-
PDF
- Split View
-
Views
-
Cite
Cite
S. Kelleher, S.M. Wishart, P.Y. Liu, L. Turner, I. Di Pierro, A.J. Conway, D.J. Handelsman, Long-term outcomes of elective human sperm cryostorage, Human Reproduction, Volume 16, Issue 12, 1 December 2001, Pages 2632–2639, https://doi.org/10.1093/humrep/16.12.2632
Close - Share Icon Share
Abstract
BACKGROUND: Sperm cryopreservation allows men with threatened fertility to preserve their progenitive potential, but there is little data on long-term outcomes of elective sperm cryostorage programmes. METHODS AND RESULTS: Over 22 years, 930 men sought semen cryostorage in a single academic hospital, of which 833 (90%) had spermatozoa cryostored. Among 692 (74%) men surviving their illness, sperm samples were discarded for 193 (21% of all applicants, 28% of survivors) and cryostored spermatozoa were used for 64 men (7% of all applicants, 9% of survivors) in 85 treatment cycles commencing at a median of 36 months post-storage (range 12–180 months) with nearly 90% of usage started within 10 years of storage and none after 15 years. Pregnancy was most efficiently produced by intracytoplasmic sperm injection (median three cycles) compared with conventional IVF (median eight cycles) or artificial insemination (median more than six cycles; P < 0.05). A total of 141 (15%) of men had died and of these, 120 (85% of those dying) had their spermatozoa discarded; requests to prolong cryostorage were received from relatives of 21 men (2% of all applicants, 15% of deceased) of which three cases had spermatozoa transferred for use with no pregnancies reported. Sperm concentration was lower for all cryostorage groups compared with healthy sperm donor controls (P < 0.05). Following orchidectomy, men with testicular cancer had sperm density approximately half that of all other groups of men seeking cryostorage (P < 0.05), the lowering attributable to removal of one testis rather than in defects in spermatogenesis. CONCLUSION: Elective sperm cryopreservation is an effective, if sparsely used, form of fertility insurance for men whose fertility is threatened by medical treatment and is an essential part of any comprehensive cancer care programme.
Introduction
Male infertility is an expected consequence following treatment of most malignancies with chemotherapy and/or radiotherapy (Redman et al., 1987). While infertility may be reversible for some cancer treatment regimens (notably testis cancer), sustained infertility develops in 50–95% of malignancies, particularly when combination therapies or bone marrow transplants have been used (Hendry et al., 1983; Selby et al., 1988; Meirow and Schenker, 1995). In young men with either testicular tumours or Hodgkin's disease, low sperm counts have been observed on presentation of the illness, indicating that these diseases or their investigations have impaired spermatogenesis even before the commencement of treatment (Sanger et al., 1980; Hendry et al., 1983; Scammell et al., 1985; Meirow and Schenker, 1995; Padron et al., 1997). Historically, before IVF techniques were applied to male infertility, semen cryopreservation was not offered to men whose sperm count was very low as this material was deemed unlikely to produce a pregnancy using artificial insemination by the husband (AIH) (Sanger et al., 1980; Rhodes et al., 1985; Reed et al., 1986). Advanced IVF techniques such as intracytoplasmic sperm injection (ICSI) have meant that semen cryopreservation may be offered so long as there are spermatozoa present in the ejaculate (Sanger et al., 1992; Meirow and Schenker, 1995; Hallak et al., 1998).
Previous analyses of electively cryostored semen use describe successful pregnancies resulting from AIH since 1983 (Hendry et al., 1983), IVF since 1992 (Tournaye et al., 1991) and ICSI since 1994 (Wennerholm et al., 2000). ICSI using cryopreserved spermatozoa extracted directly from the testis has also successfully resulted in pregnancy (Yavetz et al., 1997). Offspring from either AIH or IVF technologies (for infertile couples) appear to have no greater risk of abnormalities than normally conceived babies (Padron et al., 1997). Similarly, naturally occurring pregnancies after recovery from cancer treatment have generally no increase in malformation rates (Senturia et al., 1985; Li et al., 1987; Meirow and Schenker, 1995). However, an increased risk of spontaneous abortion has been reported in partners of men who have had combined chemotherapy and radiotherapy as treatment for Hodgkin's disease (Holmes and Holmes, 1978). Few data are available for artificial reproductive technologies after cancer treatment. Despite previous research identifying ICSI as being associated with a higher incidence of multiple births and genetic defects affecting reproductive function (Sutcliffe, 2000; Wennerholm et al., 2000), no substantial data on ICSI babies conceived with semen stored pre-cancer treatment is available. The present paper reviews 22 years of experience in a single teaching hospital centre involving elective sperm cryopreservation for 930 men prior to undergoing treatment likely to cause infertility.
Materials and Methods
Patients and Procedures
Sperm cryostorage was provided by a single laboratory at a large teaching hospital for hospitals in the states of New South Wales and the Australian Capital Territory. Originally the only service in the State, during the 1980s additional cryostorage services were developed. From inception until the early 1980s, spermatozoa were cryostored only if the semen analysis had at least several million spermatozoa/ml and a non-zero post-thaw motility, as it was then considered that these were requirements for effective use for homologous insemination, which was the only available artificial reproductive technology. In the early 1980s the policy changed to cryostorage of all specimens containing motile spermatozoa. All procedures including clinical evaluation, semen analysis and cryostorage were provided without additional charge to patients being treated under the hospital-based national health scheme.
Through close liaison with medical and nursing staff in medical and radiation oncology and haematology departments, men were referred early during evaluation for cancer treatments. The standard protocol for elective sperm cryostorage involves three semen collections at 2 day intervals. Each man and his partner, if available, are seen by an andrologist who obtains the social, medical and reproductive history, undertakes a brief medical examination including measurement of testis volume with a Prader orchidometer and provides advice regarding prognosis for recovery of spermatogenesis and fertility, risk of malformations, need for contraception, schedule of follow-up visits and options to use spermatozoa. After provision of written and verbal information, men are required to sign a cryostorage consent form which states that spermatozoa will be discarded upon the man's death or loss to follow-up. As a courtesy and without legal basis, the agreement states that the cryostorage service `...will make reasonable efforts to consult with...wife or de facto wife...prior to the termination of the cryostorage.' Men are required to maintain at least yearly contact following the completion of their treatment. At each annual follow-up visit a semen analysis, a blood sample for plasma hormone measurements and orchidometry are repeated and a decision is made regarding the need for ongoing cryostorage and/or advice on potential use of stored material. If a couple want to attempt a pregnancy before the return of spermatogenesis and fertility, they are referred and semen transported to an appropriate fertility service.
Spermatozoa may be discarded with the man's agreement if cryostorage is no longer required based on their desire for further fertility and/or recovery of spermatogenesis. In the case of patients lost to follow-up, contact is made with the hospital and/or treating doctor to establish their health status. If there is written confirmation of the man's death, contact is made with the man's partner (if any known) with a view to discarding the stored spermatozoa. If the man is overdue to attend for annual follow-up, further contact is pursued by telephone and a registered letter to their last known address, search of the telephone books and electoral rolls for a possible new address, and if still not located, placing an advertisement in a national newspaper requesting they contact the hospital. After all avenues for contact have been exhausted, the material may be discarded with approval of the institutional Ethics Committee.
