-
PDF
- Split View
-
Views
-
Cite
Cite
S.A. Brigham, C. Conlon, R.G. Farquharson, A longitudinal study of pregnancy outcome following idiopathic recurrent miscarriage, Human Reproduction, Volume 14, Issue 11, November 1999, Pages 2868–2871, https://doi.org/10.1093/humrep/14.11.2868
Close - Share Icon Share
Abstract
Recurrent miscarriage is a difficult clinical problem occurring in ~1–2% of fertile women. Following investigation, most cases fail to reveal an identifiable cause and are therefore classified as idiopathic. The aim of this study was to identify important gestational milestones for pregnancy success prediction in women following idiopathic recurrent miscarriage. A total of 325 consecutive patients with idiopathic recurrent miscarriage was involved in a prospective longitudinal observational study. Patients were identified from a miscarriage database of 716 patients. Preconceptual presentation and investigation excluded patients from the study sample with known associations of recurrent pregnancy loss, such as antiphosholipid syndrome, oligomenorrhoea, mid-trimester loss and other rare causes, e.g. abnormal parental karyotype. Following early presentation in a subsequent pregnancy, all patients followed a standard clinic protocol including fetal viability ultrasonography on a fortnightly basis throughout the first trimester. Kaplan–Meier curves were constructed for pregnancy outcome. Out of 325 idiopathic cases, 70% (n = 226) conceived, with a 75% success rate. Of 55 miscarriages, longitudinal assessment showed that six losses occurred following detection of fetal cardiac activity (3%). Data from this large study group have enabled accurate prediction of future pregnancy success and have established important gestational milestones for women with idiopathic recurrent miscarriage.
Introduction
Spontaneous miscarriage occurs in ~15% of all pregnancies, as recorded by hospital episode statistics. The actual figure, from community-based assessment, may well be higher than this as some women miscarry at home and remain unreported to hospital (Everett, 1997). Between 1 and 2% of fertile women will experience recurring pregnancy loss and despite a wide range of investigations, no apparent cause can be found in ~50% of cases (Stirrat, 1990; Quenby and Farquharson, 1993). Recurrent loss of pregnancy is distressing for the patient and frustrating for the clinician, especially where treatment options are limited as in idiopathic recurrent miscarriage. The mainstay of management of these patients is empirically based upon tender loving care and emotional support.
In the absence of predicted success rates with idiopathic recurrent miscarriage, the clinician is at a disadvantage in the miscarriage clinic setting, where the most commonly posed question concerns the chance of future pregnancy success. Previous population studies are small, and few have documented sufficient patient numbers to generate confidence with clinical prediction of future pregnancy outcome, in terms of success or failure. The effect of emotional support, supplemented by ultrasound in early pregnancy gives `success rates' of between 70 and 80% (Stray-Pedersen and Stray-Pedersen, 1984; Liddell et al., 1991; Clifford et al., 1997). As important as an overall success rate, however, is the significance of each gestational milestone attained in the first trimester, which has not been previously determined.
In this large prospective study, an attempt has been made to identify important gestational milestones for women presenting with idiopathic recurrent miscarriage and used the data analysis to predict future pregnancy success based on gestational age, maternal age and miscarriage history.
Materials and methods
All women attending a dedicated Miscarriage Clinic in a University Teaching Hospital (Liverpool Women's Hospital, Liverpool, UK) were entered on a live, i.e. constantly updated, miscarriage database over a 10 year period. Patient information was entered onto a spreadsheet database, with the findings checked by a second doctor. The majority of the 716 patients included in the database (76%) had a history of at least three consecutive miscarriages. Due to patient demand for investigation, some patients were seen in the clinic with a history of two consecutive miscarriages (172 out of 716, 24%).
Following preconceptual presentation to the clinic, an accurate patient history was taken and investigations performed to exclude known associations of recurrent pregnancy loss, such as antiphospholipid syndrome, oligomenorrhoea (Quemby and Farquharson, 1993; Hasegawa et al., 1996; Drakeley et al., 1998), cervical weakness and other rarer causes, for example, abnormal parental chromosome karyotype, as previously described (Drakeley et al., 1998). Patients with identified causes for their pregnancy loss, those who had a history of second trimester loss and those who had completed successful treatment of an abnormal finding were then excluded from the study sample, leaving the `idiopathic' recurrent miscarriage patients. A separate database was then set up for these patients (Li, 1998) and all results of the investigations performed were recorded including number of previous miscarriages and live births. Further differentiation of the group was made into primary losers (n = 173, those with no previous live births) and secondary losers (n = 152, those with previous live births).
