-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer L. Wolff, Erin R. Giovannetti, Cynthia M. Boyd, Lisa Reider, Sara Palmer, Daniel Scharfstein, Jill Marsteller, Stephen T. Wegener, Katherine Frey, Bruce Leff, Kevin D. Frick, Chad Boult, Effects of Guided Care on Family Caregivers, The Gerontologist, Volume 50, Issue 4, August 2010, Pages 459–470, https://doi.org/10.1093/geront/gnp124
Close - Share Icon Share
Abstract
Purpose: Guided Care (GC) is a model of health care for multimorbid older adults that is provided by a registered nurse who works with the patients’ primary care physician (PCP). The purpose of this study was to determine whether GC improves patients’ primary caregivers’ depressive symptoms, strain, productivity, and perceptions of the quality of care recipients’ chronic illness care. Design and Methods: A cluster-randomized controlled trial of GC was conducted within 14 PCP teams. The study sample included 196 primary caregivers who completed baseline and 18-month surveys and whose care recipients remained alive and enrolled in the GC study for 18 months. Caregiver outcomes included the following: depressive symptoms (Center for Epidemiological Studies-Depression scale), strain (Modified Caregiver Strain Index), the quality of care recipients’ chronic illness care [Patient Assessment of Chronic Illness Care (PACIC)], and personal productivity (Work Productivity and Activity Impairment questionnaire, adapted for caregiving). Results: In multivariate regression models, between-group differences in depression, strain, work productivity, and regular activity productivity were not statistically significant after 18 months, but GC caregivers reported the overall quality of their recipients’ chronic illness care to be significantly higher (adjusted beta = 0.40, 95% confidence interval : 0.14–0.67). Quality was significantly higher in 4 of 5 PACIC subscales, reflecting the dimensions of goal setting, coordination of care, decision support, and patient activation. Implications: GC improved the quality of chronic illness care received by multimorbid care recipients but did not improve caregivers’ depressive symptoms, affect, or productivity.
The role of families as long-term caregivers is well established, although the scope and implications of associated responsibilities continues to evolve. Increases in longevity, advances in medical technologies, and reimbursement systems that favor lower levels of care collectively provide reason to believe that families have assumed a more active role in older adults’ health care. Evidence that families participate in health care decision making (Deimling, Smerglia, & Barresi, 1990; Ishikawa, Roter, Yamazaki, & Takayama, 2005), coordinate services between providers and settings of care (DesRoches, Blendon, Young, Scoles, & Kim, 2002; Weinberg, Lusenhop, Gittell, & Kautz, 2007), assist with medication administration and wound care (Donelan et al., 2002; Wolff & Kasper, 2006), and communicate directly with patients and providers during medical visits (Clayman, Roter, Wissow, & Bandeen-Roche, 2005; Wolff & Roter, 2008) supports the assertion that they are engaged in chronic as well as acute health care processes. Despite a consensus regarding the importance and pervasiveness of families’ roles in older adults’ health care (Institute Of Medicine, 2008), they typically are not educated by health care professionals to perform the tasks they are expected to assume (Donelan et al., 2002; Thielemann, 2000). Perhaps not surprisingly then, families report being insufficiently prepared when confronted with the need to manage tasks such as home-based technologies (Silver, Wellman, Galindo-Ciocon, & Johnson, 2004; Winkler, Ross, Piamjariyakul, Gajewski, & Smith, 2006) and postacute care (DesRoches et al.; Weinberg et al.).
The attributes, responsibilities, and needs of families who share health care responsibilities with chronically ill older adults have not been well described to date (Silliman, 2000). Studies of family caregivers typically have been restricted to individuals assisting patients with a single disease requiring long-term or episodic assistance, such as dementia, stroke, or cancer (Sorensen, Pinquart, & Duberstein, 2002; Wolff, 2007), and have most often focused on a narrow range of outcomes related to physical, emotional, and financial burdens associated with care provision (Emanuel, Fairclough, Slutsman, & Emanuel, 2000; Pinquart & Sorensen, 2003b; Sorensen et al.; Vitaliano, Zhang, & Scanlan, 2003). Strong evidence substantiating caregiving-related burdens has motivated investigation of the causal pathway by which care provision influences health and well-being as well as the development of intervention strategies.
A theoretical framework has been articulated that differentiates between objective burdens associated with intensity of caregiving-related demands (e.g., time, physical exertion, money, privacy, personal freedom) and subjective burdens tied to emotional worry, frustration, and anxiety related to caring for someone with physical and/or cognitive deficits (Montgomery, Gonyea, & Hooyman, 1985; Pearlin, Mullan, Semple, & Skaff, 1990). Contextual factors such as the knowledge and skills needed to perform tasks, involvement of secondary caregivers and/or formal service providers, family attributes, such as cohesion or conflict, and social support are posited to moderate the effects of caregiving on caregivers and to explain individual heterogeneity (Pearlin; Yates, Tennstedt, & Chang, 1999). Thus far, interventions seeking to minimize objective effects (e.g., respite care, adult day care) and/or subjective effects (e.g., psychotherapy, support groups) associated with care provision have met with limited success (Sorensen et al., 2002).
This article bridges the literatures on family caregiving and chronic care by discussing results from a cluster-randomized controlled trial of a primary care-based, nurse-facilitated, chronic care intervention, “Guided Care,” that includes training and supporting patients’ family caregivers. Guided Care (GC) was designed to address deficiencies in the quality of chronic care delivery by facilitating coordinated, comprehensive, evidence-based health care for multimorbid adults (Boyd et al., 2007). In GC, a registered nurse, who has completed a supplemental educational curriculum and joined a primary care practice, works closely with several primary care physicians (PCPs) to meet the health needs of 50–60 chronically ill patients who are at high risk for heavy use of health services during the coming year. Using health information technology, the Guided Care Nurse (GCN) collaborates with the patient’s PCP to provide eight clinical processes: (a) assessing the patient at home, (b) creating an evidence-based care plan, (c) promoting patient self-management, (d) proactively monitoring the patient’s conditions, (e) coaching the patient to practice healthy behaviors, (f) coordinating patient’s transitions between sites and providers of care, (g) facilitating access to community resources, and (h) educating and supporting patients’ family caregivers (“The Guided Care Program for Families and Friends” [GCPFF]).
As detailed elsewhere, the GCPFF melds support for family caregivers with the delivery of coordinated and comprehensive chronic care to patients and seeks to concurrently improve the health and well-being of patients and their family caregivers (Wolff et al., 2009). Building on evidence that individually tailored multicomponent family caregiver interventions are most successful (Acton & Kang, 2001; Knight, Lutzky, & Macofsky-Urban, 1993; Sorensen et al., 2002), the GCPFF includes the following: (a) an initial one-on-one assessment of the patient’s primary caregiver, (b) education and referral of the caregiver to community resources, (c) ongoing “coaching” of the caregiver, (d) six 90-min caregiver workshop sessions guided by the philosophy and approach of chronic disease self-management (Lorig et al., 2001), and (e) unstructured monthly support group meetings of 1 hr in duration, all facilitated by the GC nurse. The GCPFF attempts to meet family caregivers’ diverse needs by making available a range of services targeting both objective burden (e.g., by referral to community services, greater proficiency in meeting recipient’s medical needs) and subjective burden (e.g., through exposure to cognitive-behavioral coping techniques and emotional support offered in the caregiver workshop and support groups) to alleviate caregiving-related stressors and improve appraisal.
We conducted a cluster-randomized controlled trial to measure the effects of GC on patients, caregivers, primary care practices, physicians, nurses, and health care insurers. Preliminary data at 6-month follow-up indicate that GC patients were twice as likely to rate the quality of their care highly (Boult et al., 2008) and to have experienced fewer hospital days, skilled nursing facility days, emergency room visits, and home health episodes than usual care (UC) patients (Leff et al., 2009). A paper describing the GCPFF model and its early effects on primary caregivers reported that the intervention was associated with modest reduction in depressive symptoms and strain after 6 months and that the effect of the intervention was stronger among caregivers who were providing higher levels of assistance at baseline (Wolff et al., 2009). In this article, we expand on these findings by exploring a more comprehensive range of outcomes after 18-month follow-up. More specifically, we investigate the hypothesis that by providing caregivers with education, guidance, and support, while simultaneously improving patients’ physical and mental health, GC improves caregiver depression, strain, and productivity as well as their perceptions of the quality of patients’ chronic illness care.
Methods
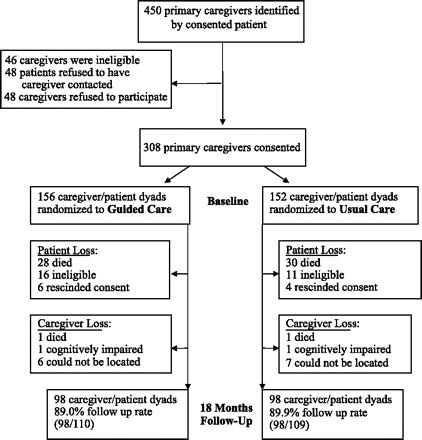
A cluster-randomized trial of GC was initiated in 2006. Seven nurses were recruited, trained, and integrated into seven randomly selected PCP teams (from a pool of 14) in three Mid-Atlantic health care delivery systems (Boult et al., 2008). Caregiver participants were identified after patients in participating physician practices were consented as follows.
