether spermatogenesis recovery is a function of baseline semen quality, we divided each group into two subgroups by pre-therapy total sperm count: subgroup A, <40 × 106/ejaculate (26 patients for CH group and 31 for RT group) and subgroup B, ≥40 × 106/ejaculate (64 patients for RT and 45 for CH), according to WHO (1999).
We also evaluated spermatogenesis recovery versus number of chemotherapy cycles (two, three or four) or radiation intensity (≤2600 or >2600 cGy) based on the mean dose used to treat our patients (2600 rad, range 1460–4200 rad).
Statistical analysis
Quantitative results are expressed as mean and SD for the entire patient group and the subgroups CH and RT. Before comparing the various groups considered (by therapy, dosage and pre-therapy sperm concentration), uniformity tests were performed on the variables observed at base time (t0). To compare the results for the two groups, we subsequently calculated the relative efficacy for sperm parameter X as (Xt – X0)/X0, where X0 is the pre-therapy value and Xt is the value of the sperm parameter at time t. The Student’s t-test for paired or unpaired data was used to evaluate the differences between two mean values. Non-parametric tests (Wilcoxon and Mann–Whitney tests) were also calculated where necessary. A two-tailed P-value below 0.05 was considered as statistically significant.
To confirm the results obtained by comparing sperm parameters at different times, we repeated the same statistical analysis, limited to patients who had undergone all follow-up sperm examinations.
To evaluate the association of semen variables and the different covariates, we performed analyses of variance (ANOVA) for repeated measures, using each semen parameter as the dependent variable, therapy type as the grouping variable and the number of chemotherapy cycles or radiotherapy intensity, abstinence duration, age and time as covariates. The significance of covariates included in the model was tested by Wald test (Armitage and Colton, 1998).
All statistical analyses were performed using BMDP dynamic software (Dixon, 1992; Dixon and Merdian, 1992).
Results
The mean age ± SD of CH and RT group patients was 26.7 ± 4.4 (range 14–40) and 29.8 ± 4.9 (range 20–43) years, respectively; this difference is not significant. The mean period of abstinence for the CH and RT groups was 3.7 ± 1.1 and 4.3 ± 1.4 days, respectively; this difference is not significant. Patients becoming azoospermic during the follow-up period were evaluated separately (Table I). For the CH group, 15 of 40 patients (37%) were azoospermic 3 months after the end of therapy, 11 of 32 (34%) after 6 months, 5 of 42 (12%) after 9 months and only 3 of 46 (6%) after 1 year, and just 1 of 33 patients (3%) was still azoospermic after 2 years.
Azoospermic patients at t3, t6, t9, t12 and t24
| Months . | Chemotherapy . | Radiotherapy . | ||
|---|---|---|---|---|
| . | . | . | ||
| . | Total patients . | Azoospermic patients [n (%)] . | Total patients . | Azoospermic patients [n (%)] . |
| 3 | 40 | 15 (37) | 44 | 2 (4) |
| 6 | 32 | 11 (34) | 43 | 11 (26) |
| 9 | 42 | 5 (12) | 46 | 9 (19) |
| 12 | 46 | 3 (6) | 69 | 6 (9) |
| 24 | 33 | 1 (3) | 57 | 3 (6) |
| Months . | Chemotherapy . | Radiotherapy . | ||
|---|---|---|---|---|
| . | . | . | ||
| . | Total patients . | Azoospermic patients [n (%)] . | Total patients . | Azoospermic patients [n (%)] . |
| 3 | 40 | 15 (37) | 44 | 2 (4) |
| 6 | 32 | 11 (34) | 43 | 11 (26) |
| 9 | 42 | 5 (12) | 46 | 9 (19) |
| 12 | 46 | 3 (6) | 69 | 6 (9) |
| 24 | 33 | 1 (3) | 57 | 3 (6) |
Azoospermic patients at t3, t6, t9, t12 and t24
| Months . | Chemotherapy . | Radiotherapy . | ||
|---|---|---|---|---|
| . | . | . | ||
| . | Total patients . | Azoospermic patients [n (%)] . | Total patients . | Azoospermic patients [n (%)] . |
| 3 | 40 | 15 (37) | 44 | 2 (4) |
| 6 | 32 | 11 (34) | 43 | 11 (26) |
| 9 | 42 | 5 (12) | 46 | 9 (19) |
| 12 | 46 | 3 (6) | 69 | 6 (9) |
| 24 | 33 | 1 (3) | 57 | 3 (6) |
| Months . | Chemotherapy . | Radiotherapy . | ||
|---|---|---|---|---|
| . | . | . | ||
| . | Total patients . | Azoospermic patients [n (%)] . | Total patients . | Azoospermic patients [n (%)] . |
| 3 | 40 | 15 (37) | 44 | 2 (4) |
| 6 | 32 | 11 (34) | 43 | 11 (26) |
| 9 | 42 | 5 (12) | 46 | 9 (19) |
| 12 | 46 | 3 (6) | 69 | 6 (9) |
| 24 | 33 | 1 (3) | 57 | 3 (6) |
For the RT group, 2 of 44 patients (4%) were azoospermic 3 months after the end of therapy, 11 of 43 (26%) after 6 months, 9 of 46 (19%) after 9 months and 6 of 69 (9%) after 1 year, and just 3 of 57 patients (6%) were still azoospermic after 2 years. Seminal parameters at t0, t3, t6, t9, t12 and t24 of patients becoming azoospermic after therapy were not included in any statistical analysis to avoid underestimation of mean values. Differences in mean sperm parameters between the CH and RT groups at t0 were not significant, that is, the groups were sufficiently homogenous.
