-
PDF
- Split View
-
Views
-
Cite
Cite
Ngai-Yin Chan, Chi-Chung Choy, Chun-Leung Lau, Ying-Keung Lo, Pui-Shan Chu, Ho-Chuen Yuen, Ngai-Shing Mok, Ping-Tim Tsui, Suet-Ting Lau, Persistent iatrogenic atrial septal defect after pulmonary vein isolation by cryoballoon: an under-recognized complication, EP Europace, Volume 13, Issue 10, October 2011, Pages 1406–1410, https://doi.org/10.1093/europace/eur138
Close - Share Icon Share
Abstract
Iatrogenic atrial septal defect (IASD) has been reported as a complication of transseptal puncture. This study aims to investigate the incidence, echocardiographic characteristics, and clinical outcome of persistent IASD after pulmonary vein isolation (PVI) by cryoballoon catheter delivered by a large transseptal sheath.
Thirteen patients (9 males, mean age 54.9 ± 13.0) with paroxysmal (10) or persistent (3) atrial fibrillation underwent PVI with cryoballoon catheter. Single transseptal puncture was performed with a BRK-1 shaped Brockenbrough needle and an 8 F sheath which was exchanged for a steerable transseptal sheath (15 F outer diameter and 12 F inner diameter) with the support of a stiff guidewire. Pulmonary vein isolation was performed with a 28 mm cryoballoon catheter. The incidence of persistent IASD was evaluated by transoesophageal echocardiography performed at 6 and 9 months after the procedure. At 6 months, five (38%) patients had persistent IASD with left-to-right shunt. The mean size of the IASD was 5.5 ± 2.4 mm. At 9 months, one patient had closure of the IASD and four (31%) patients had persistent IASD with mean size of 4.6 ± 1.4 mm. No patient died or suffered clinically from paradoxical embolism.
Persistent IASD is a common complication after PVI by cryoballoon catheter. Only left-to-right, but not right-to-left, interatrial shunting occurred as a result of the IASD. There was no clinical occurrence of paradoxical embolism. Patients should be screened for this complication after cryoballoon procedures and regular reassessment with echocardiographic or other techniques should be performed for monitoring.
Introduction
Iatrogenic atrial septal defect (IASD) has been reported as a complication of transseptal puncture (TsP) performed in different types of cardiological procedures.1–6 Catheter ablation for paroxysmal or persistent atrial fibrillation (AF), requiring left atrial (LA) access by TsP, is now a treatment option for symptomatic patients especially when they are refractory to anti-arrhythmic medications.7 Iatrogenic atrial septal defect has been shown to persist beyond 9 months in 30% of patients undergoing pulmonary vein isolation (PVI) procedures with placement of two catheters across a single TsP site.1 In contrast, in the same study, no persistent IASD was observed in patients with double TsP.
Despite the observation of persistent IASD as a complication of PVI procedures, it is rarely reported systematically in AF ablation studies. According to a worldwide survey,8 a major complication rate of 4.5%, including 0.04% of atrio-oesophageal fistula, 0.94% of stroke or transient ischaemic attack, and 0.29% of severe pulmonary vein (PV) stenosis, but not IASD, has been reported for AF ablation procedures.
A novel cryoballoon catheter is recently available for PVI. It has been shown to be safe and effective in early European and American experience.9,10 For delivery and manipulation of the cryoballoon catheter in the LA, a large size steerable transseptal sheath with an outer diameter of 15 F has to be used.9 The incidence, echocardiographic characteristics, and clinical outcome of persistent IASD after PVI with cryoballoon catheter have not been investigated. The purpose of this study therefore is to investigate prospectively and serially the complication of IASD after the use of a large size transseptal sheath in patients with AF and PVI achieved by cryoballoon catheter ablation.
Methods
Study population and pre-ablation management
The study protocol has been approved by the Ethics Committee of the Investigation Centre. All patients gave written informed consent before recruitment into the study. Thirteen patients with symptomatic paroxysmal or persistent AF refractory to at least one antiarrhythmic drug and underwent PVI with cryoballoon catheter were included in this study. Exclusion criteria include inability to undergo transoesophageal echocardiography (TEE), pre-existing atrial septal defect (ASD) and other congenital heart disease, previous LA ablation for AF or other transseptal procedures, LA thrombus, pregnancy, or other severe comorbidity. The patients were on therapeutic anticoagulation with international normalized ratio (INR) maintained between 2.0 and 3.0 with warfarin. Warfarin was stopped 3 days before ablation procedure and baseline TEE was performed the day before the procedure to rule out pre-existing ASD and LA thrombus. All antiarrhythmic drugs including amiodarone were discontinued at least 5 days before the procedure.