Study Participant Recruitment
Patients of participating physicians were eligible for the study if they were at least 65 years of age and ranked in the upper quartile of risk for using health services heavily during the coming year according to their hierarchical condition category (HCC) predictive model scores based on health insurance claims’ diagnoses (Pope et al., 2004). A total of 904 high-risk patients of the 14 physician teams provided informed consent and completed a baseline in-person interview with a professional interviewer. At the time of the baseline interview, patient participants who reported receiving health-related assistance from another person were asked to identify their primary caregiver as the family member or unpaid friend who helped “the most.” As outlined in Figure 1, among 450 patient participants who were receiving help at the time of their baseline interview, 308 primary caregivers met the study’s eligibility criteria (serving as an unpaid or family caregiver to a patient participant), provided informed consent, and were interviewed. Upon completion of baseline interviews, caregivers were assigned to the same group as their care recipients, who were cluster randomized by physician team to receive either GC (n = 156 caregivers) or UC (n = 152 caregivers).
Attrition of patients through the course of 18 months occurred as a result of death (n = 58), loss of study eligibility (n = 27), and rescinding of consent (n = 10). A total of 213 of the 308 patient participants with a participating primary caregiver at baseline remained alive and enrolled in the study at follow-up. Among these 213 patient-caregiver dyads, 4 caregivers died or were too cognitively impaired to be interviewed and 13 primary caregivers could not be located; thus, 196 primary caregivers completed the 18-month follow-up interview. There were no statistically significant differences between the dyads who completed the 18-month interview and those who did not with regard to characteristics of the patients (age, HCC score, numbers of chronic conditions, Short-Form 36 physical and mental health component scores, gender, marital status, race, self-rated health, functional limitations, education, Medicaid enrollment) or the caregivers (age, gender, marital status, relationship to patient, employment, frequency providing assistance, self-rated health, depression, and strain).
Intervention
Seven registered nurses were hired and prepared to provide all the services included in GC (Boult et al., 2008). GCNs were all female but were diverse in age (range: 32–57 years), race (five Caucasian and two African American), clinical background (including inpatient medical–surgical, case management, psychiatric nursing, and disease management), and years of nursing experience (mean of 16 years; range: 4–31). The GCN educational program included the topics of educating and supporting family caregivers, as well as comprehensive patient assessment, evidence-based guidelines for chronic conditions, motivational interviewing for health behavior change, collaborative care, chronic disease self-management, elder abuse, cultural competence, community resources, and using the GC electronic health record. The curriculum included case-based interactive seminars and workshops, supplemented by readings and brief recorded lectures. Successful completion of the program required demonstration of GC competencies during a practicum with simulated patients.
In practice, GCNs carried caseloads of 50–60 patients and 5–26 primary caregivers. GCNs were responsible for making all the GCPFF services available to all consented caregivers, but the use of these services by caregivers was voluntary.
Data Collection and Measurement
Baseline interviews of patients and primary caregiver participants were conducted in-person, and 18-month follow-up interviews were conducted by telephone by rigorously trained closely supervised professional interviewers who were masked to group assignment. Computer-assisted interviewing technology was used, and 10% of interviews underwent reliability testing. At baseline, caregivers reported their sociodemographic characteristics, employment status, health, and time devoted to caregiving. A criticism of caregiver intervention research has been that it is too narrow in its ascertainment of outcomes (Sorensen et al., 2002). To address this concern, four constructs of interest were examined from the caregiver perspective: mental health (i.e., depression), strain, the quality of recipient’s chronic illness care, and caregiving-related personal productivity loss.
Caregiver depressive symptoms were ascertained with the Center for Epidemiological Studies-Depression scale (CES-D; Weissman, Sholomskas, Pottenger, Prusoff, & Locke, 1977), a 20-item self-reported symptom rating scale. Each item is measured on a 4-point Likert scale, where “0” = rarely or none of the time (<1 day/week), “1” = some or a little of the time (1–2 days/week), “2” = occasionally or a moderate amount (3–4 days/week), and “3” = most or almost all of the time (5–7 days/week). Scores range in value from 0 to 60, with higher values indicating more depressive symptoms. The CES-D has been shown to be reliable and valid in relation to other self-report measures as well as clinicians’ ascertainment (Shafer, 2006; Weissman et al.).
Caregiver strain related to care provision was measured with the Modified Caregiver Strain Index (CSI; Robinson, 1983; Thornton & Travis, 2003), a 13-item instrument that ascertains caregiving-related strain across domains of employment, finances, physical health, and social relationships. The Modified CSI employs a 2-point scoring system for each item, yielding total scores that range from 0 to 26, with higher scores reflecting greater strain. The original instrument has been widely used to ascertain caregiver burden (Pinquart & Sorensen, 2003a), and the Modified CSI has been reported to perform well and to possess improved internal reliability as compared with the original instrument (Thornton & Travis).
Quality of Chronic Illness Care was ascertained using a modified version of the Patient Assessment of Chronic Illness Care (PACIC; Glasgow, Wagner et al., 2005). In the unmodified version, patients report how often within the past 6 months they have received care that is congruent with 20 attributes of high-quality chronic care, ranging from 1 (no or never) to 5 (yes or always). Responses are combined to produce a single summary score and five subscale scores: patient activation, decision support, goal setting, problem solving, and coordination of care. Validation studies indicate that the PACIC is reliable and valid (Glasgow, Wagner et al. 2005) and correlates well with patient ratings of health care and quality of life (Glasgow, Whitesides, Nelson, & King, 2005; Schmittdiel et al., 2008).
PACIC question wording was modified in this study to reflect caregiver, rather than patient, perceptions of the quality of chronic illness care. Rather than the patient being asked “Over the past six months, when I received care for my chronic illness, I was … ,” the caregiver was asked “Over the past six months, when (PATIENT) received care for his/her chronic illness, he/she was ….” Although there was modest correlation between patient and caregiver aggregate PACIC scores at baseline (r = .36), this outcome was evaluated as a reflection of caregivers’ perceptions of the quality of chronic illness care delivered to patients not as proxy reports to substitute for patient responses (Snow, Cook, Lin, Morgan, & Magaziner, 2005). Mean scores for individual PACIC subscales are reported as well as values for the aggregate summary score.
Caregivers’ Productivity Loss was ascertained with the Work Productivity and Activity Impairment questionnaire, as adapted for caregiving (WPAI:CG; Giovannetti, Wolff, Frick, & Boult, 2009; Reilly, Zbrozek, & Dukes, 1993). Regular activity productivity loss represents the degree to which care provision affected regular (i.e., nonemployment related) activities during the past 7 days on a scale from 1 to 10. Work productivity loss was assessed among the subset of caregivers who were employed for pay. Work productivity loss aggregates the amount of time missed from work (absenteeism) due to care provision along with caregiving-related reductions in productivity while at work (presenteeism) in the past 7 days on a scale from 1 to 10. The WPAI:CG has been validated as an instrument that produces separate estimates of caregiving-related productivity loss in work and in regular activities, with higher values indicating greater caregiving-related productivity loss (Giovannetti et al.).
Analysis
As discussed elsewhere (Boult et al., 2008; Wolff et al., 2009), chained equations were used to impute values for caregiver responses that were missing at baseline; five imputed data sets were generated and results combined across data sets using Rubin’s combining rules (Royston, 2005; Rubin, 1987). Intervention and control groups were compared at baseline using logistic regression for categorical variables and linear regression for continuous variables; all comparisons were adjusted for site. To test the hypothesis that GC improves primary caregivers’ depression, strain, productivity, and perceptions of the quality of care recipients’ chronic illness care at 18 months, multivariate linear regression models were fit that accounted for study site and baseline scale scores for each outcome measure as well as patient gender and education. To clarify the magnitude of the effects of GC on scaled scores, effect sizes were calculated using Hedges’ d, taking into account the baseline values, study site, and patient gender and education (Nakagawa & Cuthill, 2007). All scale scores were computed as recommended by the originators of the scales. Unless explicitly stated, between-group differences described in the text were limited to those that were differentiated by tests of statistical significance at p < .05.
In a previous study, we reported that caregiver participants provided a median of 14 hr of weekly assistance to patient participants at baseline (Wolff et al., 2009), with some caregivers providing little or no assistance. Given the broad definition used to identify caregivers for the GC study, it is not surprising that some caregivers were providing limited assistance to patients at baseline. However, depression, strain, and hours of care are highly intercorrelated (Pinquart & Sorensen, 2003a). For this reason, we conducted analyses that were designed a priori, using a consistent cut-point of 14 hr, to understand whether the effects of GC were greater among caregivers who were providing assistance to patients at a sufficient level so as to potentially benefit from the intervention. Analyses were performed in Stata version 9 statistical software (StataCorp, College Station, TX).
Results
Description of Study Sample
The analytic sample included caregivers whose care recipients remained alive and enrolled in the study at 18-month follow-up and who completed both baseline and 18-month surveys (N = 196; Figure 1). Patient participants were, on average, 78 years of age and afflicted with 4.6 chronic conditions; approximately half were limited in one or more activities of daily living (Table 1). Caregiver participants were, on average, 61 years of age. Many were female (70.4%), married (72.5%), and spouses or partners (47.9%) or adult children (43.9%) of patients (Table 2). Caregivers helped patients an average of 20.6 hr/week at baseline. More than half of caregivers assisted daily (55.7%). A total of 43.9% of caregivers were employed for pay outside the home. UC patients were more likely to be female and less educated than GC patients; however, no other statistically significant differences were observed between GC and UC patient or caregiver participant attributes at baseline.