CH group
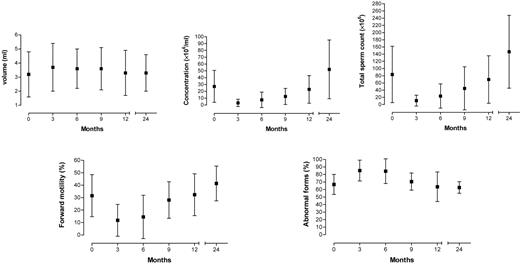
Means, SDs and significance of sperm parameter variations (volume, concentration per ml and total sperm count, percentage forward motility and percentage abnormal forms) between t0 and follow-up periods are reported in Table II. There was a statistically significant decrease in sperm concentration per ml, total sperm count and forward motility and a statistically significant increase in abnormal forms up to t9. Differences between sperm parameters at t0 and t12 were not statistically significant, indicating that sperm quality had returned to pre-chemotherapy values. Further significant improvements in sperm concentration per ml, total sperm count and forward motility were found at t24. The difference in abnormal forms between t0 and t24 was not significant. No significant differences in ejaculate volume were found at any of the follow-ups.
Comparisons of mean sperm parameters between baseline and follow-up (0/3, 0/6, 0/9, 0/12 and 0/24) of chemotherapy patients
| Sperm parameter . | Baseline (n = 71) . | 3 months (n = 25) . | 6 months (n = 21) . | 9 months (n = 37) . | 12 months (n = 43) . | 24 months (n = 32) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± S.D. . | Significance . | M ± S.D. . | Significance . |
| Volume (ml) | 3.2 ± 1.6 | 3.7 ± 1.7 | NS | 3.6 ± 1.4 | NS | 3.6 ± 1.5 | NS | 3.3 ± 1.6 | NS | 3.3 ± 1.3 | NS |
| Concentration (×106/ml) | 27.2 ± 23.4 | 3.0 ± 5.4 | ** | 7.5 ± 11.5 | *** | 12.5 ± 11.8 | *** | 22.9 ± 20.2 | NS | 52.2 ± 43.1 | ** |
| Total sperm count (x106) | 83.6 ± 78.3 | 10.9 ± 15.2 | *** | 23.6 ± 33.8 | *** | 45.1 ± 59.9 | *** | 69.7 ± 66.0 | NS | 146.8 ± 101.4 | ** |
| Forward motility (%) | 31.6 ± 16.9 | 11.8 ± 12.8 | ** | 14.5 ± 17.4 | * | 28.1 ± 14.7 | * | 32.4 ± 16.8 | NS | 41.4 ± 14.0 | ** |
| Abnormal forms (%) | 66.8 ± 13.3 | 85.2 ± 13.8 | *** | 84.4 ± 16.4 | *** | 70.6 ± 11.4 | ** | 63.7 ± 19.6 | NS | 62.7 ± 7.7 | NS |
| Sperm parameter . | Baseline (n = 71) . | 3 months (n = 25) . | 6 months (n = 21) . | 9 months (n = 37) . | 12 months (n = 43) . | 24 months (n = 32) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± S.D. . | Significance . | M ± S.D. . | Significance . |
| Volume (ml) | 3.2 ± 1.6 | 3.7 ± 1.7 | NS | 3.6 ± 1.4 | NS | 3.6 ± 1.5 | NS | 3.3 ± 1.6 | NS | 3.3 ± 1.3 | NS |
| Concentration (×106/ml) | 27.2 ± 23.4 | 3.0 ± 5.4 | ** | 7.5 ± 11.5 | *** | 12.5 ± 11.8 | *** | 22.9 ± 20.2 | NS | 52.2 ± 43.1 | ** |
| Total sperm count (x106) | 83.6 ± 78.3 | 10.9 ± 15.2 | *** | 23.6 ± 33.8 | *** | 45.1 ± 59.9 | *** | 69.7 ± 66.0 | NS | 146.8 ± 101.4 | ** |
| Forward motility (%) | 31.6 ± 16.9 | 11.8 ± 12.8 | ** | 14.5 ± 17.4 | * | 28.1 ± 14.7 | * | 32.4 ± 16.8 | NS | 41.4 ± 14.0 | ** |
| Abnormal forms (%) | 66.8 ± 13.3 | 85.2 ± 13.8 | *** | 84.4 ± 16.4 | *** | 70.6 ± 11.4 | ** | 63.7 ± 19.6 | NS | 62.7 ± 7.7 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
Comparisons of mean sperm parameters between baseline and follow-up (0/3, 0/6, 0/9, 0/12 and 0/24) of chemotherapy patients
| Sperm parameter . | Baseline (n = 71) . | 3 months (n = 25) . | 6 months (n = 21) . | 9 months (n = 37) . | 12 months (n = 43) . | 24 months (n = 32) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± S.D. . | Significance . | M ± S.D. . | Significance . |
| Volume (ml) | 3.2 ± 1.6 | 3.7 ± 1.7 | NS | 3.6 ± 1.4 | NS | 3.6 ± 1.5 | NS | 3.3 ± 1.6 | NS | 3.3 ± 1.3 | NS |