Following early presentation to the clinic in a subsequent pregnancy, all patients followed a standardized clinic protocol including transvaginal ultrasonography using transducers of 7.5–5 MHz to assess fetal viability on a fortnightly basis until 12 weeks gestation. Thereafter, they were followed up in the Pregnancy Support Antenatal Clinic. The gestation at which cardiac activity was initially seen was recorded on the database along with the outcome of the pregnancy. A successful outcome was regarded as survival beyond 24 weeks. A record was made of the gestational age at which cardiac activity was lost. Ectopic pregnancy and termination of pregnancy in the subsequent pregnancy were excluded from the study sample.
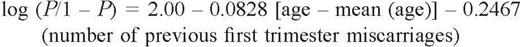
Results
On the live database, 716 consecutive patients were entered with a history of recurrent miscarriage and 325 of these were identified as having `idiopathic recurrent miscarriage', 23 of whom were lost to follow-up. Following postal contact, 76 patients reported no further pregnancy. Of the remainder, 226/325 (70%) subsequently achieved a further pregnancy, two of which were found to be ectopic, and two patients had termination of pregnancy. The majority of patients presented to the dedicated clinic by 8 weeks gestation (90%) and by 10 weeks 98% had presented.
The mean age of the study sample was 32 years (range 17–45 years) and the mean number of previous miscarriages was three (range 2–10) (Table I).
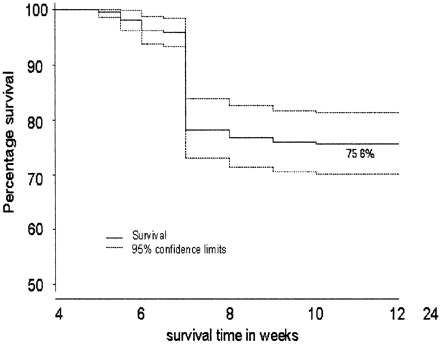
Of the patients achieving a further pregnancy, 167/222 (75%) had a successful outcome with survival beyond 24 weeks (Figure 1). A fetal survival curve was also constructed for the subset of women with at least three previous miscarriages. It was found identical to that for the population as a whole. There was also no statistical difference in outcome between women who had two and those who had three previous miscarriages.
There was no statistically significant difference in outcome between primary (77%) and secondary loser (74%), i.e. a previous live birth did not confer a greater chance of success in a subsequent pregnancy.
The entire group of 222 patients suffered 55 (25%) further miscarriages, 54 in the first trimester and one in the second trimester. Of these 55 miscarriages, six (3% of all pregnancies) occurred following detection of fetal cardiac activity.
Using the Kaplan–Meier curve (Figure 1), time-dependent survival, in terms of gestational age, was demonstrated. It was clear from this survival curve that the most perilous time for women with a history of idiopathic recurrent miscarriage was between 6 and 8 weeks gestation. Between these gestations, 78% of the pregnancy losses occurred, 89% of which occurred without the detection of fetal cardiac activity (embryo loss).
Fetal cardiac activity had been identified in 90% of the pregnancies by 8 weeks, rising to 98% by 10 weeks. Consequently, by 8 weeks gestation, if a fetal heart beat had been identified, the chances of a successful outcome in a subsequent pregnancy were 98%, climbing to 99.4% at 10 weeks gestation (Table II).
Previous miscarriage history and age of the patient significantly affected the chances of a successful outcome, age being slightly more significant than previous number of miscarriages (P = 0.0329 and P = 0.00318 respectively). Women with a history of two previous miscarriages had similar chances of success in a subsequent pregnancy to those that had a history of three previous miscarriages (76 versus 79% respectively, as calculated from the logistic regression model) (Table III).