Pulmonary vein isolation by cryoballoon catheter ablation
The ablation procedures were performed under local anaesthesia and conscious sedation with midazolam and fentanyl which were given whenever necessary. Infusion of heparin was given to maintain an activated clotting time (ACT) of >300 s. Measurement of the ACT was performed every 30 min.
The 6 F diagnostic catheters were positioned in the coronary sinus (decapolar), the His bundle region (quadripolar), and the right atrium (quadripolar). The diagnostic catheters served as landmarks for TsP and allowed pacing during mapping of PV ostia. A single TsP was performed by the Brockenbrough needle and an 8 F sheath (Mullin transseptal guiding introducer, St Jude Medical, Minnetonka, MN, USA) was introduced into the LA. The 8 F sheath was then exchanged for the steerable transseptal sheath, with an outer diameter of 15 F and an inner diameter of 12 F (FlexCath, Medtronic Cryocath, Minneapolis, MN, USA) with the Amplatz extra-stiff guidewire (Cook Incorporated, Bloomington, IN, USA). All the PVs were catheterized by the steerable transseptal sheath and PV angiography was performed. Baseline mapping of each PV ostia was performed by using a multi-polar circular catheter (Inquiry Optima, St Jude Medical, Irvine, CA, USA).
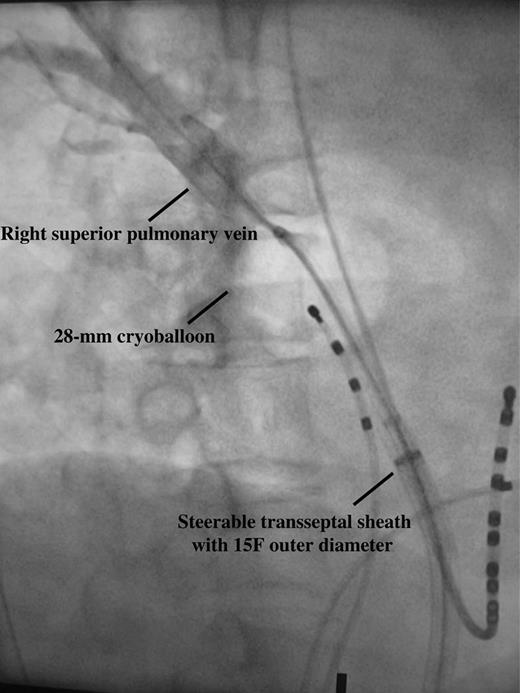
The 10.5 F 28 mm cryoballoon catheter (Arctic Front, Medtronic Cryocath) was used for PVI in all patients. With the deflated cryoballoon catheter inside the steerable transseptal sheath, the Amplatz extra-stiff guidewire was positioned in a branch of a left-sided PV. The cryoballoon catheter was then advanced towards the LA and inflated. The inflated cryoballoon catheter was pushed against the PV ostium and an excellent occlusion with full retention of contrast medium distal to the catheter tip during injection of 50% diluted contrast medium through the central lumen was aimed (Figure 1). The duration of each freezing cycle was 300 s and a minimum of two consecutive freezing cycles for each targeted PV were delivered. For patients who remained in AF after PVI of all PVs, external biphasic electrical cardioversion was performed for conversion to sinus rhythm. Mapping with the multi-polar circular catheter (Inquiry Optima) after ablation of all the PVs was performed. Successful PVI was defined as loss of all signals or evidence of entrance block at the level of PV ostia. An 8 mm tip cryoablation catheter (Freezor Max, Medtronic Cryocath) would be used to achieve PVI if repeated cryoballoon ablation failed to do so.
Excellent occlusion of right superior pulmonary vein with 28 mm cryoballoon with full retention of contrast medium distally; taken at right anterior oblique 40° projection.
Echocardiographic examination
All patients underwent transthoracic echocardiography (TTE) before PVI. A commercially available equipment (Vivid 7, GE Medical Systems, Milwaukee, WI, USA) with a 1.7/3.4 MHz harmonic transducer was used. In particular, LA size, left ventricular ejection fraction, right ventricular dilatation, or dysfunction and elevated pulmonary artery pressure of >30 mmHg was recorded.