Baseline Characteristics of Care Recipients Who Completed Baseline and 18-Month Follow-up Surveys (N = 196)
| Usual care (n = 98), % or M (SE) | Guided Care (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 77.9 (0.74) | 78.0 (0.61) | 77.9 (0.48) |
| HCC score | 2.2 (0.15) | 2.4 (0.13) | 2.3 (0.10) |
| Number of chronic conditions | 4.3 (0.19) | 4.9 (0.2) | 4.6 (0.14) |
| Quality of care (aggregate PACIC score) | 2.6 (0.08) | 2.6 (0.07) | 2.6 (0.05) |
| SF-36 Physical Component score | 35.9 (0.99) | 35.8 (1.09) | 35.8 (0.73) |
| SF-36 Mental Component score | 47.9 (1.25) | 47.1 (1.37) | 47.5 (0.92) |
| Femalea | 61.2 | 48.9 | 55.1 |
| Married | 56.1 | 55.1 | 55.6 |
| Caucasian | 46.9 | 54.1 | 50.5 |
| Excellent, very good, or good self-rated health | 46.9 | 48.9 | 47.9 |
| At least one ADL limitation | 46.9 | 46.9 | 46.9 |
| Patient has dementia | 5.1 | 4.1 | 4.6 |
| Could use more support with regular activities | 26.7 | 34.1 | 30.4 |
| Patient helps care for someone else | 16.7 | 15.4 | 16.0 |
| Has at least a high school educationa | 68.4 | 78.6 | 73.5 |
| Not enough money to make ends meet | 14.9 | 10.4 | 12.7 |
| Enrolled in Medicaid | 19.4 | 12.2 | 15.8 |
| Usual care (n = 98), % or M (SE) | Guided Care (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 77.9 (0.74) | 78.0 (0.61) | 77.9 (0.48) |
| HCC score | 2.2 (0.15) | 2.4 (0.13) | 2.3 (0.10) |
| Number of chronic conditions | 4.3 (0.19) | 4.9 (0.2) | 4.6 (0.14) |
| Quality of care (aggregate PACIC score) | 2.6 (0.08) | 2.6 (0.07) | 2.6 (0.05) |
| SF-36 Physical Component score | 35.9 (0.99) | 35.8 (1.09) | 35.8 (0.73) |
| SF-36 Mental Component score | 47.9 (1.25) | 47.1 (1.37) | 47.5 (0.92) |
| Femalea | 61.2 | 48.9 | 55.1 |
| Married | 56.1 | 55.1 | 55.6 |
| Caucasian | 46.9 | 54.1 | 50.5 |
| Excellent, very good, or good self-rated health | 46.9 | 48.9 | 47.9 |
| At least one ADL limitation | 46.9 | 46.9 | 46.9 |
| Patient has dementia | 5.1 | 4.1 | 4.6 |
| Could use more support with regular activities | 26.7 | 34.1 | 30.4 |
| Patient helps care for someone else | 16.7 | 15.4 | 16.0 |
| Has at least a high school educationa | 68.4 | 78.6 | 73.5 |
| Not enough money to make ends meet | 14.9 | 10.4 | 12.7 |
| Enrolled in Medicaid | 19.4 | 12.2 | 15.8 |
Notes: ADL = activity of daily living; HCC = hierarchical condition category (1.0 = average risk of heavy future use of health services); SF-36 = Short-Form 36 (range: 0[poor function] − 100[excellent function]).
Significant difference between control and intervention groups, adjusted for site p < .05.
Baseline Characteristics of Care Recipients Who Completed Baseline and 18-Month Follow-up Surveys (N = 196)
| Usual care (n = 98), % or M (SE) | Guided Care (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 77.9 (0.74) | 78.0 (0.61) | 77.9 (0.48) |
| HCC score | 2.2 (0.15) | 2.4 (0.13) | 2.3 (0.10) |
| Number of chronic conditions | 4.3 (0.19) | 4.9 (0.2) | 4.6 (0.14) |
| Quality of care (aggregate PACIC score) | 2.6 (0.08) | 2.6 (0.07) | 2.6 (0.05) |
| SF-36 Physical Component score | 35.9 (0.99) | 35.8 (1.09) | 35.8 (0.73) |
| SF-36 Mental Component score | 47.9 (1.25) | 47.1 (1.37) | 47.5 (0.92) |
| Femalea | 61.2 | 48.9 | 55.1 |
| Married | 56.1 | 55.1 | 55.6 |
| Caucasian | 46.9 | 54.1 | 50.5 |
| Excellent, very good, or good self-rated health | 46.9 | 48.9 | 47.9 |
| At least one ADL limitation | 46.9 | 46.9 | 46.9 |
| Patient has dementia | 5.1 | 4.1 | 4.6 |
| Could use more support with regular activities | 26.7 | 34.1 | 30.4 |
| Patient helps care for someone else | 16.7 | 15.4 | 16.0 |
| Has at least a high school educationa | 68.4 | 78.6 | 73.5 |
| Not enough money to make ends meet | 14.9 | 10.4 | 12.7 |
| Enrolled in Medicaid | 19.4 | 12.2 | 15.8 |
| Usual care (n = 98), % or M (SE) | Guided Care (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 77.9 (0.74) | 78.0 (0.61) | 77.9 (0.48) |
| HCC score | 2.2 (0.15) | 2.4 (0.13) | 2.3 (0.10) |
| Number of chronic conditions | 4.3 (0.19) | 4.9 (0.2) | 4.6 (0.14) |
| Quality of care (aggregate PACIC score) | 2.6 (0.08) | 2.6 (0.07) | 2.6 (0.05) |
| SF-36 Physical Component score | 35.9 (0.99) | 35.8 (1.09) | 35.8 (0.73) |
| SF-36 Mental Component score | 47.9 (1.25) | 47.1 (1.37) | 47.5 (0.92) |
| Femalea | 61.2 | 48.9 | 55.1 |
| Married | 56.1 | 55.1 | 55.6 |
| Caucasian | 46.9 | 54.1 | 50.5 |
| Excellent, very good, or good self-rated health | 46.9 | 48.9 | 47.9 |
| At least one ADL limitation | 46.9 | 46.9 | 46.9 |
| Patient has dementia | 5.1 | 4.1 | 4.6 |
| Could use more support with regular activities | 26.7 | 34.1 | 30.4 |
| Patient helps care for someone else | 16.7 | 15.4 | 16.0 |
| Has at least a high school educationa | 68.4 | 78.6 | 73.5 |
| Not enough money to make ends meet | 14.9 | 10.4 | 12.7 |
| Enrolled in Medicaid | 19.4 | 12.2 | 15.8 |
Notes: ADL = activity of daily living; HCC = hierarchical condition category (1.0 = average risk of heavy future use of health services); SF-36 = Short-Form 36 (range: 0[poor function] − 100[excellent function]).
Significant difference between control and intervention groups, adjusted for site p < .05.
Baseline Characteristics of Primary Caregivers Who Completed Baseline and 18-Month Follow-up Surveys (N = 196)
| UC (n = 98), % or M (SE) | GC (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 61.6 (1.51) | 60.9 (1.53) | 61.3 (1.07) |
| Female gender | 70.4 | 70.4 | 70.4 |
| Married | 68.4 | 76.5 | 72.5 |
| Adult child caregiver | 46.9 | 40.8 | 43.9 |
| Spousal caregiver | 42.9 | 53.1 | 47.9 |
| Employed for pay | 47.9 | 39.8 | 43.9 |
| Helped patient daily | 57.4 | 54.1 | 55.7 |
| Excellent, very good, or good self-rated health | 78.6 | 84.7 | 81.6 |
| Average hours of assistance per week | 18.8 (2.21) | 22.5 (2.61) | 20.6 (1.71) |
| Depression (CES-D) | 7.1 (0.82) | 6.4 (0.59) | 6.7 (0.5) |
| Strain (CSI) | 6.6 (0.59) | 6.5 (0.54) | 6.6 (0.4) |
| UC (n = 98), % or M (SE) | GC (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 61.6 (1.51) | 60.9 (1.53) | 61.3 (1.07) |
| Female gender | 70.4 | 70.4 | 70.4 |
| Married | 68.4 | 76.5 | 72.5 |
| Adult child caregiver | 46.9 | 40.8 | 43.9 |
| Spousal caregiver | 42.9 | 53.1 | 47.9 |
| Employed for pay | 47.9 | 39.8 | 43.9 |
| Helped patient daily | 57.4 | 54.1 | 55.7 |
| Excellent, very good, or good self-rated health | 78.6 | 84.7 | 81.6 |
| Average hours of assistance per week | 18.8 (2.21) | 22.5 (2.61) | 20.6 (1.71) |
| Depression (CES-D) | 7.1 (0.82) | 6.4 (0.59) | 6.7 (0.5) |
| Strain (CSI) | 6.6 (0.59) | 6.5 (0.54) | 6.6 (0.4) |
Notes: Differences between characteristics of the UC and GC groups were not statistically significant at the level of p < .05. CES-D = Center for Epidemiological Studies-Depression scale (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26); GC = Guided Care; UC = usual care.