Semen
There have been three methods of cryopreserving semen throughout the history of the programme. During the period of 1980–1985, cryoprotectant medium 199/FS was used. This consisted of Medium 199 supplemented with fasting human serum, glycerol and glycine. Semen samples were mixed in a 1:1 ratio with media and then frozen using a –70°C ultra-cold freezer and then stored in liquid nitrogen. In 1986 the cryoprotectant media changed to glycerol egg-yolk citrate (GEYC) media which involved a two-step preparation prior to freezing the semen. In late 1989 this method was changed to a one-step preparation to allow media to be kept frozen prior to use and a static vapour freezing method was introduced to replace the –70°C ultra-cold freeze step. From 1992 the static vapour gradient freezing method was used uniformly. Currently, cryopreservation of semen is undertaken using a modified Ackerman's cryopreservation media of GEYC formulation, 0.5 ml straws and static vapour phase cooling. If sufficient viable spermatozoa are present, one straw from each batch is allocated to perform post-thaw motility, which is assessed after thawing the semen to an ambient temperature for 5 min and then to 37°C. Semen analysis was performed according to the contemporaneous World Health Organization (WHO) laboratory standards (World Health Organization, 1999). Due to changes in WHO manual morphology criteria over the study period, sperm morphology was not analysed. Data from the sperm donor population (n = 548) recruited in the same laboratory are included as a contemporaneous control group using identical sperm cryostorage methods throughout the history of the cryostorage programme (Handelsman, 1997).
Data analysis
For semen analysis the first sample was used. Age is defined as that when the patient first presented for semen cryostorage. Kaplan–Meier survival analysis was used to analyse the outcome of the insemination cycles of men who used their material. A Cox proportional hazards model examined whether age, partner's age, year of usage or duration of cryostorage could predict outcome. Categorical, nonparametric rank tests and analysis of varience (ANOVA) using posthoc tests adjusting for unequal variance and multiple comparisons were used for statistical analysis as appropriate. P values of < 0.05 were considered significant. Analyses were performed using the Statistics Package for Social Sciences and StatXact.
Results
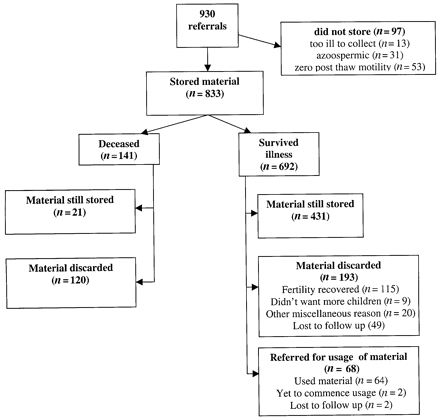
The diagnosis of the 930 men who requested sperm cryostorage included testicular tumours (n = 348), Hodgkin's and non-Hodgkin's lymphoma (n = 230), sarcoma, leukaemia or other metastatic disease (n = 281), and non-malignant diseases (renal, endocrine, immune, neurological) scheduled to undergo surgery, radiotherapy and/or cytotoxic drug treatment likely to compromise fertility (n = 71). Of 930 men requesting sperm cryostorage, 833 had semen cryostored. The remaining 97 men were either too ill to collect a semen sample (n = 13, 1.4%), azoospermic (n = 31, 3.3%), or the spermatozoa had zero post-thaw motility (n = 53, 5.6%) (Figure 1).
The semen analyses of the first semen specimen for all 833 men having elective cryostorage, together with 548 men screened as potential sperm donors, is shown according to underlying disease and whether they used the material during life or were subject to a posthumous request (Table I). Among men with testis cancer, 87% had undergone unilateral orchidectomy prior to cryostorage. As indicated by this table, potential sperm donors differed significantly in age and semen analysis from the population of men who cryostored (P < 0.05). They were older (mean age 33.6 versus 28.0 years) and had higher sperm densities (88.5 versus 56.9× 106/ml), counts (284.9 versus 149.3×106 spermatozoa) and pre-freeze motility (61.0 versus 46.7%; P < 0.05). Although mean semen volume (3.2 versus 2.7 ml) also appeared higher, this was not significant. Post hoc testing indicated that the sperm densities of donors were significantly higher for every diagnostic group except those with sarcoma or with `other cancers'. Men with testicular cancers had significantly lower sperm concentrations and densities compared with men who cryostored for any other reason or sperm donors (P < 0.05). As men with testis cancers had semen analysis after orchidectomy, analysis of sperm output per testis showed no significant difference between men with testis or other cancers, indicating that the reduced sperm output in men with testis cancer was attributable to their solitary testis rather than intrinsic defects in spermatogenesis. Men with sarcoma were younger and those with `other cancers' were older than the remaining diagnostic groups (P < 0.05). Pre-freeze and post-thaw sperm motility did not differ between diagnostic groups.
At the time of this study, 692 men continued to survive and 141 men were deceased. Of the deceased men, cryostored semen was discarded with consent of the closest relative for 120 while for the other 21 (14.9% of deceased) the closest relative, usually a wife or de facto wife (but on three occasions, a mother) requested continuation of sperm cryostorage. Although not legally binding, these wishes were acceded to unless or until they agreed to the discarding as specified in the consent forms. In these instances counselling was provided including the suggestion that specific fertility treatments be deferred until after their bereavement had resolved.
Among those who survived, material remained in ongoing storage for 431 men, while 193 agreed to discard their specimens and 68 have initiated usage of their cryostored material. The reasons for discarding cryostored spermatozoa were that fertility had recovered or family formation completed (124), straws rendered unusable (five), patients declined consent (five), fertility not impaired by treatment so cryostorage unnecessary (five), and patients expressed a wish to discard without giving a reason (five), whereas 49 specimens were discarded (with Ethics Committee approval) due to loss to follow-up after extensive searching and many years' failure to locate the individual.
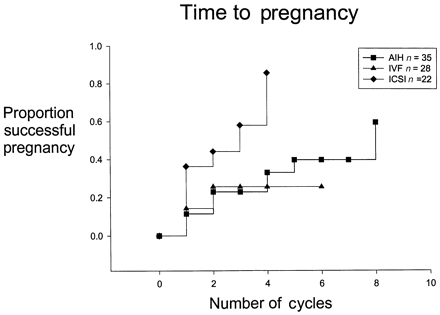
Of the 68 couples who requested to use their stored material, 64 have commenced usage of their spermatozoa, two have become lost to follow-up, and two have yet to commence fertility treatment. In total 85 cycles of treatment were administered resulting in 29 pregnancies and 39 births commencing at a median of 36 months (range 12–180) after cryostorage, with cumulative rates of start of usage of 45 (70%), 57 (89%) and 63 (98%) after 5, 10 and 12 years respectively and none after 15 years. One infant had a non-genetic abnormality (AIH), one of triplets was stillborn (ICSI), and one infant from another set of triplets to a 38-year-old mother had Down's Syndrome (ICSI). Successful usage occurred at 11 years with no further usage after 15 years. A median of three (approximated SE = 1) cycles for the couples using ICSI (12 pregnancies from 28 cycles) and eight (approximated SE = 3) cycles for those using AIH (11 pregnancies from 35 cycles) was required to achieve pregnancy. IVF was less successful (median more than six cycles) with six pregnancies from 28 IVF cycles (Figure 2). These differences in time to pregnancy were significant (P < 0.001, log-rank test). Age, partner's age, year of use and duration of cryostorage did not significantly influence time to achieve pregnancy in a Cox proportional hazards model (data not shown).