| Concentration (×106/ml) | 27.2 ± 23.4 | 3.0 ± 5.4 | ** | 7.5 ± 11.5 | *** | 12.5 ± 11.8 | *** | 22.9 ± 20.2 | NS | 52.2 ± 43.1 | ** |
| Total sperm count (x106) | 83.6 ± 78.3 | 10.9 ± 15.2 | *** | 23.6 ± 33.8 | *** | 45.1 ± 59.9 | *** | 69.7 ± 66.0 | NS | 146.8 ± 101.4 | ** |
| Forward motility (%) | 31.6 ± 16.9 | 11.8 ± 12.8 | ** | 14.5 ± 17.4 | * | 28.1 ± 14.7 | * | 32.4 ± 16.8 | NS | 41.4 ± 14.0 | ** |
| Abnormal forms (%) | 66.8 ± 13.3 | 85.2 ± 13.8 | *** | 84.4 ± 16.4 | *** | 70.6 ± 11.4 | ** | 63.7 ± 19.6 | NS | 62.7 ± 7.7 | NS |
| Sperm parameter . | Baseline (n = 71) . | 3 months (n = 25) . | 6 months (n = 21) . | 9 months (n = 37) . | 12 months (n = 43) . | 24 months (n = 32) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± S.D. . | Significance . | M ± S.D. . | Significance . |
| Volume (ml) | 3.2 ± 1.6 | 3.7 ± 1.7 | NS | 3.6 ± 1.4 | NS | 3.6 ± 1.5 | NS | 3.3 ± 1.6 | NS | 3.3 ± 1.3 | NS |
| Concentration (×106/ml) | 27.2 ± 23.4 | 3.0 ± 5.4 | ** | 7.5 ± 11.5 | *** | 12.5 ± 11.8 | *** | 22.9 ± 20.2 | NS | 52.2 ± 43.1 | ** |
| Total sperm count (x106) | 83.6 ± 78.3 | 10.9 ± 15.2 | *** | 23.6 ± 33.8 | *** | 45.1 ± 59.9 | *** | 69.7 ± 66.0 | NS | 146.8 ± 101.4 | ** |
| Forward motility (%) | 31.6 ± 16.9 | 11.8 ± 12.8 | ** | 14.5 ± 17.4 | * | 28.1 ± 14.7 | * | 32.4 ± 16.8 | NS | 41.4 ± 14.0 | ** |
| Abnormal forms (%) | 66.8 ± 13.3 | 85.2 ± 13.8 | *** | 84.4 ± 16.4 | *** | 70.6 ± 11.4 | ** | 63.7 ± 19.6 | NS | 62.7 ± 7.7 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
Semen evaluation at each follow-up showed that alteration in sperm parameters was most significant 3 months after the end of chemotherapy.
RT group
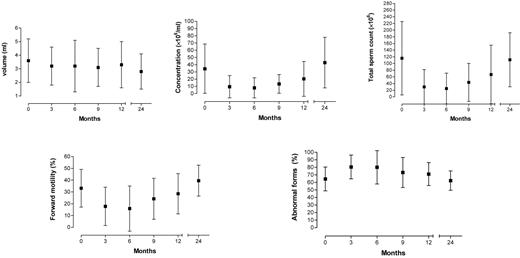
Means, SDs and significance of sperm parameter variations (volume, concentration per ml and total sperm count, percentage forward motility and percentage abnormal forms) between t0 and follow-up periods are reported in Table III. There was a statistically significant decrease in ejaculate volume, sperm concentration per ml, total sperm count and forward motility and a statistically significant increase in abnormal forms up to t12. Differences at t0 and t24 were not statistically significant for any parameter except volume, indicating that sperm quality had returned to pre-radiotherapy values. However, even after 24 months, volume was found to be significantly decreased from t0.
Comparisons of mean sperm parameters between baseline and follow-up (0/3, 0/6, 0/9, 0/12 and 0/24) of radiotherapy patients
| Sperm parameter . | Baseline (n = 95) . | 3 months (n = 42) . | 6 months (n = 32) . | 9 months (n = 37) . | 12 months (n = 63) . | 24 months (n = 54) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.6 ± 1.6 | 3.2 ± 1.4 | * | 3.2 ± 1.9 | * | 3.1 ± 1.4 | ** | 3.3 ± 1.7 | ** | 2.8 ± 1.3 | * |
| Concentration (x106/ml) | 34.4 ± 34.2 | 9.5 ± 15.4 | *** | 7.9 ± 14.0 | *** | 13.4 ± 13.0 | *** | 20.4 ± 24.2 | *** | 42.9 ± 35.0 | NS |
| Total sperm count (x106) | 115.9 ± 110.1 | 30.0 ± 51.9 | ** | 25.1 ± 46.1 | *** | 43.3 ± 57.1 | *** | 67.0 ± 87.9 | ** | 111.2 ± 81.1 | NS |
| Forward motility (%) | 33.2 ± 16.0 | 17.8 ± 16.3 | *** | 15.9 ± 19.2 | ** | 24.2 ± 17.4 | * | 28.5 ± 17.1 | * | 39.6 ± 13.1 | NS |
| Abnormal forms (%) | 64.6 ± 15.8 | 80.5 ± 15.8 | *** | 80.1 ± 22.1 | *** | 73.2 ± 19.9 | *** | 71.1 ± 15.2 | *** | 62.4 ± 12.8 | NS |