Baseline Characteristics of Primary Caregivers Who Completed Baseline and 18-Month Follow-up Surveys (N = 196)
| UC (n = 98), % or M (SE) | GC (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 61.6 (1.51) | 60.9 (1.53) | 61.3 (1.07) |
| Female gender | 70.4 | 70.4 | 70.4 |
| Married | 68.4 | 76.5 | 72.5 |
| Adult child caregiver | 46.9 | 40.8 | 43.9 |
| Spousal caregiver | 42.9 | 53.1 | 47.9 |
| Employed for pay | 47.9 | 39.8 | 43.9 |
| Helped patient daily | 57.4 | 54.1 | 55.7 |
| Excellent, very good, or good self-rated health | 78.6 | 84.7 | 81.6 |
| Average hours of assistance per week | 18.8 (2.21) | 22.5 (2.61) | 20.6 (1.71) |
| Depression (CES-D) | 7.1 (0.82) | 6.4 (0.59) | 6.7 (0.5) |
| Strain (CSI) | 6.6 (0.59) | 6.5 (0.54) | 6.6 (0.4) |
| UC (n = 98), % or M (SE) | GC (n = 98), % or M (SE) | Total (N = 196), % or M (SE) | |
| Age | 61.6 (1.51) | 60.9 (1.53) | 61.3 (1.07) |
| Female gender | 70.4 | 70.4 | 70.4 |
| Married | 68.4 | 76.5 | 72.5 |
| Adult child caregiver | 46.9 | 40.8 | 43.9 |
| Spousal caregiver | 42.9 | 53.1 | 47.9 |
| Employed for pay | 47.9 | 39.8 | 43.9 |
| Helped patient daily | 57.4 | 54.1 | 55.7 |
| Excellent, very good, or good self-rated health | 78.6 | 84.7 | 81.6 |
| Average hours of assistance per week | 18.8 (2.21) | 22.5 (2.61) | 20.6 (1.71) |
| Depression (CES-D) | 7.1 (0.82) | 6.4 (0.59) | 6.7 (0.5) |
| Strain (CSI) | 6.6 (0.59) | 6.5 (0.54) | 6.6 (0.4) |
Notes: Differences between characteristics of the UC and GC groups were not statistically significant at the level of p < .05. CES-D = Center for Epidemiological Studies-Depression scale (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26); GC = Guided Care; UC = usual care.
Eighteen-Month Outcomes
Multivariate linear regression models were used to test the hypothesis that GC improves primary caregivers’ depression, strain, productivity, and perceptions of the quality of care recipients’ chronic illness care at 18 months. Results of these models, estimated for each outcome measure, are shown in Table 3. Between baseline and 18-month follow-up, mean CSI scores increased from 6.5 to 6.7 among GC caregivers and from 6.6 to 7.7 among UC caregivers. Mean CES-D scores changed from 6.4 to 6.8 among GC caregivers and from 7.1 to 5.8 among UC caregivers. Between-group differences in depression and strain were not statistically significant at 18 months in multivariate regression models that accounted for study site, baseline scale scores, and patient gender and education.
Effects of GC on Family Caregivers’ Strain, Depression, Productivity, and Perceptions of Recipients’ Quality of Chronic Care (N = 196)
| Measure | UC | GC | αβ | 95% CI (αβ) | ESa | 95% CI (ES) | ||
| Baseline, M (SE) | 18 months, M (SE) | Baseline, M (SE) | 18 months, M (SE) | |||||
| Caregiver strain | ||||||||
| CSI score (n = 194) | 6.6 (0.59) | 7.7 (0.61) | 6.5 (0.54) | 6.7 (0.55) | −0.38 | −1.69 to 0.92 | −0.08 | −0.37 to 0.20 |
| Caregiver depression | ||||||||
| CES-D score (n = 194) | 7.1 (0.82) | 5.8 (0.68) | 6.4 (0.59) | 6.8 (0.68) | 1.42 | −0.37 to 3.21 | 0.23 | −0.06 to 0.51 |
| Quality of chronic care (PACIC) | ||||||||
| Goal setting (n = 161) | 2.6 (0.11) | 2.7 (0.13) | 2.5 (0.12) | 3.1 (0.13) | 0.50 | 0.17 to 0.83 | 0.47 | 0.15 to 0.79 |
| Coordination of care (n = 165) | 2.5 (0.11) | 2.6 (0.12) | 2.6 (0.11) | 3.1 (0.13) | 0.47 | 0.14 to 0.81 | 0.43 | 0.12 to 0.75 |
| Decision support (n = 159) | 3.3 (0.10) | 3.5 (0.12) | 3.2 (0.10) | 3.9 (0.10) | 0.40 | 0.10 to 0.70 | 0.41 | 0.09 to 0.74 |
| Problem solving (n = 159) | 2.9 (0.12) | 3.2 (0.13) | 2.9 (0.13) | 3.3 (0.13) | 0.18 | −0.15 to 0.51 | 0.17 | −0.14 to 0.49 |
| Patient activation (n = 153) | 2.9 (0.14) | 2.9 (0.15) | 2.8 (0.14) | 3.3 (0.13) | 0.51 | 0.16 to 0.85 | 0.47 | 0.14 to 0.80 |
| Aggregate quality (n = 164) | 2.8 (0.09) | 2.9 (0.11) | 2.8 (0.1) | 3.3 (0.11) | 0.40 | 0.14 to 0.67 | 0.47 | 0.15 to 0.78 |
| Productivity loss (WPAI:CG) | ||||||||
| Regular activity (n = 195) | 0.24 (0.03) | 0.23 (0.03) | 0.22 (0.03) | 0.23 (0.03) | −0.05 | −0.13 to 0.04 | −0.26 | −0.74 to 0.22 |
| Work productivity (n = 70) | 0.18 (0.05) | 0.16 (0.04) | 0.15 (0.03) | 0.08 (0.02) | 0.00 | −0.08 to 0.08 | 0.01 | −0.28 to 0.30 |
| Measure | UC | GC | αβ | 95% CI (αβ) | ESa | 95% CI (ES) | ||
| Baseline, M (SE) | 18 months, M (SE) | Baseline, M (SE) | 18 months, M (SE) | |||||
| Caregiver strain | ||||||||
| CSI score (n = 194) | 6.6 (0.59) | 7.7 (0.61) | 6.5 (0.54) | 6.7 (0.55) | −0.38 | −1.69 to 0.92 | −0.08 | −0.37 to 0.20 |
| Caregiver depression | ||||||||
| CES-D score (n = 194) | 7.1 (0.82) | 5.8 (0.68) | 6.4 (0.59) | 6.8 (0.68) | 1.42 | −0.37 to 3.21 | 0.23 | −0.06 to 0.51 |
| Quality of chronic care (PACIC) | ||||||||
| Goal setting (n = 161) | 2.6 (0.11) | 2.7 (0.13) | 2.5 (0.12) | 3.1 (0.13) | 0.50 | 0.17 to 0.83 | 0.47 | 0.15 to 0.79 |
| Coordination of care (n = 165) | 2.5 (0.11) | 2.6 (0.12) | 2.6 (0.11) | 3.1 (0.13) | 0.47 | 0.14 to 0.81 | 0.43 | 0.12 to 0.75 |
| Decision support (n = 159) | 3.3 (0.10) | 3.5 (0.12) | 3.2 (0.10) | 3.9 (0.10) | 0.40 | 0.10 to 0.70 | 0.41 | 0.09 to 0.74 |
| Problem solving (n = 159) | 2.9 (0.12) | 3.2 (0.13) | 2.9 (0.13) | 3.3 (0.13) | 0.18 | −0.15 to 0.51 | 0.17 | −0.14 to 0.49 |
| Patient activation (n = 153) | 2.9 (0.14) | 2.9 (0.15) | 2.8 (0.14) | 3.3 (0.13) | 0.51 | 0.16 to 0.85 | 0.47 | 0.14 to 0.80 |
| Aggregate quality (n = 164) | 2.8 (0.09) | 2.9 (0.11) | 2.8 (0.1) | 3.3 (0.11) | 0.40 | 0.14 to 0.67 | 0.47 | 0.15 to 0.78 |
| Productivity loss (WPAI:CG) | ||||||||
| Regular activity (n = 195) | 0.24 (0.03) | 0.23 (0.03) | 0.22 (0.03) | 0.23 (0.03) | −0.05 | −0.13 to 0.04 | −0.26 | −0.74 to 0.22 |
| Work productivity (n = 70) | 0.18 (0.05) | 0.16 (0.04) | 0.15 (0.03) | 0.08 (0.02) | 0.00 | −0.08 to 0.08 | 0.01 | −0.28 to 0.30 |
Notes: 95% CI = 95% confidence interval; CES-D = Center for Epidemiological Studies-Depression scale (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26); GC = Guided Care; ES = effect size; PACIC = Patient Assessment of Chronic Illness Care; WPAI:CG = Work Productivity and Activity Impairment, as adapted for Caregiving; UC = usual care; αβ = the regression coefficient for group assignment (GC = 1; UC = 0) in models of 18-month scores, adjusting for baseline score, patient gender, patient education, and study site.
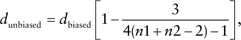
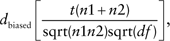
where n1 and n2 are the numbers of sample size in two groups and df is the degrees of freedom used for a corresponding t value in a linear model.