There were 121 deaths of men who cryostored during the study period. For the total group, deaths occurred at a median of 9 months after storage (range 1–158). Among the 21 instances where a request to prolong sperm cryostorage after death was received, initial contact with the closest relative was received shortly after death (median 1 week, range 1–30). The decision to accept discarding of spermatozoa by seven close relatives took a median of 192 weeks (range 92–244). Material from three men was transferred to fertility services with no pregnancies reported. Spermatozoa from the other 11 men remains in cryostorage at a median of 45 weeks (range 4–240) after post-mortem contact (data not shown).
Men for whom requests were made to use spermatozoa during life or after death were significantly different from other cryostorage candidates in marital status, diagnosis, planned treatment and number of specimens provided for storage. Men with interest in using specimens were more likely to be married, (64% for pre-mortem usage, 71% for post-mortem request, versus 42% for the whole population; Table II) and provided more specimens (47% of men who sought to use spermatozoa during their lives and 43% of men whose spermatozoa was requested for post-mortem storage provided four or more specimens compared with 24% of the whole group of men undergoing cryostorage, Table III). Furthermore, men who used their stored material were significantly more likely to have had a lymphoma (40 versus 21%) especially Hodgkin's disease (29 versus 12%); men whose wives made a posthumous request for continued usage were more likely to have other cancer (28 versus 11%, Table II). These skewed diagnostic patterns corresponded to the higher than expected proportions having planned treatment regimes likely to cause severe spermatogenic damage (Table II). The men for whom pre- or post-mortem requests were made with regard to use of their spermatozoa did not differ according to other personal, demographic or medical factors, notably desired fertility, prior fertility or infertility.
Discussion
Modern treatment for cancer and some non-malignant diseases employs combination chemotherapy and/or radiotherapy which produce severe cytotoxic effects on the testis. Elective sperm cryopreservation programmes provide such men, likely to be rendered temporarily or permanently infertile, the opportunity for paternity in a timely fashion. Both the numbers referred for cryostorage, and of those seeking to use cryostored material, have increased progressively over recent years. The increasing use of elective cryostorage facilities is probably due to better awareness by doctors, nurses, patients and their families that virtually any stored semen now has the potential to achieve a pregnancy resulting from a concomitant advance in reproductive techniques (Sanger et al., 1992). Furthermore, the improved survival following treatment for many cancers mean that men whose diagnosis once had an expectation of imminent death, are now often treated with curative intent (Meirow and Schenker, 1995). Even men with a poor prognosis often seek cryostorage as a form of psychologically reassuring planning for recovery. This is reinforced by the observation that the converse is not true—men in remission from cancer but with poor cryostorage specimens rarely agree to restore fresh specimens in anticipation of potential relapse. The key implication for elective semen cryopreservation programmes is that all men who wish to preserve their progenitive potential can and should be offered such facilities as part of a comprehensive cancer care programme (Tournaye et al., 1991; Shekarriz et al., 1995; Hallak et al., 1998). For testicular cancers, cryostorage can occur shortly after orchidectomy with no apparent loss in sperm quality (Sibert et al., 1999). Despite the good prognosis for eventual recovery of spermatogenesis and fertility for men with testicular cancer, the variability in extent or severity and time-lines of recovery make it prudent to offer semen cryostorage.
The present study demonstrates the feasibility of elective cryostorage for nearly all men in the reproductive age group who have malignant or non-malignant disorders for whom treatment is likely to cause prolonged or permanent infertility. The medico–legal prudence of offering such fertility insurance has been long established (Brahams, 1992). Despite this, the utilization of such facilities is less than expected according to the numbers of new cancers of men in the reproductive age group (15–50 years). The most frequent cancers affecting Australian men in the reproductive age group in ascending order of frequency are testis cancer, melanoma, haematological, and `other internal cancers' (Coates and Tracey, 2000). Yet neither the distributions of cancers nor the numbers seeking cryostorage at our centre reflect these figures. The under-representation of melanoma and other internal cancers are likely to be attributable to the first-line treatment being surgery, which itself rarely compromises fertility, making elective cryostorage appear unnecessary; nevertheless, the need for subsequent cytotoxic treatment is often difficult to predict at initial presentation. There may be many reasons for the lower usage of the cryostorage service than would be expected based on new diagnoses of haematological and testis cancers. These include inaccessibility or use of other facilities as well as unawareness of the need for elective cryostorage. The latter includes inappropriate reasons (patient unmarried, no immediate need for fertility), over-reliance on allegedly less cytotoxic regimens no longer causing testicular damage and/or misapprehensions that spontaneous recovery of fertility, or use of IVF after cancer treatment, can provide sufficiently reliable and timely assurance of fertility. The unpredictability of the ultimate treatment required, together with its variable impact on individuals, makes it appropriate to offer cryostorage for men at risk of severe spermatogenic damage from essentially all modern combination chemotherapy and/or radiotherapy regimens. The costs of sperm banking are modest compared with the investigations, treatment and hospitalizations required for modern comprehensive cancer care.
Our experience is that few couples (7.7%, 64/833) ever seek to use their cryostored material to achieve a pregnancy. Although this is higher than the next largest published series of 231 men which reported a usage of only 2.6% (Lass et al., 1998) such low rates of use has significance for the cost-effectiveness of cryostorage programmes. Such undertakings require not only long-term planning but also effective follow-up, most reliably provided by academic medical centres within a national health scheme. Regional differences in cancer prevalence and treatments may contribute to variations in the extent to which cryostored sperm is used; nevertheless usage appears uniformly relatively low. In the absence of effective follow-up, cryostored semen samples may accumulate despite being no longer needed or likely to be used. Our data shows that those who use their stored semen will do so in the first 3 years and that very few will do so after 10 years. In our experience, stored sperm has not been used after 15 years or used successfully after 11 years of storage, therefore serious consideration should be given to discarding sperm after 10 years.
Future technological refinement may alter the cost-effectiveness and rationale for prolonged cryostorage. Sperm lyophilization (Katayose et al., 1992; Hoshi et al., 1994; Wakayama and Yanagimachi, 1998) may allow simpler, more secure storage. Autologous germ cell transplantation (Schlatt, 1999), if and when established as a practical method, could reduce or eliminate the need for semen samples and thereby also potentially preserve fertility for prepubertal boys in whom semen cryostorage is not feasible. Other methods may preserve fertility and reduce the need for fertility insurance. For example, adjuvant cytoprotection treatments aiming to reduce cytotoxin-induced spermatogenic damage (Johnson et al., 1985; Waxman et al., 1987; Fossa et al., 1988; Kreusser et al., 1990; Brennemann et al., 1994; Masala et al., 1997) could potentially reduce need, or provide better semen samples, for long-term storage. Despite isolated reports (Masala et al., 1997), hormonal cytoprotection methods to suppress spermatogenesis and ameliorate cytotoxin-induced spermatogenic damage have been uniformly unsuccessful (Johnson et al., 1985; Waxman et al., 1987; Fossa et al., 1988; Kreusser et al., 1990; Brennemann et al., 1994). Experimentally, while hormonal suppression of spermatogenesis prior to cytotoxin damage may be ineffective (Crawford et al., 1998), more recent novel approaches of post-chemotherapy hormonal suppression (Meistrich, 1999) may hold further promise.