| Sperm parameter . | Baseline (n = 95) . | 3 months (n = 42) . | 6 months (n = 32) . | 9 months (n = 37) . | 12 months (n = 63) . | 24 months (n = 54) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.6 ± 1.6 | 3.2 ± 1.4 | * | 3.2 ± 1.9 | * | 3.1 ± 1.4 | ** | 3.3 ± 1.7 | ** | 2.8 ± 1.3 | * |
| Concentration (x106/ml) | 34.4 ± 34.2 | 9.5 ± 15.4 | *** | 7.9 ± 14.0 | *** | 13.4 ± 13.0 | *** | 20.4 ± 24.2 | *** | 42.9 ± 35.0 | NS |
| Total sperm count (x106) | 115.9 ± 110.1 | 30.0 ± 51.9 | ** | 25.1 ± 46.1 | *** | 43.3 ± 57.1 | *** | 67.0 ± 87.9 | ** | 111.2 ± 81.1 | NS |
| Forward motility (%) | 33.2 ± 16.0 | 17.8 ± 16.3 | *** | 15.9 ± 19.2 | ** | 24.2 ± 17.4 | * | 28.5 ± 17.1 | * | 39.6 ± 13.1 | NS |
| Abnormal forms (%) | 64.6 ± 15.8 | 80.5 ± 15.8 | *** | 80.1 ± 22.1 | *** | 73.2 ± 19.9 | *** | 71.1 ± 15.2 | *** | 62.4 ± 12.8 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
Comparisons of mean sperm parameters between baseline and follow-up (0/3, 0/6, 0/9, 0/12 and 0/24) of radiotherapy patients
| Sperm parameter . | Baseline (n = 95) . | 3 months (n = 42) . | 6 months (n = 32) . | 9 months (n = 37) . | 12 months (n = 63) . | 24 months (n = 54) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.6 ± 1.6 | 3.2 ± 1.4 | * | 3.2 ± 1.9 | * | 3.1 ± 1.4 | ** | 3.3 ± 1.7 | ** | 2.8 ± 1.3 | * |
| Concentration (x106/ml) | 34.4 ± 34.2 | 9.5 ± 15.4 | *** | 7.9 ± 14.0 | *** | 13.4 ± 13.0 | *** | 20.4 ± 24.2 | *** | 42.9 ± 35.0 | NS |
| Total sperm count (x106) | 115.9 ± 110.1 | 30.0 ± 51.9 | ** | 25.1 ± 46.1 | *** | 43.3 ± 57.1 | *** | 67.0 ± 87.9 | ** | 111.2 ± 81.1 | NS |
| Forward motility (%) | 33.2 ± 16.0 | 17.8 ± 16.3 | *** | 15.9 ± 19.2 | ** | 24.2 ± 17.4 | * | 28.5 ± 17.1 | * | 39.6 ± 13.1 | NS |
| Abnormal forms (%) | 64.6 ± 15.8 | 80.5 ± 15.8 | *** | 80.1 ± 22.1 | *** | 73.2 ± 19.9 | *** | 71.1 ± 15.2 | *** | 62.4 ± 12.8 | NS |
| Sperm parameter . | Baseline (n = 95) . | 3 months (n = 42) . | 6 months (n = 32) . | 9 months (n = 37) . | 12 months (n = 63) . | 24 months (n = 54) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | |||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.6 ± 1.6 | 3.2 ± 1.4 | * | 3.2 ± 1.9 | * | 3.1 ± 1.4 | ** | 3.3 ± 1.7 | ** | 2.8 ± 1.3 | * |
| Concentration (x106/ml) | 34.4 ± 34.2 | 9.5 ± 15.4 | *** | 7.9 ± 14.0 | *** | 13.4 ± 13.0 | *** | 20.4 ± 24.2 | *** | 42.9 ± 35.0 | NS |
| Total sperm count (x106) | 115.9 ± 110.1 | 30.0 ± 51.9 | ** | 25.1 ± 46.1 | *** | 43.3 ± 57.1 | *** | 67.0 ± 87.9 | ** | 111.2 ± 81.1 | NS |
| Forward motility (%) | 33.2 ± 16.0 | 17.8 ± 16.3 | *** | 15.9 ± 19.2 | ** | 24.2 ± 17.4 | * | 28.5 ± 17.1 | * | 39.6 ± 13.1 | NS |
| Abnormal forms (%) | 64.6 ± 15.8 | 80.5 ± 15.8 | *** | 80.1 ± 22.1 | *** | 73.2 ± 19.9 | *** | 71.1 ± 15.2 | *** | 62.4 ± 12.8 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
In contrast with the CH group, alteration in sperm parameters was most relevant 6 months after the end of radiotherapy. Figures 1 and 2 illustrate the progress of sperm parameter means and SDs over the follow-up period, confirming the deterioration seen in intermediate months and subsequent recovery 12–24 months after the end of therapy.
Variation of seminal parameters after chemotherapy over time (in months).
Variation of seminal parameters after radiotherapy over time (in months).
Recovery of spermatogenesis as a function of total pre-therapy sperm count
CH and RT groups were divided into two subgroups according to total sperm count (A, <40 × 106; B, ≥40 × 106). Results obtained in the subgroups are reported in Tables IV and V.