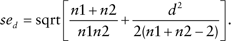
Effects of GC on Family Caregivers’ Strain, Depression, Productivity, and Perceptions of Recipients’ Quality of Chronic Care (N = 196)
| Measure | UC | GC | αβ | 95% CI (αβ) | ESa | 95% CI (ES) | ||
| Baseline, M (SE) | 18 months, M (SE) | Baseline, M (SE) | 18 months, M (SE) | |||||
| Caregiver strain | ||||||||
| CSI score (n = 194) | 6.6 (0.59) | 7.7 (0.61) | 6.5 (0.54) | 6.7 (0.55) | −0.38 | −1.69 to 0.92 | −0.08 | −0.37 to 0.20 |
| Caregiver depression | ||||||||
| CES-D score (n = 194) | 7.1 (0.82) | 5.8 (0.68) | 6.4 (0.59) | 6.8 (0.68) | 1.42 | −0.37 to 3.21 | 0.23 | −0.06 to 0.51 |
| Quality of chronic care (PACIC) | ||||||||
| Goal setting (n = 161) | 2.6 (0.11) | 2.7 (0.13) | 2.5 (0.12) | 3.1 (0.13) | 0.50 | 0.17 to 0.83 | 0.47 | 0.15 to 0.79 |
| Coordination of care (n = 165) | 2.5 (0.11) | 2.6 (0.12) | 2.6 (0.11) | 3.1 (0.13) | 0.47 | 0.14 to 0.81 | 0.43 | 0.12 to 0.75 |
| Decision support (n = 159) | 3.3 (0.10) | 3.5 (0.12) | 3.2 (0.10) | 3.9 (0.10) | 0.40 | 0.10 to 0.70 | 0.41 | 0.09 to 0.74 |
| Problem solving (n = 159) | 2.9 (0.12) | 3.2 (0.13) | 2.9 (0.13) | 3.3 (0.13) | 0.18 | −0.15 to 0.51 | 0.17 | −0.14 to 0.49 |
| Patient activation (n = 153) | 2.9 (0.14) | 2.9 (0.15) | 2.8 (0.14) | 3.3 (0.13) | 0.51 | 0.16 to 0.85 | 0.47 | 0.14 to 0.80 |
| Aggregate quality (n = 164) | 2.8 (0.09) | 2.9 (0.11) | 2.8 (0.1) | 3.3 (0.11) | 0.40 | 0.14 to 0.67 | 0.47 | 0.15 to 0.78 |
| Productivity loss (WPAI:CG) | ||||||||
| Regular activity (n = 195) | 0.24 (0.03) | 0.23 (0.03) | 0.22 (0.03) | 0.23 (0.03) | −0.05 | −0.13 to 0.04 | −0.26 | −0.74 to 0.22 |
| Work productivity (n = 70) | 0.18 (0.05) | 0.16 (0.04) | 0.15 (0.03) | 0.08 (0.02) | 0.00 | −0.08 to 0.08 | 0.01 | −0.28 to 0.30 |
| Measure | UC | GC | αβ | 95% CI (αβ) | ESa | 95% CI (ES) | ||
| Baseline, M (SE) | 18 months, M (SE) | Baseline, M (SE) | 18 months, M (SE) | |||||
| Caregiver strain | ||||||||
| CSI score (n = 194) | 6.6 (0.59) | 7.7 (0.61) | 6.5 (0.54) | 6.7 (0.55) | −0.38 | −1.69 to 0.92 | −0.08 | −0.37 to 0.20 |
| Caregiver depression | ||||||||
| CES-D score (n = 194) | 7.1 (0.82) | 5.8 (0.68) | 6.4 (0.59) | 6.8 (0.68) | 1.42 | −0.37 to 3.21 | 0.23 | −0.06 to 0.51 |
| Quality of chronic care (PACIC) | ||||||||
| Goal setting (n = 161) | 2.6 (0.11) | 2.7 (0.13) | 2.5 (0.12) | 3.1 (0.13) | 0.50 | 0.17 to 0.83 | 0.47 | 0.15 to 0.79 |
| Coordination of care (n = 165) | 2.5 (0.11) | 2.6 (0.12) | 2.6 (0.11) | 3.1 (0.13) | 0.47 | 0.14 to 0.81 | 0.43 | 0.12 to 0.75 |
| Decision support (n = 159) | 3.3 (0.10) | 3.5 (0.12) | 3.2 (0.10) | 3.9 (0.10) | 0.40 | 0.10 to 0.70 | 0.41 | 0.09 to 0.74 |
| Problem solving (n = 159) | 2.9 (0.12) | 3.2 (0.13) | 2.9 (0.13) | 3.3 (0.13) | 0.18 | −0.15 to 0.51 | 0.17 | −0.14 to 0.49 |
| Patient activation (n = 153) | 2.9 (0.14) | 2.9 (0.15) | 2.8 (0.14) | 3.3 (0.13) | 0.51 | 0.16 to 0.85 | 0.47 | 0.14 to 0.80 |
| Aggregate quality (n = 164) | 2.8 (0.09) | 2.9 (0.11) | 2.8 (0.1) | 3.3 (0.11) | 0.40 | 0.14 to 0.67 | 0.47 | 0.15 to 0.78 |
| Productivity loss (WPAI:CG) | ||||||||
| Regular activity (n = 195) | 0.24 (0.03) | 0.23 (0.03) | 0.22 (0.03) | 0.23 (0.03) | −0.05 | −0.13 to 0.04 | −0.26 | −0.74 to 0.22 |
| Work productivity (n = 70) | 0.18 (0.05) | 0.16 (0.04) | 0.15 (0.03) | 0.08 (0.02) | 0.00 | −0.08 to 0.08 | 0.01 | −0.28 to 0.30 |
Notes: 95% CI = 95% confidence interval; CES-D = Center for Epidemiological Studies-Depression scale (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26); GC = Guided Care; ES = effect size; PACIC = Patient Assessment of Chronic Illness Care; WPAI:CG = Work Productivity and Activity Impairment, as adapted for Caregiving; UC = usual care; αβ = the regression coefficient for group assignment (GC = 1; UC = 0) in models of 18-month scores, adjusting for baseline score, patient gender, patient education, and study site.
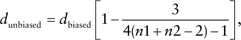
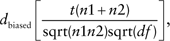
where n1 and n2 are the numbers of sample size in two groups and df is the degrees of freedom used for a corresponding t value in a linear model.
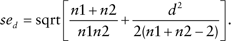
Caregiver reports of the aggregate quality of chronic illness care provided to their care recipients were higher among GC caregivers than UC caregivers at 18-month follow-up (adjusted beta [αβ] = 0.40; 95% confidence interval [CI]: 0.14–0.67), a difference that was statistically significant (p < .001) after adjusting for baseline PACIC aggregate rating, study site, and patient gender and education. GC caregivers’ ratings of chronic illness care quality were higher at 18 months than at baseline on all five PACIC subscales. GC caregivers reported significantly higher quality of chronic illness care for four of the five PACIC subscales: goal setting (αβ = 0.50; 95% CI: 0.17–0.83), coordination of care (αβ = 0.47; 95% CI: 0.14–0.81), decision support (αβ = 0.40; 95% CI: 0.10–0.70), and patient activation (αβ = 0.51; 95% CI: 0.16–0.85) after adjusting for baseline ratings, study site, and patient gender and education. The mean problem solving subscale score was higher for both GC and UC groups at 18 months, but the magnitude of difference between groups was not statistically significant.
Regular activity productivity loss associated with care provision was comparable at baseline and 18-month follow-up for the GC and UC caregivers, but work productivity loss among employed caregivers was lower at 18 months in both groups. The magnitude of decline in work productivity loss at 18 months was more substantial among GC caregivers (14.6%–8.4%) than UC caregivers (18.2%–16.1%), resulting from lower presenteeism in the GC group (Table 4). Absenteeism was comparable and low for UC and GC groups at both points in time, but presenteeism declined from 16.7% to 11.9% among UC caregivers and from 12.9% to 5.3% among GC caregivers.
Effects of Guided Care (GC) on Work Productivity Loss Among Working Caregivers (N = 70)
| Usual care % or M (SE) | GC % or M (SE) | |||
| Baseline | 18 months | Baseline | 18 months | |
| Absenteeism | ||||
| Hours missed | ||||
| Due to caregiving (N = 70) | 1.3 (0.59) | 1.5 (0.48) | 0.9 (0.40) | 1.1 (0.42) |
| For other reasons (N = 69) | 2.5 (1.25) | 2.2 (0.73) | 2.2 (0.79) | 4.6 (2.97) |
| Hours worked (N = 70) | 31.1 (3.05) | 38.5 (2.93) | 36.2 (2.30) | 33.5 (2.56) |
| Overall absenteeism (N = 70) | 5.5 | 5.1 | 2.2 | 3.7 |
| Presenteeism | ||||
| Productivity loss while at work (0–10; N = 70) | 16.7 | 11.9 | 12.9 | 5.3 |
| Total work productivity loss (N = 70) | 18.2 | 16.1 | 14.6 | 8.4 |
| Usual care % or M (SE) | GC % or M (SE) | |||
| Baseline | 18 months | Baseline | 18 months | |
| Absenteeism | ||||
| Hours missed | ||||
| Due to caregiving (N = 70) | 1.3 (0.59) | 1.5 (0.48) | 0.9 (0.40) | 1.1 (0.42) |
| For other reasons (N = 69) | 2.5 (1.25) | 2.2 (0.73) | 2.2 (0.79) | 4.6 (2.97) |
| Hours worked (N = 70) | 31.1 (3.05) | 38.5 (2.93) | 36.2 (2.30) | 33.5 (2.56) |
| Overall absenteeism (N = 70) | 5.5 | 5.1 | 2.2 | 3.7 |
| Presenteeism | ||||
| Productivity loss while at work (0–10; N = 70) | 16.7 | 11.9 | 12.9 | 5.3 |
| Total work productivity loss (N = 70) | 18.2 | 16.1 | 14.6 | 8.4 |
Effects of Guided Care (GC) on Work Productivity Loss Among Working Caregivers (N = 70)
| Usual care % or M (SE) | GC % or M (SE) | |||
| Baseline | 18 months | Baseline | 18 months | |
| Absenteeism | ||||
| Hours missed | ||||
| Due to caregiving (N = 70) | 1.3 (0.59) | 1.5 (0.48) | 0.9 (0.40) | 1.1 (0.42) |
| For other reasons (N = 69) | 2.5 (1.25) | 2.2 (0.73) | 2.2 (0.79) | 4.6 (2.97) |
| Hours worked (N = 70) | 31.1 (3.05) | 38.5 (2.93) | 36.2 (2.30) | 33.5 (2.56) |
| Overall absenteeism (N = 70) | 5.5 | 5.1 | 2.2 | 3.7 |
| Presenteeism | ||||
| Productivity loss while at work (0–10; N = 70) | 16.7 | 11.9 | 12.9 | 5.3 |
| Total work productivity loss (N = 70) | 18.2 | 16.1 | 14.6 | 8.4 |
| Usual care % or M (SE) | GC % or M (SE) | |||
| Baseline | 18 months | Baseline | 18 months | |
| Absenteeism | ||||
| Hours missed | ||||
| Due to caregiving (N = 70) | 1.3 (0.59) | 1.5 (0.48) | 0.9 (0.40) | 1.1 (0.42) |
| For other reasons (N = 69) | 2.5 (1.25) | 2.2 (0.73) | 2.2 (0.79) | 4.6 (2.97) |
| Hours worked (N = 70) | 31.1 (3.05) | 38.5 (2.93) | 36.2 (2.30) | 33.5 (2.56) |
| Overall absenteeism (N = 70) | 5.5 | 5.1 | 2.2 | 3.7 |
| Presenteeism | ||||
| Productivity loss while at work (0–10; N = 70) | 16.7 | 11.9 | 12.9 | 5.3 |
| Total work productivity loss (N = 70) | 18.2 | 16.1 | 14.6 | 8.4 |
Stratified Analyses
Given the broad definition used to identify caregivers in this study and anecdotal reports that some consented caregivers provided minimal assistance to patient participants (Wolff et al., 2009), stratified analyses were conducted based on whether caregiver participants reported the provision of 14 or more hours of assistance per week at baseline (n = 91) versus fewer than 14 hr (n = 105). At baseline, depression, strain, and productivity loss were generally higher and the quality of patients’ chronic illness care was lower, among caregivers providing 14 or more hours of assistance at baseline than among lower intensity caregivers (Table 5).