Discussion
Important gestational milestones
The appearance of fetal cardiac activity is an important fundamental observation to clinicians and patients. Following detection of fetal cardiac activity, an anticipated fetal loss rate of between 2 and 5% has been quoted by retrospective analysis in normal low risk antenatal populations (Cashner, 1987; Mackenzie et al., 1988). In women with recurrent miscarriage, a small prospective study (n = 42) demonstrated a 10-fold increase of loss rates (22%), when the appearance of fetal heart activity was studied longitudinally in the early first trimester (Opsahl and Petit, 1993). By contrast, our prospective study of a larger population showed a fetal loss rate of 3% (6/222) after the initial detection of fetal cardiac activity, perhaps related to a lower than average maternal age than the aforementioned study. Details from this and other studies are shown in Table IV. Embryo loss and fetal loss rates vary between different population types, for example our recurrent miscarriage population showed six fetal losses (3%) and 49 embryo losses (22%). This contrasts with previous work in unselected populations, where fetal loss is more likely (Hill et al., 1991; Goldstein, 1994).
No statistically significant difference was found between the two groups of primary and secondary loser groups. This observation was also made in a low risk, prospectively studied antenatal population (Goldstein, 1994). Similarly, in a recurring miscarriage population, there seems to be no obvious benefit of having had a previous live birth on improving subsequent obstetric performance.
The concept of gestational milestones has been used to predict pregnancy success at 6, 8 and 10 weeks gestation. For the entire population there was a 22% loss rate at 6 weeks gestation, which dramatically fell to 2% at 8 weeks and subsequently at 10 weeks gestation fell to 0.6% of the remaining population (Table I). The conclusion would be that the most perilous time of gestation for women with idiopathic recurrent miscarriage is between 6 and 8 weeks.
Maternal age
Increasing maternal age reduces the chance of a pregnancy success. This has been confirmed in 201 women undergoing fertility treatment by ovulation induction (Smith and Buyalos, 1996). These authors clearly showed an increasing rate of pregnancy loss from 2.1% at less than 30 years to 20% in women over 40 years of age. Furthermore, the impact of age is profound within large infertility populations undergoing in-vitro fertilization (IVF) (Templeton et al., 1996). This study concluded that maternal age is singularly the most important determinant in predicting pregnancy success in an IVF population.
The profound impact of maternal age on pregnancy outcome is similarly demonstrated in the present study. For example, a woman aged 20 years with two previous miscarriages has a 92% [confidence interval (CI) 86–98] chance of success in a subsequent pregnancy. This, however, falls dramatically to 60% (CI 41–79) in a woman with a similar loss history who is aged 45 years (Table II). Although the confidence intervals for the success prediction are wide at the extreme ends of the age spectrum, there is little doubt that maternal age has a significant impact on future success in the recurrent miscarriage population.
Pregnancy support
The effect of the provision of tender loving care and emotional support on loss rates in recurrent miscarriage populations has been previously evaluated. The first large population study, utilizing tender loving care and emotional support in the first trimester, showed an 80% success rate in patients with idiopathic recurrent miscarriage (Stray-Pedersen and Stray-Pedersen, 1983). This study, however, identified 85 out of 195 couples as having `idiopathic recurrent miscarriage' and the population was quasi-randomized, based purely upon geographical location. A separate study, in the absence of tender loving care, showed an 80% success rate, when studied in a smaller population (n = 24) with similar characteristics (Vlaanderen et al., 1987). A more recent study reported an 86% success rate with tender loving care (n = 33), as opposed to only 33% in the absence of emotional support (n = 9), in an unrandomized population (Liddell et al., 1991). Both these recent studies are restricted by small numbers, in contrast to the present study of 222 consecutive pregnancies from which a 75% success rate has been obtained with the provision of tender loving care and ultrasound in early pregnancy.
Patient empowerment
Women with a history of idiopathic recurrent miscarriage, understandably exhibit a marked stress reaction following early diagnosis of a subsequent pregnancy. Ultrasound reassurance and emotional support in a specialized Miscarriage Clinic may address this problem and go some way to alleviating this stress. The present large population study, as well as determining success rates for the group as a whole, has also identified important gestational milestones for success prediction. These milestones can empower patients to gain increasing reassurance of a potential successful pregnancy outcome, as advancing gestation is reached. Clinicians can also gain confidence from this data to predict the future chances of pregnancy success in women with a history of idiopathic recurring miscarriage.