From the first reports of pregnancy produced from the cryostored sperm of men with malignancy using cervical insemination (Hendry et al., 1983; Scammell et al., 1985) and subsequently IVF (Rowland et al., 1985; Davis et al., 1990; Tournaye et al., 1991), few reports have had sufficient cases for life-table analysis of outcomes (Sanger et al., 1992). In our experience, ICSI has been the most successful artificial reproductive technology for use with electively cryostored sperm, with a median of three cycles needed to achieve a pregnancy. By comparison, conventional cervical insemination required a median of eight cycles and conventional IVF at least six cycles. Overall, one-third of all usages resulted in pregnancies, but as these observations arise from an uncontrolled audit of fertility treatments rather than from a formal randomized clinical trial, the reliability of relative estimates of time to conception with different treatment modalities must be viewed with caution. Nevertheless, our findings in general provide reassurance regarding the possible longevity of cryostored sperm since the duration of storage was not a significant determinant of time to pregnancy. Although fertilizing ability of sperm may not be reduced by cryostorage of up to 11 years, the prevalence of transmissible genetic defects cannot be excluded from the still relatively few normal births this study covers.
In the present survey, 2.3% (21/930) of partners requested maintenance of semen storage after the death of their spouse. Posthumous requests to continue sperm cryostorage were also related to diagnosis, treatment and marital status. The men involved were more likely to be married, and to have disease requiring extensive cytotoxic anticancer treatment. In any cryostorage programme, it is inevitable that some men who cryostore will die before using their material. The legal issues arising from post-mortem insemination such as guardianship, inheritance and maintenance remain unresolved. Increasingly, however, spouses (and other relatives) request the material to be kept for possible future usage. In Anglo Saxon common law, body parts, notably spermatozoa, are not considered to be property capable of having its ownership legally transferred at any time including after death. Our consent procedures stipulate written agreement in advance that spermatozoa will be discarded after death; however, although not legally mandated, it has been considered compassionate to seek consent for disposal of spermatozoa. In most instances this is agreed without demurral by the wife. In a minority, women in the immediate bereavement period have requested prolongation of sperm cryostorage. Mostly these requests are to maintain options during a stressful period and have ultimately been rescinded; nevertheless, making such a decision is quite protracted. Whether such deferral of sperm disposal, when it has been agreed in advance by the man, constitutes anything other than aggravation of bereavement is far from clear. In rare instances, other first degree relatives (mothers) have requested posthumous continuation of cryostorage either against the wishes of the man's spouse or where the man died without a conjugal partner. In addition, we have recently had requests for sperm harvesting without consent from men who have just died, one during an IVF treatment cycle. The appropriate management of such situations is even less clear when consent to sperm procurement and storage are absent; it remains unclear if consent to use of such sperm could be considered reasonable, but legal guidelines for such cases remain to be defined (Bahadur, 1996; Kahan et al., 1999).
This review of long-term cryostorage outcomes affords an opportunity to examine semen variables in a large cohort of men with malignant and non-malignant disease prior to treatment together with healthy sperm donor controls. Overall, men seeking cryostorage have lower sperm density compared with sperm donors. Although our sperm donor controls comprise all men screened as potential sperm donors (rather than the selected sperm donors) within the same laboratory, this self-selected volunteer population cannot be considered representative of the general male population (Handelsman, 1997). Hence it remains unclear if the apparent lowering of sperm output among men who requested cryostorage is genuine or due to upward bias from recruitment of volunteers as potential sperm donors. Similar reservations apply to claims of reduced sperm output in previous studies which have also used opportunistic controls (Sanger et al., 1980; Marmor et al., 1986; Redman et al., 1987; Shekarriz et al., 1995; Agarwal et al., 1996). Among men who cryostore, our study confirms that men with sarcoma have apparently higher sperm output at presentation than men with haematological or testis cancers (Meistrich et al., 1992). The reduced sperm output of men with testis cancer in this study was fully accounted for by the presence of a solitary testis after orchidectomy and did not support claims of intrinsic defects in spermatogenesis in the non-tumorous testis (Berthelsen et al., 1979; Berthelsen and Skakkebaek, 1983; Berthelsen, 1984). This interpretation is supported by the findings of previous studies of men with testis compared with other cancers (Sanger et al., 1980; Hendry et al., 1983; Reed et al., 1986; Agarwal et al., 1996; Padron et al., 1997; Lass et al., 1998). This is not to deny that carcinoma in situ may affect the other testis (Kliesch et al., 1997), but this is relatively rare and does not change the dominant impression that the contralateral testis functions normally.
The profiling of personal and medical characteristics provided only very limited basis to identify men most likely to use their cryostored spermatozoa. Married men and those with diseases requiring the most toxic regimens (Hodgkin's disease, leukaemia and bone marrow transplantation) were more likely to seek use of their stored spermatozoa, but these criteria did not provide practical guidelines to reduce the amount of cryostorage. Whilst the extent and duration of cytotoxin-induced spermatogenic damage is roughly dose dependent, resultant infertility varies between individuals receiving the same treatment (Meirow and Schenker, 1995). Although newer regimens such as adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) (Viviani et al., 1985) is less toxic than mustine, vincristine, procarbazine and prednisolone (MOPP) for Hodgkin's disease, sperm cryostorage remains a necessary option for all such men due to the unpredictability of rate and extent of spermatogenic recovery (Shekarriz et al., 1995; Padron et al., 1997). Priming regimens employed prior to bone marrow transplantation (Chatterjee et al., 1994) also cause severe and probably irreversible spermatogenic damage. We conclude that it remains impractical to identify men or treatment regimens that need, or do not need, use of cryostorage prior to cytotoxic medical treatment for cancers. It therefore remains essential that cryostorage be offered to all such men with malignant disease where iatrogenic male infertility for prolonged periods cannot be reliably excluded. Neither improvements in cancer or fertility treatment nor cryostorage technology or alternatives to it are sufficiently well established to obviate the need for sperm cryostorage to remain an important adjunct to modern cancer treatment in the foreseeable future.