Effects of GC on Family Caregivers’ Strain, Depression, Productivity, and Perceptions of Recipients’ Quality of Chronic Care, Stratified by Hours of Care at Baseline
| Measure | UC M (SE) | GC M (SE) | αβ | 95% CI | ES | 95% CI | ||
| Baseline | 18 months | Baseline | 18 months | |||||
| Caregivers who provided 14+ hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 89) | 8.6 (0.96) | 9.8 (0.9) | 7.3 (0.82) | 7.9 (0.88) | 0.10 | −2.1 to 2.3 | 0.02 | −0.41 to 0.44 |
| Caregiver depression | ||||||||
| CES-D score (n = 90) | 7.0 (1.1) | 6.4 (1.1) | 7.0 (0.92) | 8.1 (0.99) | 2.07 | −0.71 to 4.84 | 0.31 | −0.11 to 0.74 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 79) | 2.5 (0.15) | 2.6 (0.18) | 2.4 (0.16) | 2.9 (0.19) | 0.52 | 0.04 to 1.00 | 0.48 | 0.03 to 0.94 |
| Coordination of care (n = 81) | 2.5 (0.15) | 2.6 (0.17) | 2.5 (0.15) | 2.9 (0.17) | 0.35 | −0.12 to 0.83 | 0.33 | −0.12 to 0.78 |
| Decision support (n = 78) | 3.0 (0.15) | 3.6 (0.18) | 3.1 (0.14) | 3.8 (0.14) | 0.37 | −0.08 to 0.83 | 0.37 | −0.09 to 0.83 |
| Problem solving (n = 79) | 2.7 (0.16) | 3.2 (0.18) | 2.6 (1.50) | 3.2 (0.20) | 0.32 | −0.17 to 0.82 | 0.29 | −0.16 to 0.75 |
| Patient activation (n = 77) | 2.7 (0.17) | 2.8 (0.21) | 2.6 (0.17) | 3.3 (0.17) | 0.66 | 0.20 to 1.12 | 0.65 | 0.18 to 1.12 |
| Aggregate quality (n = 81) | 2.6 (0.13) | 2.9 (0.15) | 2.6 (0.13) | 3.2 (0.15) | 0.37 | −0.02 to 0.76 | 0.42 | −0.03 to 0.87 |
| Regular activity productivity loss | ||||||||
| WPAI:CG (n = 90) | 0.35 (0.05) | 0.31 (0.05) | 0.30 (0.04) | 0.27 (0.04) | −0.03 | −0.17 to 0.10 | −0.10 | −0.52 to 0.32 |
| Caregivers who provided <14 hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 105) | 5.1 (0.68) | 5.9 (0.75) | 5.6 (0.69) | 5.5 (0.65) | −0.38 | −1.98 to 1.22 | −0.09 | −0.48 to 0.30 |
| Caregiver depression | ||||||||
| CES-D score (n = 104) | 7.2 (1.12) | 5.3 (0.89) | 5.8 (0.74) | 5.5 (0.91) | 0.50 | −2.05 to 3.06 | 0.08 | −0.32 to 0.47 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 82) | 2.7 (0.15) | 2.7 (0.19) | 2.7 (0.17) | 3.3 (0.19) | 0.53 | 0.04 to 1.03 | 0.47 | 0.03 to 0.92 |
| Coordination of care (n = 84) | 2.5 (0.15) | 2.6 (0.18) | 2.6 (0.15) | 3.2 (0.18) | 0.53 | 0.02 to 1.04 | 0.45 | 0.01 to 0.89 |
| Decision support (n = 81) | 3.6 (0.12) | 3.5 (0.16) | 3.4 (0.14) | 3.9 (0.14) | 0.42 | 0.00 to 0.84 | 0.44 | −0.01 to 0.89 |
| Problem solving (n = 80) | 3.2 (0.17) | 3.3 (0.18) | 3.1 (0.19) | 3.4 (0.17) | 0.06 | −0.41 to 0.54 | 0.06 | −0.39 to 0.51 |
| Patient activation (n = 76) | 3.0 (0.22) | 3.0 (0.21) | 3.1 (0.2) | 3.4 (0.19) | 0.37 | −0.17 to 0.91 | 0.32 | −0.15 to 0.78 |
| Aggregate quality (n = 83) | 2.9 (0.84) | 2.9 (0.99) | 2.9 (0.95) | 3.4 (0.95) | 0.45 | 0.07 to 0.83 | 0.51 | 0.07 to 0.96 |
| Regular activity productivity loss | ||||||||
| WPAI: CG (n = 105) | 0.15 (0.03) | 0.18 (0.04) | 0.15 (0.03) | 0.20 (0.04) | 0.04 | −0.06 to 0.14 | 0.16 | −0.23 to 0.56 |
| Measure | UC M (SE) | GC M (SE) | αβ | 95% CI | ES | 95% CI | ||
| Baseline | 18 months | Baseline | 18 months | |||||
| Caregivers who provided 14+ hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 89) | 8.6 (0.96) | 9.8 (0.9) | 7.3 (0.82) | 7.9 (0.88) | 0.10 | −2.1 to 2.3 | 0.02 | −0.41 to 0.44 |
| Caregiver depression | ||||||||
| CES-D score (n = 90) | 7.0 (1.1) | 6.4 (1.1) | 7.0 (0.92) | 8.1 (0.99) | 2.07 | −0.71 to 4.84 | 0.31 | −0.11 to 0.74 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 79) | 2.5 (0.15) | 2.6 (0.18) | 2.4 (0.16) | 2.9 (0.19) | 0.52 | 0.04 to 1.00 | 0.48 | 0.03 to 0.94 |
| Coordination of care (n = 81) | 2.5 (0.15) | 2.6 (0.17) | 2.5 (0.15) | 2.9 (0.17) | 0.35 | −0.12 to 0.83 | 0.33 | −0.12 to 0.78 |
| Decision support (n = 78) | 3.0 (0.15) | 3.6 (0.18) | 3.1 (0.14) | 3.8 (0.14) | 0.37 | −0.08 to 0.83 | 0.37 | −0.09 to 0.83 |
| Problem solving (n = 79) | 2.7 (0.16) | 3.2 (0.18) | 2.6 (1.50) | 3.2 (0.20) | 0.32 | −0.17 to 0.82 | 0.29 | −0.16 to 0.75 |
| Patient activation (n = 77) | 2.7 (0.17) | 2.8 (0.21) | 2.6 (0.17) | 3.3 (0.17) | 0.66 | 0.20 to 1.12 | 0.65 | 0.18 to 1.12 |
| Aggregate quality (n = 81) | 2.6 (0.13) | 2.9 (0.15) | 2.6 (0.13) | 3.2 (0.15) | 0.37 | −0.02 to 0.76 | 0.42 | −0.03 to 0.87 |
| Regular activity productivity loss | ||||||||
| WPAI:CG (n = 90) | 0.35 (0.05) | 0.31 (0.05) | 0.30 (0.04) | 0.27 (0.04) | −0.03 | −0.17 to 0.10 | −0.10 | −0.52 to 0.32 |
| Caregivers who provided <14 hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 105) | 5.1 (0.68) | 5.9 (0.75) | 5.6 (0.69) | 5.5 (0.65) | −0.38 | −1.98 to 1.22 | −0.09 | −0.48 to 0.30 |
| Caregiver depression | ||||||||
| CES-D score (n = 104) | 7.2 (1.12) | 5.3 (0.89) | 5.8 (0.74) | 5.5 (0.91) | 0.50 | −2.05 to 3.06 | 0.08 | −0.32 to 0.47 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 82) | 2.7 (0.15) | 2.7 (0.19) | 2.7 (0.17) | 3.3 (0.19) | 0.53 | 0.04 to 1.03 | 0.47 | 0.03 to 0.92 |
| Coordination of care (n = 84) | 2.5 (0.15) | 2.6 (0.18) | 2.6 (0.15) | 3.2 (0.18) | 0.53 | 0.02 to 1.04 | 0.45 | 0.01 to 0.89 |
| Decision support (n = 81) | 3.6 (0.12) | 3.5 (0.16) | 3.4 (0.14) | 3.9 (0.14) | 0.42 | 0.00 to 0.84 | 0.44 | −0.01 to 0.89 |
| Problem solving (n = 80) | 3.2 (0.17) | 3.3 (0.18) | 3.1 (0.19) | 3.4 (0.17) | 0.06 | −0.41 to 0.54 | 0.06 | −0.39 to 0.51 |
| Patient activation (n = 76) | 3.0 (0.22) | 3.0 (0.21) | 3.1 (0.2) | 3.4 (0.19) | 0.37 | −0.17 to 0.91 | 0.32 | −0.15 to 0.78 |
| Aggregate quality (n = 83) | 2.9 (0.84) | 2.9 (0.99) | 2.9 (0.95) | 3.4 (0.95) | 0.45 | 0.07 to 0.83 | 0.51 | 0.07 to 0.96 |
| Regular activity productivity loss | ||||||||
| WPAI: CG (n = 105) | 0.15 (0.03) | 0.18 (0.04) | 0.15 (0.03) | 0.20 (0.04) | 0.04 | −0.06 to 0.14 | 0.16 | −0.23 to 0.56 |
Notes: 95% CI = 95% confidence interval; CES-D = Center for Epidemiological Studies-Depression scale (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26); ES = effect size; GC = Guided Care; PACIC = Patient Assessment of Chronic Illness Care; WPAI:CG = Work Productivity and Activity Impairment, as adapted for Caregiving; UC = usual care; αβ = the regression coefficient for group assignment (GC = 1; UC = 0) in models of 18-month scores, adjusting for baseline score, patient gender, patient education, and study site.