Previous miscarriage history
| Number of previous miscarriages . | Patient number (%) . |
|---|---|
| 2 | 79 (24) |
| 3 | 157 (48) |
| 4 | 43 (13) |
| 5 | 25 (8) |
| >5 | 21 (6) |
| Number of previous miscarriages . | Patient number (%) . |
|---|---|
| 2 | 79 (24) |
| 3 | 157 (48) |
| 4 | 43 (13) |
| 5 | 25 (8) |
| >5 | 21 (6) |
Previous miscarriage history
| Number of previous miscarriages . | Patient number (%) . |
|---|---|
| 2 | 79 (24) |
| 3 | 157 (48) |
| 4 | 43 (13) |
| 5 | 25 (8) |
| >5 | 21 (6) |
| Number of previous miscarriages . | Patient number (%) . |
|---|---|
| 2 | 79 (24) |
| 3 | 157 (48) |
| 4 | 43 (13) |
| 5 | 25 (8) |
| >5 | 21 (6) |
Important gestational milestones for success and loss prediction
| Gestational age (weeks) . | Success rate (%) . | Miscarriage rate (%) . |
|---|---|---|
| 6 | 78 | 22 |
| 8 | 98 | 2 |
| 10 | 99.4 | 0.6 |
| Gestational age (weeks) . | Success rate (%) . | Miscarriage rate (%) . |
|---|---|---|
| 6 | 78 | 22 |
| 8 | 98 | 2 |
| 10 | 99.4 | 0.6 |
Important gestational milestones for success and loss prediction
| Gestational age (weeks) . | Success rate (%) . | Miscarriage rate (%) . |
|---|---|---|
| 6 | 78 | 22 |
| 8 | 98 | 2 |
| 10 | 99.4 | 0.6 |
| Gestational age (weeks) . | Success rate (%) . | Miscarriage rate (%) . |
|---|---|---|
| 6 | 78 | 22 |
| 8 | 98 | 2 |
| 10 | 99.4 | 0.6 |
Predicted percentage success rate of subsequent pregnancy according to age and previous miscarriage history
| Age (years) . | Number of previous miscarriages . | |||
|---|---|---|---|---|
| . | 2 . | 3 . | 4 . | 5 . |
| Values are percentages with 95% confidence intervals (CI) shown in parentheses. Where the CI <20%, the values are shown in bold print. | ||||
| 20 | 92 | 90 | 88 | 85 |
| (86–98) | (83–97) | (79–96) | (74–96) | |
| 25 | 89 | 86 | 82 | 79 |
| (82–95) | (79–93) | (75–91) | (68–90) | |
| 30 | 84 | 80 | 76 | 71 |
| (77–90) | (74–86) | (69–83) | (61–81) | |
| 35 | 77 | 73 | 68 | 62 |
| (69–85) | (66–80) | (60–75) | (51–74) | |
| 40 | 69 | 64 | 58 | 52 |
| (57–82) | (52–76) | (45–71) | (37–67) | |
| 45 | 60 | 54 | 48 | 42 |
| (41–79) | (35–72) | (29–67) | (22–62) | |
| Age (years) . | Number of previous miscarriages . | |||
|---|---|---|---|---|
| . | 2 . | 3 . | 4 . | 5 . |
| Values are percentages with 95% confidence intervals (CI) shown in parentheses. Where the CI <20%, the values are shown in bold print. | ||||
| 20 | 92 | 90 | 88 | 85 |
| (86–98) | (83–97) | (79–96) | (74–96) | |
| 25 | 89 | 86 | 82 | 79 |
| (82–95) | (79–93) | (75–91) | (68–90) | |
| 30 | 84 | 80 | 76 | 71 |
| (77–90) | (74–86) | (69–83) | (61–81) | |
| 35 | 77 | 73 | 68 | 62 |
| (69–85) | (66–80) | (60–75) | (51–74) | |
| 40 | 69 | 64 | 58 | 52 |
| (57–82) | (52–76) | (45–71) | (37–67) | |
| 45 | 60 | 54 | 48 | 42 |
| (41–79) | (35–72) | (29–67) | (22–62) | |
Predicted percentage success rate of subsequent pregnancy according to age and previous miscarriage history
| Age (years) . | Number of previous miscarriages . | |||
|---|---|---|---|---|
| . | 2 . | 3 . | 4 . | 5 . |
| Values are percentages with 95% confidence intervals (CI) shown in parentheses. Where the CI <20%, the values are shown in bold print. | ||||
| 20 | 92 | 90 | 88 | 85 |
| (86–98) | (83–97) | (79–96) | (74–96) | |
| 25 | 89 | 86 | 82 | 79 |
| (82–95) | (79–93) | (75–91) | (68–90) | |