Comparison of mean sperm parameters between baseline and 12 and 24 months after chemotherapy for subgroups A (total sperm count <40 × 106) and B (total sperm count ≥40 × 106)
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 26) . | 12 months (n = 18) . | 24 months (n = 11) . | Baseline (n = 45) . | 12 months (n = 25) . | 24 months (n = 19) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 2.5 ± 1.7 | 3.0 ± 1.6 | NS | 2.9 ± 1.3 | NS | 3.7 ± 1.4 | 3.5 ± 1.6 | NS | 3.5 ± 1.5 | NS |
| Total sperm count (×106) | 17.3 ± 12.8 | 29.2 ± 30.0 | NS | 107.8 ± 109.1 | *** | 119.6 ± 75.4 | 96.2 ± 70.0 | NS | 158.5 ± 92.4 | NS |
| Forward motility (%) | 18.8 ± 15.0 | 23.2 ± 17.8 | NS | 37.3 ± 12.1 | ** | 38.6 ± 13.5 | 38.5 ± 13.3 | NS | 43.6 ± 14.2 | NS |
| Abnormal forms (%) | 76.1 ± 13.0 | 71.0 ± 21.3 | NS | 64.5 ± 5.4 | ** | 61.7 ± 10.5 | 62.4 ± 14.8 | NS | 62.1 ± 8.6 | NS |
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 26) . | 12 months (n = 18) . | 24 months (n = 11) . | Baseline (n = 45) . | 12 months (n = 25) . | 24 months (n = 19) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 2.5 ± 1.7 | 3.0 ± 1.6 | NS | 2.9 ± 1.3 | NS | 3.7 ± 1.4 | 3.5 ± 1.6 | NS | 3.5 ± 1.5 | NS |
| Total sperm count (×106) | 17.3 ± 12.8 | 29.2 ± 30.0 | NS | 107.8 ± 109.1 | *** | 119.6 ± 75.4 | 96.2 ± 70.0 | NS | 158.5 ± 92.4 | NS |
| Forward motility (%) | 18.8 ± 15.0 | 23.2 ± 17.8 | NS | 37.3 ± 12.1 | ** | 38.6 ± 13.5 | 38.5 ± 13.3 | NS | 43.6 ± 14.2 | NS |
| Abnormal forms (%) | 76.1 ± 13.0 | 71.0 ± 21.3 | NS | 64.5 ± 5.4 | ** | 61.7 ± 10.5 | 62.4 ± 14.8 | NS | 62.1 ± 8.6 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
Comparison of mean sperm parameters between baseline and 12 and 24 months after chemotherapy for subgroups A (total sperm count <40 × 106) and B (total sperm count ≥40 × 106)
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 26) . | 12 months (n = 18) . | 24 months (n = 11) . | Baseline (n = 45) . | 12 months (n = 25) . | 24 months (n = 19) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 2.5 ± 1.7 | 3.0 ± 1.6 | NS | 2.9 ± 1.3 | NS | 3.7 ± 1.4 | 3.5 ± 1.6 | NS | 3.5 ± 1.5 | NS |
| Total sperm count (×106) | 17.3 ± 12.8 | 29.2 ± 30.0 | NS | 107.8 ± 109.1 | *** | 119.6 ± 75.4 | 96.2 ± 70.0 | NS | 158.5 ± 92.4 | NS |
| Forward motility (%) | 18.8 ± 15.0 | 23.2 ± 17.8 | NS | 37.3 ± 12.1 | ** | 38.6 ± 13.5 | 38.5 ± 13.3 | NS | 43.6 ± 14.2 | NS |
| Abnormal forms (%) | 76.1 ± 13.0 | 71.0 ± 21.3 | NS | 64.5 ± 5.4 | ** | 61.7 ± 10.5 | 62.4 ± 14.8 | NS | 62.1 ± 8.6 | NS |
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 26) . | 12 months (n = 18) . | 24 months (n = 11) . | Baseline (n = 45) . | 12 months (n = 25) . | 24 months (n = 19) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 2.5 ± 1.7 | 3.0 ± 1.6 | NS | 2.9 ± 1.3 | NS | 3.7 ± 1.4 | 3.5 ± 1.6 | NS | 3.5 ± 1.5 | NS |
| Total sperm count (×106) | 17.3 ± 12.8 | 29.2 ± 30.0 | NS | 107.8 ± 109.1 | *** | 119.6 ± 75.4 | 96.2 ± 70.0 | NS | 158.5 ± 92.4 | NS |
| Forward motility (%) | 18.8 ± 15.0 | 23.2 ± 17.8 | NS | 37.3 ± 12.1 | ** | 38.6 ± 13.5 | 38.5 ± 13.3 | NS | 43.6 ± 14.2 | NS |
| Abnormal forms (%) | 76.1 ± 13.0 | 71.0 ± 21.3 | NS | 64.5 ± 5.4 | ** | 61.7 ± 10.5 | 62.4 ± 14.8 | NS | 62.1 ± 8.6 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
Comparison of mean sperm parameters between baseline and 12 and 24 months after radiotherapy for subgroups A (total sperm count <40 × 106) and B (total sperm count ≥40 × 106)
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 31) . | 12 months (n = 21) . | 24 months (n = 17) . | Baseline (n = 64) . | 12 months (n = 48) . | 24 months (n = 40) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.1 ± 1.4 | 2.8 ± 1.5 | NS | 2.3 ± 1.1 | NS | 3.9 ± 1.7 | 3.4 ± 1.7 | NS | 2.9 ± 1.3 | ** |
| Total sperm count (x106) | 17.1 ± 12.5 | 19.7 ± 22.4 | NS | 48.3 ± 34.0 | *** | 163.7 ± 104.3 | 84.0 ± 96.8 | *** | 127.9 ± 82.9 | NS |
| Forward motility (%) | 19.4 ± 15.2 | 20.6 ± 14.7 | NS | 30.3 ± 16.5 | * | 39.8 ± 11.4 | 30.9 ± 17.1 | ** | 42.7 ± 10.6 | NS |