Effects of GC on Family Caregivers’ Strain, Depression, Productivity, and Perceptions of Recipients’ Quality of Chronic Care, Stratified by Hours of Care at Baseline
| Measure | UC M (SE) | GC M (SE) | αβ | 95% CI | ES | 95% CI | ||
| Baseline | 18 months | Baseline | 18 months | |||||
| Caregivers who provided 14+ hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 89) | 8.6 (0.96) | 9.8 (0.9) | 7.3 (0.82) | 7.9 (0.88) | 0.10 | −2.1 to 2.3 | 0.02 | −0.41 to 0.44 |
| Caregiver depression | ||||||||
| CES-D score (n = 90) | 7.0 (1.1) | 6.4 (1.1) | 7.0 (0.92) | 8.1 (0.99) | 2.07 | −0.71 to 4.84 | 0.31 | −0.11 to 0.74 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 79) | 2.5 (0.15) | 2.6 (0.18) | 2.4 (0.16) | 2.9 (0.19) | 0.52 | 0.04 to 1.00 | 0.48 | 0.03 to 0.94 |
| Coordination of care (n = 81) | 2.5 (0.15) | 2.6 (0.17) | 2.5 (0.15) | 2.9 (0.17) | 0.35 | −0.12 to 0.83 | 0.33 | −0.12 to 0.78 |
| Decision support (n = 78) | 3.0 (0.15) | 3.6 (0.18) | 3.1 (0.14) | 3.8 (0.14) | 0.37 | −0.08 to 0.83 | 0.37 | −0.09 to 0.83 |
| Problem solving (n = 79) | 2.7 (0.16) | 3.2 (0.18) | 2.6 (1.50) | 3.2 (0.20) | 0.32 | −0.17 to 0.82 | 0.29 | −0.16 to 0.75 |
| Patient activation (n = 77) | 2.7 (0.17) | 2.8 (0.21) | 2.6 (0.17) | 3.3 (0.17) | 0.66 | 0.20 to 1.12 | 0.65 | 0.18 to 1.12 |
| Aggregate quality (n = 81) | 2.6 (0.13) | 2.9 (0.15) | 2.6 (0.13) | 3.2 (0.15) | 0.37 | −0.02 to 0.76 | 0.42 | −0.03 to 0.87 |
| Regular activity productivity loss | ||||||||
| WPAI:CG (n = 90) | 0.35 (0.05) | 0.31 (0.05) | 0.30 (0.04) | 0.27 (0.04) | −0.03 | −0.17 to 0.10 | −0.10 | −0.52 to 0.32 |
| Caregivers who provided <14 hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 105) | 5.1 (0.68) | 5.9 (0.75) | 5.6 (0.69) | 5.5 (0.65) | −0.38 | −1.98 to 1.22 | −0.09 | −0.48 to 0.30 |
| Caregiver depression | ||||||||
| CES-D score (n = 104) | 7.2 (1.12) | 5.3 (0.89) | 5.8 (0.74) | 5.5 (0.91) | 0.50 | −2.05 to 3.06 | 0.08 | −0.32 to 0.47 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 82) | 2.7 (0.15) | 2.7 (0.19) | 2.7 (0.17) | 3.3 (0.19) | 0.53 | 0.04 to 1.03 | 0.47 | 0.03 to 0.92 |
| Coordination of care (n = 84) | 2.5 (0.15) | 2.6 (0.18) | 2.6 (0.15) | 3.2 (0.18) | 0.53 | 0.02 to 1.04 | 0.45 | 0.01 to 0.89 |
| Decision support (n = 81) | 3.6 (0.12) | 3.5 (0.16) | 3.4 (0.14) | 3.9 (0.14) | 0.42 | 0.00 to 0.84 | 0.44 | −0.01 to 0.89 |
| Problem solving (n = 80) | 3.2 (0.17) | 3.3 (0.18) | 3.1 (0.19) | 3.4 (0.17) | 0.06 | −0.41 to 0.54 | 0.06 | −0.39 to 0.51 |
| Patient activation (n = 76) | 3.0 (0.22) | 3.0 (0.21) | 3.1 (0.2) | 3.4 (0.19) | 0.37 | −0.17 to 0.91 | 0.32 | −0.15 to 0.78 |
| Aggregate quality (n = 83) | 2.9 (0.84) | 2.9 (0.99) | 2.9 (0.95) | 3.4 (0.95) | 0.45 | 0.07 to 0.83 | 0.51 | 0.07 to 0.96 |
| Regular activity productivity loss | ||||||||
| WPAI: CG (n = 105) | 0.15 (0.03) | 0.18 (0.04) | 0.15 (0.03) | 0.20 (0.04) | 0.04 | −0.06 to 0.14 | 0.16 | −0.23 to 0.56 |
| Measure | UC M (SE) | GC M (SE) | αβ | 95% CI | ES | 95% CI | ||
| Baseline | 18 months | Baseline | 18 months | |||||
| Caregivers who provided 14+ hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 89) | 8.6 (0.96) | 9.8 (0.9) | 7.3 (0.82) | 7.9 (0.88) | 0.10 | −2.1 to 2.3 | 0.02 | −0.41 to 0.44 |
| Caregiver depression | ||||||||
| CES-D score (n = 90) | 7.0 (1.1) | 6.4 (1.1) | 7.0 (0.92) | 8.1 (0.99) | 2.07 | −0.71 to 4.84 | 0.31 | −0.11 to 0.74 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 79) | 2.5 (0.15) | 2.6 (0.18) | 2.4 (0.16) | 2.9 (0.19) | 0.52 | 0.04 to 1.00 | 0.48 | 0.03 to 0.94 |
| Coordination of care (n = 81) | 2.5 (0.15) | 2.6 (0.17) | 2.5 (0.15) | 2.9 (0.17) | 0.35 | −0.12 to 0.83 | 0.33 | −0.12 to 0.78 |
| Decision support (n = 78) | 3.0 (0.15) | 3.6 (0.18) | 3.1 (0.14) | 3.8 (0.14) | 0.37 | −0.08 to 0.83 | 0.37 | −0.09 to 0.83 |
| Problem solving (n = 79) | 2.7 (0.16) | 3.2 (0.18) | 2.6 (1.50) | 3.2 (0.20) | 0.32 | −0.17 to 0.82 | 0.29 | −0.16 to 0.75 |
| Patient activation (n = 77) | 2.7 (0.17) | 2.8 (0.21) | 2.6 (0.17) | 3.3 (0.17) | 0.66 | 0.20 to 1.12 | 0.65 | 0.18 to 1.12 |
| Aggregate quality (n = 81) | 2.6 (0.13) | 2.9 (0.15) | 2.6 (0.13) | 3.2 (0.15) | 0.37 | −0.02 to 0.76 | 0.42 | −0.03 to 0.87 |
| Regular activity productivity loss | ||||||||
| WPAI:CG (n = 90) | 0.35 (0.05) | 0.31 (0.05) | 0.30 (0.04) | 0.27 (0.04) | −0.03 | −0.17 to 0.10 | −0.10 | −0.52 to 0.32 |
| Caregivers who provided <14 hr of weekly assistance at baseline | ||||||||
| Caregiver strain | ||||||||
| CSI score (n = 105) | 5.1 (0.68) | 5.9 (0.75) | 5.6 (0.69) | 5.5 (0.65) | −0.38 | −1.98 to 1.22 | −0.09 | −0.48 to 0.30 |
| Caregiver depression | ||||||||
| CES-D score (n = 104) | 7.2 (1.12) | 5.3 (0.89) | 5.8 (0.74) | 5.5 (0.91) | 0.50 | −2.05 to 3.06 | 0.08 | −0.32 to 0.47 |
| Quality of chronic illness care (PACIC) | ||||||||
| Goal setting (n = 82) | 2.7 (0.15) | 2.7 (0.19) | 2.7 (0.17) | 3.3 (0.19) | 0.53 | 0.04 to 1.03 | 0.47 | 0.03 to 0.92 |
| Coordination of care (n = 84) | 2.5 (0.15) | 2.6 (0.18) | 2.6 (0.15) | 3.2 (0.18) | 0.53 | 0.02 to 1.04 | 0.45 | 0.01 to 0.89 |
| Decision support (n = 81) | 3.6 (0.12) | 3.5 (0.16) | 3.4 (0.14) | 3.9 (0.14) | 0.42 | 0.00 to 0.84 | 0.44 | −0.01 to 0.89 |
| Problem solving (n = 80) | 3.2 (0.17) | 3.3 (0.18) | 3.1 (0.19) | 3.4 (0.17) | 0.06 | −0.41 to 0.54 | 0.06 | −0.39 to 0.51 |
| Patient activation (n = 76) | 3.0 (0.22) | 3.0 (0.21) | 3.1 (0.2) | 3.4 (0.19) | 0.37 | −0.17 to 0.91 | 0.32 | −0.15 to 0.78 |
| Aggregate quality (n = 83) | 2.9 (0.84) | 2.9 (0.99) | 2.9 (0.95) | 3.4 (0.95) | 0.45 | 0.07 to 0.83 | 0.51 | 0.07 to 0.96 |
| Regular activity productivity loss | ||||||||
| WPAI: CG (n = 105) | 0.15 (0.03) | 0.18 (0.04) | 0.15 (0.03) | 0.20 (0.04) | 0.04 | −0.06 to 0.14 | 0.16 | −0.23 to 0.56 |
Notes: 95% CI = 95% confidence interval; CES-D = Center for Epidemiological Studies-Depression scale (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26); ES = effect size; GC = Guided Care; PACIC = Patient Assessment of Chronic Illness Care; WPAI:CG = Work Productivity and Activity Impairment, as adapted for Caregiving; UC = usual care; αβ = the regression coefficient for group assignment (GC = 1; UC = 0) in models of 18-month scores, adjusting for baseline score, patient gender, patient education, and study site.