Age at date of first cryostorage related to diagnosis and the quality and volume of the first sample of cryostored semen, data expressed as mean and (SEM)
| Category . | Number . | Mean age (years) . | Semen volume . | Sperm density (×106) . | Total (×106) . | Sperm motility pre-freeze . | (106/ml) post-thaw . |
|---|---|---|---|---|---|---|---|
| aSignificantly different from sperm donors (P < 0.05). | |||||||
| bSignificantly different from sperm donors and all other diagnoses in multiple pairwise comparisons (P < 0.05). | |||||||
| All cases | 901 | 28 (0.3)a | 2.7 (0.1) | 56.9 (2.2)a | 149.3 (6.8)a | 46.7 (0.9)a | 15.7 (0.5) |
| Testis cancer | 323 | 28.5 (0.4) | 3.1 (0.1) | 23.8 (3.1)b | 75.3 (10)b | 45.8 (1.2) | 14.3 (0.7) |
| Teratoma | 174 | 26.5 (0.5) | 3 (0.1) | 23.6 (4.3) | 72.6 (13.6) | 43.6 (1.7) | 13.7 (1.0) |
| Seminoma | 149 | 30.8 (0.6) | 3.2 (0.1) | 23.9 (4.6) | 78.5 (14.7) | 48.3 (1.9) | 14.9 (1.1) |
| Lymphoma | 201 | 28.1 (0.5) | 2.7 (0.1) | 57.2 (4) | 147.7 (12.7) | 48.4 (1.6) | 16.4 (0.9) |
| Hodgkin's disease | 117 | 26.4 (0.7) | 2.8 (0.2) | 56 (5.2) | 153 (16.6) | 47.7 (2.1) | 15.2 (1.2) |
| Non-Hodgkin's lymphoma | 84 | 30.3 (0.8) | 2.7 (0.2) | 58.9 (6.3) | 140.2 (19.9) | 49.4 (2.5) | 18.2 (1.4) |
| Leukaemia/bone marrow transplantation | 107 | 26.9 (0.7) | 2.6 (0.2) | 61.2 (5.5) | 149.5 (17.6) | 41.9 (2.3) | 15 (1.3) |
| Sarcoma | 71 | 24 (0.9) | 2.5 (0.2) | 73.2 (6.8) | 175.5 (21.4) | 50.8 (2.7) | 19.2 (1.4) |
| Other cancer | 99 | 31.7 (0.7) | 3.1 (0.2) | 67.3 (5.7) | 209.8 (18) | 46.9 (2.3) | 14.6 (1.2) |
| Non-malignant disease | 100 | 30 (0.9) | 2.5 (0.2) | 58.7 (5.6) | 137.9 (17.7) | 45.9 (2.2) | 14.6 (1.3) |
| Sperm used during life | 68 | 29.3 (1.5) | 2.7 (0.1) | 38.8 (9.2) | 110.9 (17) | 41.5 (3.9) | 15.2 (2.4) |
| Request for sperm post-mortem | 21 | 30.5 (0.8) | 2.7 (0.2) | 48.5 (9.2) | 114 (15.9) | 48.5 (3.8) | 15.2 (2) |
| Sperm donors | 548 | 33.6 (0.4)a | 3.2 (0.2) | 88.5 (2.9)a | 284.9 (12.1)a | 61 (0.6)a | 28.3 (1.6) |
| Category . | Number . | Mean age (years) . | Semen volume . | Sperm density (×106) . | Total (×106) . | Sperm motility pre-freeze . | (106/ml) post-thaw . |
|---|---|---|---|---|---|---|---|
| aSignificantly different from sperm donors (P < 0.05). | |||||||
| bSignificantly different from sperm donors and all other diagnoses in multiple pairwise comparisons (P < 0.05). | |||||||
| All cases | 901 | 28 (0.3)a | 2.7 (0.1) | 56.9 (2.2)a | 149.3 (6.8)a | 46.7 (0.9)a | 15.7 (0.5) |
| Testis cancer | 323 | 28.5 (0.4) | 3.1 (0.1) | 23.8 (3.1)b | 75.3 (10)b | 45.8 (1.2) | 14.3 (0.7) |
| Teratoma | 174 | 26.5 (0.5) | 3 (0.1) | 23.6 (4.3) | 72.6 (13.6) | 43.6 (1.7) | 13.7 (1.0) |
| Seminoma | 149 | 30.8 (0.6) | 3.2 (0.1) | 23.9 (4.6) | 78.5 (14.7) | 48.3 (1.9) | 14.9 (1.1) |
| Lymphoma | 201 | 28.1 (0.5) | 2.7 (0.1) | 57.2 (4) | 147.7 (12.7) | 48.4 (1.6) | 16.4 (0.9) |
| Hodgkin's disease | 117 | 26.4 (0.7) | 2.8 (0.2) | 56 (5.2) | 153 (16.6) | 47.7 (2.1) | 15.2 (1.2) |
| Non-Hodgkin's lymphoma | 84 | 30.3 (0.8) | 2.7 (0.2) | 58.9 (6.3) | 140.2 (19.9) | 49.4 (2.5) | 18.2 (1.4) |
| Leukaemia/bone marrow transplantation | 107 | 26.9 (0.7) | 2.6 (0.2) | 61.2 (5.5) | 149.5 (17.6) | 41.9 (2.3) | 15 (1.3) |
| Sarcoma | 71 | 24 (0.9) | 2.5 (0.2) | 73.2 (6.8) | 175.5 (21.4) | 50.8 (2.7) | 19.2 (1.4) |
| Other cancer | 99 | 31.7 (0.7) | 3.1 (0.2) | 67.3 (5.7) | 209.8 (18) | 46.9 (2.3) | 14.6 (1.2) |
| Non-malignant disease | 100 | 30 (0.9) | 2.5 (0.2) | 58.7 (5.6) | 137.9 (17.7) | 45.9 (2.2) | 14.6 (1.3) |
| Sperm used during life | 68 | 29.3 (1.5) | 2.7 (0.1) | 38.8 (9.2) | 110.9 (17) | 41.5 (3.9) | 15.2 (2.4) |
| Request for sperm post-mortem | 21 | 30.5 (0.8) | 2.7 (0.2) | 48.5 (9.2) | 114 (15.9) | 48.5 (3.8) | 15.2 (2) |
| Sperm donors | 548 | 33.6 (0.4)a | 3.2 (0.2) | 88.5 (2.9)a | 284.9 (12.1)a | 61 (0.6)a | 28.3 (1.6) |
Age at date of first cryostorage related to diagnosis and the quality and volume of the first sample of cryostored semen, data expressed as mean and (SEM)
| Category . | Number . | Mean age (years) . | Semen volume . | Sperm density (×106) . | Total (×106) . | Sperm motility pre-freeze . | (106/ml) post-thaw . |
|---|---|---|---|---|---|---|---|
| aSignificantly different from sperm donors (P < 0.05). | |||||||
| bSignificantly different from sperm donors and all other diagnoses in multiple pairwise comparisons (P < 0.05). | |||||||
| All cases | 901 | 28 (0.3)a | 2.7 (0.1) | 56.9 (2.2)a | 149.3 (6.8)a | 46.7 (0.9)a | 15.7 (0.5) |
| Testis cancer | 323 | 28.5 (0.4) | 3.1 (0.1) | 23.8 (3.1)b | 75.3 (10)b | 45.8 (1.2) | 14.3 (0.7) |
| Teratoma | 174 | 26.5 (0.5) | 3 (0.1) | 23.6 (4.3) | 72.6 (13.6) | 43.6 (1.7) | 13.7 (1.0) |
| Seminoma | 149 | 30.8 (0.6) | 3.2 (0.1) | 23.9 (4.6) | 78.5 (14.7) | 48.3 (1.9) | 14.9 (1.1) |
| Lymphoma | 201 | 28.1 (0.5) | 2.7 (0.1) | 57.2 (4) | 147.7 (12.7) | 48.4 (1.6) | 16.4 (0.9) |
| Hodgkin's disease | 117 | 26.4 (0.7) | 2.8 (0.2) | 56 (5.2) | 153 (16.6) | 47.7 (2.1) | 15.2 (1.2) |