| Abnormal forms (%) | 78.2 ± 14.5 | 78.7 ± 12.6 | NS | 72.1 ± 14.3 | NS | 58.6 ± 9.2 | 68.6 ± 15.2 | *** | 59.7 ± 10.9 | NS |
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 31) . | 12 months (n = 21) . | 24 months (n = 17) . | Baseline (n = 64) . | 12 months (n = 48) . | 24 months (n = 40) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.1 ± 1.4 | 2.8 ± 1.5 | NS | 2.3 ± 1.1 | NS | 3.9 ± 1.7 | 3.4 ± 1.7 | NS | 2.9 ± 1.3 | ** |
| Total sperm count (x106) | 17.1 ± 12.5 | 19.7 ± 22.4 | NS | 48.3 ± 34.0 | *** | 163.7 ± 104.3 | 84.0 ± 96.8 | *** | 127.9 ± 82.9 | NS |
| Forward motility (%) | 19.4 ± 15.2 | 20.6 ± 14.7 | NS | 30.3 ± 16.5 | * | 39.8 ± 11.4 | 30.9 ± 17.1 | ** | 42.7 ± 10.6 | NS |
| Abnormal forms (%) | 78.2 ± 14.5 | 78.7 ± 12.6 | NS | 72.1 ± 14.3 | NS | 58.6 ± 9.2 | 68.6 ± 15.2 | *** | 59.7 ± 10.9 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
Comparison of mean sperm parameters between baseline and 12 and 24 months after radiotherapy for subgroups A (total sperm count <40 × 106) and B (total sperm count ≥40 × 106)
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 31) . | 12 months (n = 21) . | 24 months (n = 17) . | Baseline (n = 64) . | 12 months (n = 48) . | 24 months (n = 40) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.1 ± 1.4 | 2.8 ± 1.5 | NS | 2.3 ± 1.1 | NS | 3.9 ± 1.7 | 3.4 ± 1.7 | NS | 2.9 ± 1.3 | ** |
| Total sperm count (x106) | 17.1 ± 12.5 | 19.7 ± 22.4 | NS | 48.3 ± 34.0 | *** | 163.7 ± 104.3 | 84.0 ± 96.8 | *** | 127.9 ± 82.9 | NS |
| Forward motility (%) | 19.4 ± 15.2 | 20.6 ± 14.7 | NS | 30.3 ± 16.5 | * | 39.8 ± 11.4 | 30.9 ± 17.1 | ** | 42.7 ± 10.6 | NS |
| Abnormal forms (%) | 78.2 ± 14.5 | 78.7 ± 12.6 | NS | 72.1 ± 14.3 | NS | 58.6 ± 9.2 | 68.6 ± 15.2 | *** | 59.7 ± 10.9 | NS |
| Sperm parameter . | Subgroup A . | Subgroup B . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ||||||||
| . | Baseline (n = 31) . | 12 months (n = 21) . | 24 months (n = 17) . | Baseline (n = 64) . | 12 months (n = 48) . | 24 months (n = 40) . | ||||
| . | . | . | . | . | . | . | ||||
| . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . | M ± SD . | M ± SD . | Significance . | M ± SD . | Significance . |
| Volume (ml) | 3.1 ± 1.4 | 2.8 ± 1.5 | NS | 2.3 ± 1.1 | NS | 3.9 ± 1.7 | 3.4 ± 1.7 | NS | 2.9 ± 1.3 | ** |
| Total sperm count (x106) | 17.1 ± 12.5 | 19.7 ± 22.4 | NS | 48.3 ± 34.0 | *** | 163.7 ± 104.3 | 84.0 ± 96.8 | *** | 127.9 ± 82.9 | NS |
| Forward motility (%) | 19.4 ± 15.2 | 20.6 ± 14.7 | NS | 30.3 ± 16.5 | * | 39.8 ± 11.4 | 30.9 ± 17.1 | ** | 42.7 ± 10.6 | NS |
| Abnormal forms (%) | 78.2 ± 14.5 | 78.7 ± 12.6 | NS | 72.1 ± 14.3 | NS | 58.6 ± 9.2 | 68.6 ± 15.2 | *** | 59.7 ± 10.9 | NS |
n, number of patients; NS, not significant.
*P < 0.05; **P < 0.01; ***P < 0.001.
For CH patients in subgroup A, the total sperm count showed a non-significant increase at t12 and a highly significant increase at t24 (P < 0.001). The same parameter in subgroup B appeared reduced at t12 and increased at t24, the difference being non-significant in both cases. There was a non-significant alteration in volume in both subgroups, whereas a significant increase at t24 was found for forward motility in subgroup A. Finally, the percentage of abnormal forms showed a statistically significant decrease at t24 for subgroup A but was unchanged in subgroup B (Table IV).
For RT patients in subgroup A, there was a non-significant increase in total sperm count from t0 to t12 and a highly significant increase from t0 to t24 (P < 0.001). The same parameter in subgroup B showed a significant decrease at t12 (P < 0.001) and non-significant decrease at t24. There was a progressive drop of volume in both subgroups, becoming significant only in subgroup B at t24. Forward motility in subgroup A was similar at t0 and t12 but showed a statistically significant increase at t24, whereas in subgroup B it decreased significantly at t12, returning to the baseline value at t24. In subgroup A, there was a non-significant reduction in abnormal forms at t12 and t24, whereas subgroup B showed a significant increase at t12, returning to the baseline value at t24 (Table V).