| 30 | 84 | 80 | 76 | 71 |
| (77–90) | (74–86) | (69–83) | (61–81) | |
| 35 | 77 | 73 | 68 | 62 |
| (69–85) | (66–80) | (60–75) | (51–74) | |
| 40 | 69 | 64 | 58 | 52 |
| (57–82) | (52–76) | (45–71) | (37–67) | |
| 45 | 60 | 54 | 48 | 42 |
| (41–79) | (35–72) | (29–67) | (22–62) | |
| Age (years) . | Number of previous miscarriages . | |||
|---|---|---|---|---|
| . | 2 . | 3 . | 4 . | 5 . |
| Values are percentages with 95% confidence intervals (CI) shown in parentheses. Where the CI <20%, the values are shown in bold print. | ||||
| 20 | 92 | 90 | 88 | 85 |
| (86–98) | (83–97) | (79–96) | (74–96) | |
| 25 | 89 | 86 | 82 | 79 |
| (82–95) | (79–93) | (75–91) | (68–90) | |
| 30 | 84 | 80 | 76 | 71 |
| (77–90) | (74–86) | (69–83) | (61–81) | |
| 35 | 77 | 73 | 68 | 62 |
| (69–85) | (66–80) | (60–75) | (51–74) | |
| 40 | 69 | 64 | 58 | 52 |
| (57–82) | (52–76) | (45–71) | (37–67) | |
| 45 | 60 | 54 | 48 | 42 |
| (41–79) | (35–72) | (29–67) | (22–62) | |
Comparison of studies identifying miscarriage rates following detection of fetal cardiac activity
| Authors . | Type of study . | No. of patients . | Population type . | Miscarriage rate (%) . |
|---|---|---|---|---|
| Brigham et al. | Prospective longitudinal | 325 | Selected recurrent miscarriage | 3 |
| Qasim et al. (1997) | Prospective | 116 | Infertility | 15 |
| Smithand Buyalos (1996) | Prospective | 201 | Infertility | 6.5 |
| Ovulation induction | ||||
| Goldstein et al. (1994) | Prospective | 232 | Unselected | 2.5 |
| Opsahl et al. (1993) | Prospective | 23 | Unselected | 21.7 |
| Recurrent miscarriage | ||||
| Lyndon et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Hill et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Cashner et al. (1987) | Prospective | 489 | Unselected from 8–12 weeks gestation | 2 |
| Liu et al. (1987) | Prospective | 1068 | Unselected | 1.5 |
| Lindand McFadyen (1986) | Prospective | 932 | Unselected | 2.3 |
| Wilson et al. (1986) | Prospective | 796 | Unselected | 2.1 |
| Gilmoreand McNay (1985) | Prospective | 1960 | Unselected after 10 weeks | 2.1 |
| Christiaens and Stoutenbeek (1984) | Prospective | 274 | Unselected after 10 weeks | 3.3 |
| Authors . | Type of study . | No. of patients . | Population type . | Miscarriage rate (%) . |
|---|---|---|---|---|
| Brigham et al. | Prospective longitudinal | 325 | Selected recurrent miscarriage | 3 |
| Qasim et al. (1997) | Prospective | 116 | Infertility | 15 |
| Smithand Buyalos (1996) | Prospective | 201 | Infertility | 6.5 |
| Ovulation induction | ||||
| Goldstein et al. (1994) | Prospective | 232 | Unselected | 2.5 |
| Opsahl et al. (1993) | Prospective | 23 | Unselected | 21.7 |
| Recurrent miscarriage | ||||
| Lyndon et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Hill et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Cashner et al. (1987) | Prospective | 489 | Unselected from 8–12 weeks gestation | 2 |
| Liu et al. (1987) | Prospective | 1068 | Unselected | 1.5 |
| Lindand McFadyen (1986) | Prospective | 932 | Unselected | 2.3 |
| Wilson et al. (1986) | Prospective | 796 | Unselected | 2.1 |
| Gilmoreand McNay (1985) | Prospective | 1960 | Unselected after 10 weeks | 2.1 |
| Christiaens and Stoutenbeek (1984) | Prospective | 274 | Unselected after 10 weeks | 3.3 |
Comparison of studies identifying miscarriage rates following detection of fetal cardiac activity