| Non-Hodgkin's lymphoma | 84 | 30.3 (0.8) | 2.7 (0.2) | 58.9 (6.3) | 140.2 (19.9) | 49.4 (2.5) | 18.2 (1.4) |
| Leukaemia/bone marrow transplantation | 107 | 26.9 (0.7) | 2.6 (0.2) | 61.2 (5.5) | 149.5 (17.6) | 41.9 (2.3) | 15 (1.3) |
| Sarcoma | 71 | 24 (0.9) | 2.5 (0.2) | 73.2 (6.8) | 175.5 (21.4) | 50.8 (2.7) | 19.2 (1.4) |
| Other cancer | 99 | 31.7 (0.7) | 3.1 (0.2) | 67.3 (5.7) | 209.8 (18) | 46.9 (2.3) | 14.6 (1.2) |
| Non-malignant disease | 100 | 30 (0.9) | 2.5 (0.2) | 58.7 (5.6) | 137.9 (17.7) | 45.9 (2.2) | 14.6 (1.3) |
| Sperm used during life | 68 | 29.3 (1.5) | 2.7 (0.1) | 38.8 (9.2) | 110.9 (17) | 41.5 (3.9) | 15.2 (2.4) |
| Request for sperm post-mortem | 21 | 30.5 (0.8) | 2.7 (0.2) | 48.5 (9.2) | 114 (15.9) | 48.5 (3.8) | 15.2 (2) |
| Sperm donors | 548 | 33.6 (0.4)a | 3.2 (0.2) | 88.5 (2.9)a | 284.9 (12.1)a | 61 (0.6)a | 28.3 (1.6) |
| Category . | Number . | Mean age (years) . | Semen volume . | Sperm density (×106) . | Total (×106) . | Sperm motility pre-freeze . | (106/ml) post-thaw . |
|---|---|---|---|---|---|---|---|
| aSignificantly different from sperm donors (P < 0.05). | |||||||
| bSignificantly different from sperm donors and all other diagnoses in multiple pairwise comparisons (P < 0.05). | |||||||
| All cases | 901 | 28 (0.3)a | 2.7 (0.1) | 56.9 (2.2)a | 149.3 (6.8)a | 46.7 (0.9)a | 15.7 (0.5) |
| Testis cancer | 323 | 28.5 (0.4) | 3.1 (0.1) | 23.8 (3.1)b | 75.3 (10)b | 45.8 (1.2) | 14.3 (0.7) |
| Teratoma | 174 | 26.5 (0.5) | 3 (0.1) | 23.6 (4.3) | 72.6 (13.6) | 43.6 (1.7) | 13.7 (1.0) |
| Seminoma | 149 | 30.8 (0.6) | 3.2 (0.1) | 23.9 (4.6) | 78.5 (14.7) | 48.3 (1.9) | 14.9 (1.1) |
| Lymphoma | 201 | 28.1 (0.5) | 2.7 (0.1) | 57.2 (4) | 147.7 (12.7) | 48.4 (1.6) | 16.4 (0.9) |
| Hodgkin's disease | 117 | 26.4 (0.7) | 2.8 (0.2) | 56 (5.2) | 153 (16.6) | 47.7 (2.1) | 15.2 (1.2) |
| Non-Hodgkin's lymphoma | 84 | 30.3 (0.8) | 2.7 (0.2) | 58.9 (6.3) | 140.2 (19.9) | 49.4 (2.5) | 18.2 (1.4) |
| Leukaemia/bone marrow transplantation | 107 | 26.9 (0.7) | 2.6 (0.2) | 61.2 (5.5) | 149.5 (17.6) | 41.9 (2.3) | 15 (1.3) |
| Sarcoma | 71 | 24 (0.9) | 2.5 (0.2) | 73.2 (6.8) | 175.5 (21.4) | 50.8 (2.7) | 19.2 (1.4) |
| Other cancer | 99 | 31.7 (0.7) | 3.1 (0.2) | 67.3 (5.7) | 209.8 (18) | 46.9 (2.3) | 14.6 (1.2) |
| Non-malignant disease | 100 | 30 (0.9) | 2.5 (0.2) | 58.7 (5.6) | 137.9 (17.7) | 45.9 (2.2) | 14.6 (1.3) |
| Sperm used during life | 68 | 29.3 (1.5) | 2.7 (0.1) | 38.8 (9.2) | 110.9 (17) | 41.5 (3.9) | 15.2 (2.4) |
| Request for sperm post-mortem | 21 | 30.5 (0.8) | 2.7 (0.2) | 48.5 (9.2) | 114 (15.9) | 48.5 (3.8) | 15.2 (2) |
| Sperm donors | 548 | 33.6 (0.4)a | 3.2 (0.2) | 88.5 (2.9)a | 284.9 (12.1)a | 61 (0.6)a | 28.3 (1.6) |
Demographic and diagnostic variables as compared with semen usage
| Variable . | Request use during life . | Request continued storage post-mortem . | All . |
|---|---|---|---|
| aSignificant result (P < 0.05). | |||
| BM = bone marrow. | |||
| n | 68 | 21 | 930 |
| Age (years) | 29.3± 1.5 | 30.5 ± 0.8 | 28.6 ± 0.7 |
| Height (cm) | 177 ± 1 | 177 ± 1.6 | 177 ± 0.3 |
| Weight (kg) | 77 ± 1.9 | 78.4 ± 3 | 77.4 ± 0.5 |
| BMI (kg/m2) | 24.5 ± 0.6 | 25 ± 1.1 | 24.4 ± 0.2 |
| Testis volume (ml) | 22 ± 1 | 22 ± 1 | 22 ± 1 |
| Cryptorchidism | 10/60 (16%) | 1/19 (5%) | 45/803 (5%) |
| Normal virilization | 60/60 (100%) | 20/20 (100%) | 853/868 (98%) |
| Smoke (any) | 14/60 (23%) | 7/20 (35%) | 241/864 (27%) |
| Alcohol (any) | 53/60 (88%) | 19/20 (90%) | 721/855 (84%) |
| Social drugs (any) | 22/61 (36%) | 11/20 (55%) | 348/871 (40%) |
| Married at cryostorage | 40/62 (64%)a | 15/21 (71%)a | 382/908 (42%) |
| Prior infertility | 4/61 (6%) | 0/20 (0%) | 37/888 (4%) |
| Prior fertility | 17/61 (27%) | 5/20 (25%) | 228/817 (27%) |
| Desired fertility | 61/61 (100%) | 19/21 (90%) | 772/898 (86%) |
| Testis cancer | |||
| Teratoma | 10/64 (15%) | 0/21 (0%)a | 178/923 (19%) |
| Seminoma | 10/64 (15%) | 2/21 (9%) | 149/923 (16%) |
| Lymphoma | |||
| Hodgkin's disease | 19/64 (29%)a | 1/21 (4%) | 99/923 (12%) |
| Non-Hodgkin's lymphoma | 7/64 (11%) | 3/21 (14%) | 88/923 (9%) |
| Leukaemia/BM transplant | 10/64 (16%) | 4/21 (19%) | 108/923 (11%) |
| Sarcoma | 2/64 (3%) | 4/21 (19%) | 73/923 (8%) |
| Other cancer | 2/64 (3%) | 6/21 (28%)a | 101/923 (11%) |
| Non-malignant disease | 4/64 (6%) | 1/21 (4%) | 107/923 (11%) |
| Scheduled chemotherapy | 59/63 (94%)a | 18/21 (86%) | 732/917 (78%) |
| Scheduled radiotherapy | 58/63 (92%) | 20/21 (95%) | 796/897 (88%) |
| Scheduled surgery | 60/63 (95%) | 16/21 (76%)a | 817/896 (91%) |
| Variable . | Request use during life . | Request continued storage post-mortem . | All . |
|---|---|---|---|
| aSignificant result (P < 0.05). | |||
| BM = bone marrow. | |||
| n | 68 | 21 | 930 |
| Age (years) | 29.3± 1.5 | 30.5 ± 0.8 | 28.6 ± 0.7 |
| Height (cm) | 177 ± 1 | 177 ± 1.6 | 177 ± 0.3 |
| Weight (kg) | 77 ± 1.9 | 78.4 ± 3 | 77.4 ± 0.5 |
| BMI (kg/m2) | 24.5 ± 0.6 | 25 ± 1.1 | 24.4 ± 0.2 |
| Testis volume (ml) | 22 ± 1 | 22 ± 1 | 22 ± 1 |