Spermatogenesis recovery as a function of treatment intensity
There was no significant difference in sperm parameters at t12 and t24 with respect to the baseline for the three CH subgroups of two, three and four therapy cycles. A significant difference (P < 0.01) was found between the two RT subgroups (≤2600 and >2600 cGy) between t0 and t12 for concentration per ml and total sperm count only, whereas there was no statistically significant difference in any parameters between t0 and t24. ANOVA confirmed that for these specific therapeutic regimens, the only covariate with a different effect on sperm parameter variations between the CH and RT groups was time. To confirm the results obtained after chemo- and radiotherapy in terms of spermatogenesis recovery, we repeated the comparisons of the mean sperm parameter values for CH and RT groups, including only those patients who had undergone all controls. Twenty-one CH and 19 RT patients underwent all controls up to t12, and 14 CH and 16 RT patients underwent all controls up to t24. The results of the analysis of these patients were similar to those obtained in the CH and RT groups as a whole (data not shown). It therefore follows that the differences seen in sperm parameter values at later follow-ups were not influenced by missed follow-up appointments.
Discussion
The prognosis of patients with testicular cancer has considerably improved in recent years: whereas in 1970 the mean survival of such patients was only 10%, since 1990 it has risen to 90%. This can be attributed to notable diagnostic and surgical advances and new radiotherapy and chemotherapy protocols, to which testicular tumours are especially sensitive.
The most commonly used chemotherapy regimen is PEB, consisting of etoposide, bleomycin and cisplatin. Etoposide works by breaking individual DNA strands and blocking the cell cycle at the S–G2 phase. Bleomycin’s cytotoxic activity is related to its ability to interact with iron and oxygen molecules, inducing free-radical production capable of fragmenting the DNA molecules and blocking cells in the G2 phase. Finally, cisplatin contains a platinum ion which forms complexes able to react with DNA, creating both intrachain and interchain cross-links. It interferes with the S phase of the cell cycle, inhibiting replication and transcription and inducing coding errors.
In contrast, radiotherapy damages the DNA, impeding the cell from replicating itself and causing its death. This therapy is especially effective in cancer cells, which replicate more quickly, but also affects normal cells, especially those with a high replication rate such as spermatogonia. Radiation induces material ionization both directly, through excitation of the atoms making up the DNA molecule, and indirectly, through its interaction with non-DNA molecules, which induce the ionization of the genetic material by emitting secondary electrons (Coogle, 1983; Chabner et al., 2001).
The cytotoxic effect of these therapies on spermatogenetic cells has led to great interest in studying post-therapy sperm parameter alterations in testicular cancer subjects. Numerous literature articles report studies of antineoplastic therapy on sperm quality but can be limited by the low number of patients examined and methodological errors which reduce their validity (Fossa et al., 1985; Aubier et al., 1989; Hansen et al., 1990; Shafford et al., 1993; Petersen et al., 1994; Palmieri et al., 1996; Lampe et al., 1997; Reiter et al., 1998; Ishikawa et al., 2004; Bahadur et al., 2005).
Lampe et al. (1997) studied 178 patients with testicular cancer, of whom 170 had recovered spermatogenesis 1 year after the end of chemotherapy. Of the 89 patients with a normal pre-therapy sperm concentration per ml, 64% had returned to normozoospermia, 16% presented oligozoospermia and 20% azoospermia. It should be noted that these authors arbitrarily designated all patients with a sperm concentration of less than 1 × 106/ml as azoospermic. The paper concludes that the recovery of spermatogenesis is tied not only to the type of treatment but also to pre-therapy sperm quality, and therefore these two factors may be predictive of spermatogenesis recovery after chemotherapy for testicular cancer.
According to other authors, the most important parameter in evaluating spermatogenesis recovery is the chemotherapeutic dose used. Petersen et al. (1994) compared a classic and high-dose PEB regimen over a 3-year follow-up, with 19% of the classic group and 47% of the high-dose group becoming azoospermic. Ishikawa et al. (2004) also found the recovery of spermatogenesis in only five of 10 patients after high doses of chemotherapy. The authors found no difference in response to cumulative therapy dose between two groups of azoospermic and non-azoospermic patients. They therefore concluded that it was not possible to establish a priori which patients would become azoospermic and which would recover spermatogenesis.
Reiter et al. (1998) reported the recovery of a normal sperm condition in 68% of 22 patients with stage I seminoma 4 years after chemotherapy. This is in contrast with the results of our study, which found the recovery of 97% of the patients within 2 years after the end of chemotherapy. It should be stressed that in our caseload, seminoma patients were subjected to radiotherapy, whereas in Reiter’s study the treatment effected was high-dose carboplatin. In fact, although radiotherapy is more damaging than chemotherapy for spermatogenesis, it can be postulated that the massive doses of carboplatin used induced far more damage.
Fossa et al. (1986) evaluated the recovery of spermatogenesis in seminoma patients treated with radiotherapy after shielding of the remaining testicle, under a regimen similar to that reported in our work but with a more restricted caseload of 29 patients. The authors concluded that spermatogenesis was much more severely affected in patients with a pre-therapy sperm concentration of <3 × 106/ml and therefore that the alterations in spermatogenesis observed 2–3 years after the end of radiotherapy were the result of differing pre-treatment sperm production rather than the level of irradiation of the remaining testicle.
The deterioration in semen quality after gonadotoxic therapy is also confirmed by the retrospective study conducted by Bahadur et al. (2005). Although this analysis of post-treatment semen data involved 314 patients, the caseload was somewhat mixed—including malignant neoplasms with unspecified location, benign pathological conditions, lymphomas and leukaemia as well as testicular tumours. Furthermore, the paper refers to a generic gonadotoxic therapy without specifying fundamental parameters such as the type and duration of the therapy undergone by the patients.