With the exception of patients’ chronic illness care quality, no statistically significant differences were observed between GC and UC caregivers at 18-month follow-up for either subgroup. Among the subgroup of caregivers providing fewer than 14 hr of weekly assistance at baseline, GC caregivers’ aggregate PACIC scores (αβ = 0.45; 95% CI: 0.07–0.83), and three of the five PACIC subscale scores (goal setting, coordination of care, and decision support) indicated statistically significantly higher quality of chronic illness care provided to care recipients at 18 months, after accounting for baseline scores, study site, and patient gender and education. Among caregivers providing more than 14 hr of weekly assistance at baseline, the goal setting and patient activation subscales of the PACIC were the only statistically significant between-group difference at 18 months; differences that favored the GC group (αβ = 0.52; 95% CI: 0.04, 1.00 and αβ = 0.66; 95% CI: 0.20, 1.12, respectively).
Discussion
The GC model was designed to benefit high-risk older adults and their family caregivers by providing comprehensive care that combines several successful chronic disease innovations. Implementation experiences and results from the cluster-randomized controlled study substantiate the feasibility of employing a nurse in primary care practice to work simultaneously with both patients and their caregivers. Early data from this cluster-randomized controlled trial indicated that benefit was experienced by the GC caregivers who remained enrolled in GC for 6 months (Wolff et al., 2009). Results presented here indicate that benefits related to reductions in depressive symptoms and strain did not persist at 18 months. Likewise, GC does not appear to have led to a statistically significant reduction in caregiving-related regular activity loss or work productivity loss. However, significant improvements were observed in GC caregiver reports of the quality of patients’ chronic illness care at 18 months, a result that is consistent with other findings regarding the effect of GC on patient and physician (Boult et al., 2008) perspectives. Collectively, results from this study suggest that the GC intervention was more successful in addressing caregivers’ appraisal of recipients’ health care, rather than the objective or subjective stresses associated with care provision.
Compared with UC, GC caregiver participants reported higher quality of chronic illness care for patient participants at 18-month follow-up. Perceptions of better chronic illness care quality were detected in the aggregate as well as for four of the five PACIC subscales, reflecting dimensions of goal setting, coordination of care, decision support, and patient activation. Family caregivers’ ratings of patients’ health care quality are important. A recent national survey found nearly 40% of older adults to be typically accompanied to routine medical encounters by family and friends and that accompaniment is more common in light of advanced age, poor health, and disability (Wolff & Roter, 2008), all of which characterize the patient participants in this study. Although not specifically investigated here, evidence that family members and friends who accompany patients to routine health care encounters actively participate in visit communication (Clayman et al., 2005; Ishikawa et al., 2005; Silliman, Bhatti, Khan, Dukes, & Sullivan, 1996) and are knowledgeable about patients’ disease and treatment (Silliman et al.) lends credence to the measurement of families’ perspectives of the quality of patients’ chronic illness care.
Regression analyses do not support the hypothesis that GC reduced depressive symptoms and strain related to care provision at 18-month follow-up. It is also noteworthy that GC caregivers did not experience increases in strain or burden relative to UC caregivers, a distinct possibility given the medical complexity and challenging care needs of study participants. This study relied on a population-based sampling strategy and defined caregivers broadly. As a consequence, enrolled caregivers did not have a high degree of depressive symptoms or strain at the outset of the study. The level of depressive symptoms reported by caregivers in this study was lower than other studies of caregivers (Belle et al., 2006; Kim, Duberstein, Sorensen, & Larson, 2005) and comparable to that reported for the general community population (Blazer, Landerman, Hays, Simonsick, & Saunders, 1998; Weissman et al., 1977). That few caregivers reported depressive symptoms or strain associated with providing assistance at baseline is likely to have had a bearing on the ability of GC to improve these outcomes.
Another factor that may have attenuated the efficacy of GC for caregivers in this study is incomplete implementation of two components of the GCPFF. For several reasons, the six-session caregiver workshops and the ongoing caregiver support groups led by the GC nurses were poorly attended by caregivers. Anecdotally, the nurses reported feeling uncomfortable in leading these activities and the caregivers reported difficulty attending because of the sessions’ timing, location, and duration. The other three components of the GCPFF (caregiver assessment, referral to community resources, and ongoing coaching of caregivers) were implemented with much greater fidelity to the GC model. Unfortunately, we did not collect research data that would allow quantitative description of the rates of implementation of GCPFF components.
Several limitations of this study merit comment. The multifaceted nature of GC and the GCPFF, as well as the heterogeneity in patient and caregiver attributes, result in several conceptual and methodological challenges to interpreting results. It is notable that we are unable to disentangle which aspects of the GC intervention did or did not work well or were most salient to patients and their family caregivers, some of whom were challenged by serious health issues of their own. Likewise, given the study context, and nature of the intervention, it is not possible for us to ascertain the extent to which nurses implemented the model of care consistently over time and across sites or in a manner that retained fidelity to the GCPFF. Conceptually, it is unclear which outcomes are most salient to caregiver participants in this study. Depression and strain are among the most widely studied outcomes of caregiver interventions. However, these measures may not be the outcomes most relevant to caregiver participants in GC, given its primary care-based focus and the relatively low levels of depression and strain reported by caregiver participants at baseline. Although several caregiver outcomes were examined, the lack of measures that encompass favorable aspects of the caregiving experience is a limitation of this study.
This study was powered on its ability to detect changes in patient, rather than caregiver, outcomes. The relatively small sample of caregivers who remained eligible and completed the 18-month interview impedes our ability to understand nuanced effects of the intervention for subgroups of caregivers. For example, we observed a trend toward less work productivity loss among GC caregivers at 18 months, but the small sample of caregivers working for pay limits our understanding of the extent to which this observation was related to the intervention or to factors beyond this study. Nevertheless, given the specific functions assumed by GCNs and the fact that both patients and their caregivers have direct access to the GCN during business hours, it is plausible that GC could improve caregivers’ work productivity.
In conclusion, results of this 18-month study support the hypothesis that employing a GC-trained nurse in primary care to work with both patients and their caregivers improves caregivers’ perceptions of the quality of care recipients’ chronic illness care but do not indicate that objective or subjective stresses associated with care provision were modified as reflected by strain, depressive symptoms, and productivity. This study provides several lessons for future caregiving intervention studies based in primary care. First, findings substantiate the importance of facilitators being fully prepared to deliver all intervention components. Second, the implementation of intervention components should be individually monitored to allow examination of model fidelity. Third, interventions should be made more broadly accessible through a variety of venues, including telephone or web-based mechanisms. Fourth, caregiver samples should be sufficiently large and diverse so as to permit meaningful subgroup analyses. Last, future research should identify end points for caregivers that are meaningful across a broader spectrum of circumstances surrounding care provision and that acknowledge caregivers’ perceptions, experiences, and roles as partners in their care recipient’s health care processes.
Funding
This work was supported by the Jacob and Valeria Langeloth Foundation (000610 to J.L.W.); The John A. Hartford Foundation (2004-0335 to C.B.); the Agency for Healthcare Research and Quality and the National Institute on Aging (R01-HS01-4580-02 to C.B.); Kaiser-Permanente of the Mid-Atlantic States; Johns Hopkins HealthCare; and the Roger C. Lipitz Center for Integrated Health Care.
The authors acknowledge the invaluable contributions to this study made by Johns Hopkins Community Physicians, Kaiser-Permanente Mid-Atlantic States, MedStar, Battelle Centers for Public Health Research, the Centers for Medicare & Medicaid Services, Accumen, ResDAC, the University of Minnesota Survey Research Center, study consultants (Jean Giddens, RN, PhD; Kate Lorig, RN, DrPH), the nurse managers (Lora Rosenthal, RN and Carol Groves, RN, MPA), and all the participating patients, caregivers, physicians, and Guided Care Nurses. Aspects of this article were presented at the 2008 annual meeting of the Gerontological Society of America.
References
Author notes
Decision Editor: William J. McAuley, PhD