| Cryptorchidism | 10/60 (16%) | 1/19 (5%) | 45/803 (5%) |
| Normal virilization | 60/60 (100%) | 20/20 (100%) | 853/868 (98%) |
| Smoke (any) | 14/60 (23%) | 7/20 (35%) | 241/864 (27%) |
| Alcohol (any) | 53/60 (88%) | 19/20 (90%) | 721/855 (84%) |
| Social drugs (any) | 22/61 (36%) | 11/20 (55%) | 348/871 (40%) |
| Married at cryostorage | 40/62 (64%)a | 15/21 (71%)a | 382/908 (42%) |
| Prior infertility | 4/61 (6%) | 0/20 (0%) | 37/888 (4%) |
| Prior fertility | 17/61 (27%) | 5/20 (25%) | 228/817 (27%) |
| Desired fertility | 61/61 (100%) | 19/21 (90%) | 772/898 (86%) |
| Testis cancer | |||
| Teratoma | 10/64 (15%) | 0/21 (0%)a | 178/923 (19%) |
| Seminoma | 10/64 (15%) | 2/21 (9%) | 149/923 (16%) |
| Lymphoma | |||
| Hodgkin's disease | 19/64 (29%)a | 1/21 (4%) | 99/923 (12%) |
| Non-Hodgkin's lymphoma | 7/64 (11%) | 3/21 (14%) | 88/923 (9%) |
| Leukaemia/BM transplant | 10/64 (16%) | 4/21 (19%) | 108/923 (11%) |
| Sarcoma | 2/64 (3%) | 4/21 (19%) | 73/923 (8%) |
| Other cancer | 2/64 (3%) | 6/21 (28%)a | 101/923 (11%) |
| Non-malignant disease | 4/64 (6%) | 1/21 (4%) | 107/923 (11%) |
| Scheduled chemotherapy | 59/63 (94%)a | 18/21 (86%) | 732/917 (78%) |
| Scheduled radiotherapy | 58/63 (92%) | 20/21 (95%) | 796/897 (88%) |
| Scheduled surgery | 60/63 (95%) | 16/21 (76%)a | 817/896 (91%) |
Demographic and diagnostic variables as compared with semen usage
| Variable . | Request use during life . | Request continued storage post-mortem . | All . |
|---|---|---|---|
| aSignificant result (P < 0.05). | |||
| BM = bone marrow. | |||
| n | 68 | 21 | 930 |
| Age (years) | 29.3± 1.5 | 30.5 ± 0.8 | 28.6 ± 0.7 |
| Height (cm) | 177 ± 1 | 177 ± 1.6 | 177 ± 0.3 |
| Weight (kg) | 77 ± 1.9 | 78.4 ± 3 | 77.4 ± 0.5 |
| BMI (kg/m2) | 24.5 ± 0.6 | 25 ± 1.1 | 24.4 ± 0.2 |
| Testis volume (ml) | 22 ± 1 | 22 ± 1 | 22 ± 1 |
| Cryptorchidism | 10/60 (16%) | 1/19 (5%) | 45/803 (5%) |
| Normal virilization | 60/60 (100%) | 20/20 (100%) | 853/868 (98%) |
| Smoke (any) | 14/60 (23%) | 7/20 (35%) | 241/864 (27%) |
| Alcohol (any) | 53/60 (88%) | 19/20 (90%) | 721/855 (84%) |
| Social drugs (any) | 22/61 (36%) | 11/20 (55%) | 348/871 (40%) |
| Married at cryostorage | 40/62 (64%)a | 15/21 (71%)a | 382/908 (42%) |
| Prior infertility | 4/61 (6%) | 0/20 (0%) | 37/888 (4%) |
| Prior fertility | 17/61 (27%) | 5/20 (25%) | 228/817 (27%) |
| Desired fertility | 61/61 (100%) | 19/21 (90%) | 772/898 (86%) |
| Testis cancer | |||
| Teratoma | 10/64 (15%) | 0/21 (0%)a | 178/923 (19%) |
| Seminoma | 10/64 (15%) | 2/21 (9%) | 149/923 (16%) |
| Lymphoma | |||
| Hodgkin's disease | 19/64 (29%)a | 1/21 (4%) | 99/923 (12%) |
| Non-Hodgkin's lymphoma | 7/64 (11%) | 3/21 (14%) | 88/923 (9%) |
| Leukaemia/BM transplant | 10/64 (16%) | 4/21 (19%) | 108/923 (11%) |
| Sarcoma | 2/64 (3%) | 4/21 (19%) | 73/923 (8%) |
| Other cancer | 2/64 (3%) | 6/21 (28%)a | 101/923 (11%) |
| Non-malignant disease | 4/64 (6%) | 1/21 (4%) | 107/923 (11%) |
| Scheduled chemotherapy | 59/63 (94%)a | 18/21 (86%) | 732/917 (78%) |
| Scheduled radiotherapy | 58/63 (92%) | 20/21 (95%) | 796/897 (88%) |
| Scheduled surgery | 60/63 (95%) | 16/21 (76%)a | 817/896 (91%) |
| Variable . | Request use during life . | Request continued storage post-mortem . | All . |
|---|---|---|---|
| aSignificant result (P < 0.05). | |||
| BM = bone marrow. | |||
| n | 68 | 21 | 930 |
| Age (years) | 29.3± 1.5 | 30.5 ± 0.8 | 28.6 ± 0.7 |
| Height (cm) | 177 ± 1 | 177 ± 1.6 | 177 ± 0.3 |
| Weight (kg) | 77 ± 1.9 | 78.4 ± 3 | 77.4 ± 0.5 |
| BMI (kg/m2) | 24.5 ± 0.6 | 25 ± 1.1 | 24.4 ± 0.2 |
| Testis volume (ml) | 22 ± 1 | 22 ± 1 | 22 ± 1 |
| Cryptorchidism | 10/60 (16%) | 1/19 (5%) | 45/803 (5%) |
| Normal virilization | 60/60 (100%) | 20/20 (100%) | 853/868 (98%) |
| Smoke (any) | 14/60 (23%) | 7/20 (35%) | 241/864 (27%) |
| Alcohol (any) | 53/60 (88%) | 19/20 (90%) | 721/855 (84%) |
| Social drugs (any) | 22/61 (36%) | 11/20 (55%) | 348/871 (40%) |
| Married at cryostorage | 40/62 (64%)a | 15/21 (71%)a | 382/908 (42%) |
| Prior infertility | 4/61 (6%) | 0/20 (0%) | 37/888 (4%) |
| Prior fertility | 17/61 (27%) | 5/20 (25%) | 228/817 (27%) |
| Desired fertility | 61/61 (100%) | 19/21 (90%) | 772/898 (86%) |
| Testis cancer | |||
| Teratoma | 10/64 (15%) | 0/21 (0%)a | 178/923 (19%) |
| Seminoma | 10/64 (15%) | 2/21 (9%) | 149/923 (16%) |
| Lymphoma | |||
| Hodgkin's disease | 19/64 (29%)a | 1/21 (4%) | 99/923 (12%) |
| Non-Hodgkin's lymphoma | 7/64 (11%) | 3/21 (14%) | 88/923 (9%) |
| Leukaemia/BM transplant | 10/64 (16%) | 4/21 (19%) | 108/923 (11%) |
| Sarcoma | 2/64 (3%) | 4/21 (19%) | 73/923 (8%) |
| Other cancer | 2/64 (3%) | 6/21 (28%)a | 101/923 (11%) |
| Non-malignant disease | 4/64 (6%) | 1/21 (4%) | 107/923 (11%) |
| Scheduled chemotherapy | 59/63 (94%)a | 18/21 (86%) | 732/917 (78%) |
| Scheduled radiotherapy | 58/63 (92%) | 20/21 (95%) | 796/897 (88%) |
| Scheduled surgery | 60/63 (95%) | 16/21 (76%)a | 817/896 (91%) |
Number of semen specimens collected according to usage category.