| Authors . | Type of study . | No. of patients . | Population type . | Miscarriage rate (%) . |
|---|---|---|---|---|
| Brigham et al. | Prospective longitudinal | 325 | Selected recurrent miscarriage | 3 |
| Qasim et al. (1997) | Prospective | 116 | Infertility | 15 |
| Smithand Buyalos (1996) | Prospective | 201 | Infertility | 6.5 |
| Ovulation induction | ||||
| Goldstein et al. (1994) | Prospective | 232 | Unselected | 2.5 |
| Opsahl et al. (1993) | Prospective | 23 | Unselected | 21.7 |
| Recurrent miscarriage | ||||
| Lyndon et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Hill et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Cashner et al. (1987) | Prospective | 489 | Unselected from 8–12 weeks gestation | 2 |
| Liu et al. (1987) | Prospective | 1068 | Unselected | 1.5 |
| Lindand McFadyen (1986) | Prospective | 932 | Unselected | 2.3 |
| Wilson et al. (1986) | Prospective | 796 | Unselected | 2.1 |
| Gilmoreand McNay (1985) | Prospective | 1960 | Unselected after 10 weeks | 2.1 |
| Christiaens and Stoutenbeek (1984) | Prospective | 274 | Unselected after 10 weeks | 3.3 |
| Authors . | Type of study . | No. of patients . | Population type . | Miscarriage rate (%) . |
|---|---|---|---|---|
| Brigham et al. | Prospective longitudinal | 325 | Selected recurrent miscarriage | 3 |
| Qasim et al. (1997) | Prospective | 116 | Infertility | 15 |
| Smithand Buyalos (1996) | Prospective | 201 | Infertility | 6.5 |
| Ovulation induction | ||||
| Goldstein et al. (1994) | Prospective | 232 | Unselected | 2.5 |
| Opsahl et al. (1993) | Prospective | 23 | Unselected | 21.7 |
| Recurrent miscarriage | ||||
| Lyndon et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Hill et al. (1991) | Prospective | 347 | Unselected | 6.1 |
| Cashner et al. (1987) | Prospective | 489 | Unselected from 8–12 weeks gestation | 2 |
| Liu et al. (1987) | Prospective | 1068 | Unselected | 1.5 |
| Lindand McFadyen (1986) | Prospective | 932 | Unselected | 2.3 |
| Wilson et al. (1986) | Prospective | 796 | Unselected | 2.1 |
| Gilmoreand McNay (1985) | Prospective | 1960 | Unselected after 10 weeks | 2.1 |
| Christiaens and Stoutenbeek (1984) | Prospective | 274 | Unselected after 10 weeks | 3.3 |
Fetal survival in women with a history of idiopathic recurrent miscarriage (n = 222).
To whom correspondence should be addressed
The authors would like to thank Paul Monaghan and Dr Simon Fear (Department of Statistics) for their assistance with data analysis.
References
Cashner, K.A., Christopher, C.R. and Dysert, G.A. (
Christiaens, G.C. and Stoutenbeek, P.H. (
Clifford, K., Rai, R. and Regan, L. (
Drakeley, A.J, Quenby, S. and Farquharson, R.G. (
Everett, C. (
Gilmore, D.H. and McNay, M.B. (
Goldstein, S.R. (
Hasegawa, I., Takakuwa, K. and Tanaka, K. (
Hill, L.M., Guzick, D., Fries, J. and Hixon, J. (
Li, T.C. (
Liddell, H.S., Pattison, N.S. and Zanderigo, A. (
Liu, D.T.Y., Jeavons, B., Preston, C. et al. (
Lyndon, M., Hill, M.D., Guzick, D. et al. (
Mackenzie, W.E., Holmes, D.S. and Newton, J.R. (
Opsahl, M.S. and Petit, D.C. (
Qasim, S.M., Sachdev, R., Trias, A. et al. (
Quenby, S. and Farquharson, R.G. (
Smith, K.E. and Buyalos, R.P. (
Stray-Pedersen, B. and Stray-Pedersen, S. (
Templeton, A., Morris, J.K. and Parslow, W. (
Vlaanderen, W. and Treffers, P.E. (