Our study was conducted on a uniform caseload of 166 patients. For the chemotherapy group, the number of azoospermic patients was highest 3 months post-treatment, slightly lower at t6 and more significantly reduced from t9 onwards. This leads us to suppose that the cytostatic effect of the drugs used is more selectively targeted at cells in the premeiotic phase—spermatogonia and primary spermatocytes—which suffer serious nuclear damage with a subsequent block of replication and thus of the spermatogenesis cascade. This halt explains the absence of sperm in the first 3 months after therapy.
The contrasting progress over time between chemotherapy and radiotherapy should be stressed. In fact, the peak in azoospermic patients was delayed in the RT group (t6) compared with the CH group (t3).
This leads us to hypothesize that not only spermatogonia, the most radiosensitive cells due to their intense mitotic activity, but also spermatids are affected by ionizing radiation. The latter are unprotected, because of the loss of their DNA damage repair mechanisms caused by post-meiotic differentiation and chromatin condensation. The persistence of an adequate population of spermatocytes surviving the radioactive impact and able to mature and produce spermatozoa could explain the greater time needed to reveal the damage.
Spermatogenesis in chemotherapy patients considerably worsened in the 3 months after treatment, characterized by a severe oligoasthenoteratozoospermia exactly coinciding with the peak in numbers of azoospermic patients. This again can be explained by the antineoplastic effect of these substances on spermatogenesis, especially on the spermatogonia and primary spermatocytes, cells with a higher mitotic activity. Spermatogenesis then shows a steady recovery from 9 to 12 months, and at t24, these patients presented a sperm concentration twice that of the analysis made on the day of cryopreservation.
Spermatogenesis in radiotherapy patients considerably worsened 6 months after treatment, in line with the number of subjects becoming azoospermic. This too is explained by the hypothesis of radiation-induced damage to the spermatogonia and spermatids as well as the persistence of an adequate population of spermatocytes in the maturation phase. In this group too, most patients had recovered spermatogenesis similar to the baseline at t24: it is self-evident that protection of the remaining testicle from radiation absorption, as demonstrated by Hansen et al. (1990) and carried out on all our patients, is essential to preserve spermatogenesis.
One more important finding of our study is the lack of correlation between pre-therapy sperm parameters and post-therapy spermatogenesis recovery. In fact, subgroup A (pre-therapy total sperm count <40 × 106) of both the CH and RT groups showed improved sperm parameters over the baseline at t24. To explain this, it should be remembered that some patients may show a poorer sperm quality at the time of cryopreservation due to the surgical stress of the orchiectomy, the subsequent antibiotic therapy and the psychological stress related to diagnosis of cancer and the need for major therapeutic procedures.
Subgroup B (pre-therapy total sperm count ≥40 × 106) of both the CH and RT groups recovered their baseline sperm quality at t24. Variations with respect to the baseline were not statistically or biologically significant. Our data demonstrate that spermatogenesis recovery is not a function of baseline spermatogenesis, and thus it is not possible to identify an indicator able to predict either possible azoospermia or the quality of post-therapy spermatogenesis recovery.
We found that the number of PEB chemotherapy cycles (two, three or four) and thus the total dose did not affect spermatogenesis recovery. There was no statistically significant difference in recovery between t0 and t12 and t24 (data not shown) among the three groups. This agrees with literature data, which describe worsened spermatogenesis with more than four therapy cycles, if the cumulative cisplatin and etoposide dose reaches around twice that taken by the patients enrolled in our study.
A different result was seen for radiotherapy, considering the mean dose (2600 rad) as the discriminating value. At t12, the reduction in total sperm count is statistically significant in subjects having undergone a total dose >2600 cGy in comparison with those subjected to a dose ≤2600 cGy. This difference is not statistically significant at t24. This confirms that the total dose of radiation administered is a discriminating and predictive factor of the time necessary to recover spermatogenesis.
Furthermore, our data demonstrate that these chemo- and radiotherapy protocols have the most detrimental effect on spermatogenesis within 3–6 months of the treatment. Spermatogenesis recovery is a function of the time since the end of the therapy, with 94% of patients treated with chemotherapy showing good recovery after 12 months and 97% after 24 months (93 and 94% for radiotherapy).
No statistically significant differences were seen between baseline and control ejaculate volume values for the CH group. The therapy probably acts only on the sperm progenitor cells, not on the accessory glands. In contrast, a reduction in ejaculate volume was seen in the RT group, remaining constant up to t24. This demonstrates the effect of radiotherapy on seminal vesicles, inducing their partial hypofunction.
In conclusion, it is currently impossible to predict a priori which patients will recover spermatogenesis and which will remain azoospermic, and there is no sperm index which might help predict which patients will remain permanently sterile. In fact, recovery of spermatogenesis after chemo- or radiotherapy in our group was not a function of pre-therapy sperm parameter quality. The detrimental effect on the chromatin condensation and DNA integrity of spermatozoa of men pre- and post-cancer therapy (Morris, 2002; Stahl et al., 2004; O’Donovan, 2005) should also be borne in mind.
Despite all of this, cryopreservation before chemo- or radiotherapy is an indispensable health tool, giving the patient the chance of fertility using cryopreserved spermatozoa even in years immediately after therapy.
Acknowledgements
The authors thank Marie-Hélène Hayles for her assistance in the English translation of the manuscript. This work was supported by a grant from the Italian Ministry of Education and Research (MIUR-COFIN) and the University of Rome ‘La Sapienza’, Faculty of Medicine.