| . | Specimens collected . | |||
|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . |
| 99% CI; P = 0.04. | ||||
| Total | 833 (100%) | 754 (90%) | 599 (72%) | 203 (24%) |
| Used (pregnancy) | 25 (100%) | 25 (100%) | 25 (100%) | 12 (48%) |
| Used (no pregnancy) | 39 (100%) | 36 (92%) | 29 (74%) | 18 (46%) |
| Posthumous request for storage | 21 (100%) | 21 (100%) | 18 (86%) | 9 (43%) |
| . | Specimens collected . | |||
|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . |
| 99% CI; P = 0.04. | ||||
| Total | 833 (100%) | 754 (90%) | 599 (72%) | 203 (24%) |
| Used (pregnancy) | 25 (100%) | 25 (100%) | 25 (100%) | 12 (48%) |
| Used (no pregnancy) | 39 (100%) | 36 (92%) | 29 (74%) | 18 (46%) |
| Posthumous request for storage | 21 (100%) | 21 (100%) | 18 (86%) | 9 (43%) |
Number of semen specimens collected according to usage category.
| . | Specimens collected . | |||
|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . |
| 99% CI; P = 0.04. | ||||
| Total | 833 (100%) | 754 (90%) | 599 (72%) | 203 (24%) |
| Used (pregnancy) | 25 (100%) | 25 (100%) | 25 (100%) | 12 (48%) |
| Used (no pregnancy) | 39 (100%) | 36 (92%) | 29 (74%) | 18 (46%) |
| Posthumous request for storage | 21 (100%) | 21 (100%) | 18 (86%) | 9 (43%) |
| . | Specimens collected . | |||
|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . |
| 99% CI; P = 0.04. | ||||
| Total | 833 (100%) | 754 (90%) | 599 (72%) | 203 (24%) |
| Used (pregnancy) | 25 (100%) | 25 (100%) | 25 (100%) | 12 (48%) |
| Used (no pregnancy) | 39 (100%) | 36 (92%) | 29 (74%) | 18 (46%) |
| Posthumous request for storage | 21 (100%) | 21 (100%) | 18 (86%) | 9 (43%) |
Diagram detailing the outcomes of 930 referrals for elective semen cryopreservation between May 1978 and August 2000.
Survival analysis of the number of cycles required to achieve a pregnancy.
To whom correspondence should be addressed at: ANZAC Research Institute, Concord Hospital, University of Sydney, Sydney NSW 2139, Australia. Email: djh@med.usyd.edu.au
References
Agarwal, A., Shekarriz, M., Sidhu, R.K. and Thomas, A.J. (
Bahadur, G. (
Berthelsen, J.G. (
Berthelsen, J.G. and Skakkebaek, N.E. (
Berthelsen, J.G., Skakkebaek, N.E., Mogensen, P. and Sorensen, B.L. (
Brennemann, W., Brensing, K.A., Leipner, N. et al. (
Chatterjee, R., Mills, W., Katz, M. et al. (
Coates, M.S. and Tracey, E.A. (2000) Cancer in New South Wales. Incidence and mortality 1997. New South Wales Cancer Council: Sydney.
Crawford, B.A., Spaliviero, J.A., Simpson and J.M., Handelsman, D.J. (
Davis, O.K., Bedford, J.M., Berkeley, A.S. et al. (
Fossa, S.D., Klepp, O. and Norman, N. (
Hallak, J., Sharma, R.K., Thomas, A.J. and Agarwal, A. (
Handelsman, D.J. (
Hendry, W.F., Stedronska, J., Jones, C.R. et al. (
Holmes, G.E. and Holmes, F.F. (
Hoshi, K., Yanagida, K., Katayose, H. and Yazawa, H. (
Johnson, D.H., Linde, R., Hainsworth, J.D. et al. (
Kahan, S.E., Seftel, A.D. and Resnick, M.I. (
Katayose, H., Matsuda, J. and Yanagimachi, R. (
Kliesch, S., Bergmann, M., Hertle, L. et al. (
Kreusser, E.D., Hetzel, W.D., Hautmann, R. and Pfeiffer, E.F. (
Lass, A., Akagbosu, F., Abusheikha, N. et al. (
Li, F.P., Gimbrere, K., Gelber, R.D. et al. (
Marmor, D., Elefant, E., Dauchez, C. and Roux, C. (
Masala, A., Faedda, R., Alagna, A. et al. (
Meistrich, M.L. (
Meistrich, M.L., Wilson, G., Brown, B.W. et al. (
Padron, O.F., Sharma, R.K., Thomas, A.J. and Agarwal, A. (
Redman, J.R., Bajorunas, D.R., Goldstein, M.C. et al. (
Reed, E., Sanger, W.G. and Armitage, J.O. (
Rhodes, E.A., Hoffman, D.J. and Kaempher, S.H. (
Rowland, G.F., Cohen, J., Steptoe, P.C. and Hewitt, J. (
Sanger, W.G., Armitage, J.O. and Schmidt, M.A. (
Sanger, W.G., Olson, J.H. and Sherman, J.K. (
Scammell, G.E., Stedronska, J., Edmonds, D.K. et al. (1985) Cryopreservation of semen in men with testicular tumour or Hodgkin's disease: results of artificial insemination of their partners.
Schlatt, S. (
Selby, P., Brada, M., Horwich, A. et al. (1988) Semen cryopreservation for patients surviving malignant disease: implications of proposed legislation.
Senturia, Y.D., Peckham, C.S. and Peckham, M.J. (1985) Children fathered by men treated for testicular cancer.
Shekarriz, M., Tolentino, M.V., Ayzman, I. et al. (
Sibert, L., Rives, N., Rey, D. et al. (
Sutcliffe, A.G. (
Tournaye, H., Camus, M., Bollen, N. et al. (
Viviani, S., Santoro, A., Ragni, G. et al. (
Wakayama, T. and Yanagimachi, R. (
Waxman, J.H., Ahmed, R., Smith, D. et al. (
Wennerholm, U.B., Bergh, C., Hamberger, L. et al. (
World Health Organization (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. Cambridge University Press, Cambridge.