All patients underwent TEE 1 day before, 6 and 9 months after the procedure. A commercially available echocardiographic equipment (Vivid 7, GE Medical Systems) with a multiplane 7 MHz transducer was used. Atrial anatomy was examined with TEE from the mid to upper oesophagus at angles ranging between 0 and 120°. Both 2D images and colour Doppler flow mapping of the interatrial septum were obtained from multiple views. Atrial septal defect was defined as interatrial shunt confirmed by Doppler flow mapping beside the fossa ovalis but not fulfilling the echocardiographic criteria for patent foramen ovale (PFO).11 In case of confirmed IASD during TEE, the size of the ASD was measured and the presence of right-to-left shunt (RLS) was examined by agitated saline contrast echocardiography before and after Valsalva manoeuvre within three cardiac cycles from right atrial opacification.
Post-ablation management and follow-up
The patients underwent continuous ECG monitoring in the coronary care unit for 24 h after the procedure. For uncomplicated procedures, patients were discharged the next day after the procedure. Oral anticoagulation with warfarin was started 1 day after PVI, targeting an INR of 2.0–3.0 for 3 months. Subsequent need for anticoagulation depended on the CHADS2 score of the patients.12 Previously used antiarrhythmic drugs were given for 3 months after the procedure. Patients were scheduled for clinical follow-up 1 month and then quarterly after the procedure. Clinical examination including full neurological assessment was performed during each follow-up. History on symptom recurrence of palpitations was specifically taken during each follow-up. A 12-lead ECG and a 24 h Holter monitoring were performed for each follow-up to look for asymptomatic recurrence of AF.
Statistical analysis
Mean and standard deviation were calculated for parametric data. Fisher exact test was used for comparison of categorical variables. The threshold of significance was set at 0.05. Statistics were performed with the Statistical Package for Social Science (IBM SPSS version 19, Chicago, IL, USA).
Results
Patients
Thirteen patients (9 males, mean age 54.9 ± 13.0) with paroxysmal (10) or persistent (3) AF underwent PVI with cryoballoon. Other clinical characteristics are given in Table 1.
Baseline characteristics
| Number of patients | 13 |
| Men/women | 9/4 |
| Age (years) | 54.9 ± 13.0 |
| Paroxysmal AF, n (%) | 10 (78) |
| Persistent AF, n (%) | 3 (22) |
| LVEF (%) | 66.6 ± 11.8 |
| LA size (cm) | 3.6 ± 0.6 |
| Lone AF, n (%) | 6 (46) |
| Co-morbidities | |
| Hypertension, n (%) | 6 (46) |
| Hyperlipidaemia, n (%) | 2 (15) |
| Ischaemic heart disease, n (%) | 1 (8) |
| Sick sinus syndrome, n (%) | 1 (8) |
| CHADS2 score | |
| 0, n (%) | 7 (54) |
| 1, n (%) | 5 (38) |
| 2, n (%) | 1 (8) |
| Number of patients | 13 |
| Men/women | 9/4 |
| Age (years) | 54.9 ± 13.0 |
| Paroxysmal AF, n (%) | 10 (78) |
| Persistent AF, n (%) | 3 (22) |
| LVEF (%) | 66.6 ± 11.8 |
| LA size (cm) | 3.6 ± 0.6 |
| Lone AF, n (%) | 6 (46) |
| Co-morbidities | |
| Hypertension, n (%) | 6 (46) |
| Hyperlipidaemia, n (%) | 2 (15) |
| Ischaemic heart disease, n (%) | 1 (8) |
| Sick sinus syndrome, n (%) | 1 (8) |
| CHADS2 score | |
| 0, n (%) | 7 (54) |
| 1, n (%) | 5 (38) |
| 2, n (%) | 1 (8) |
AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LA, left atrium; CHADS2, 1 mark for congestive heart failure, hypertension, age or diabetes mellitus, 2 marks for stroke or transient ischaemic event.
Baseline characteristics
| Number of patients | 13 |
| Men/women | 9/4 |
| Age (years) | 54.9 ± 13.0 |
| Paroxysmal AF, n (%) | 10 (78) |
| Persistent AF, n (%) | 3 (22) |
| LVEF (%) | 66.6 ± 11.8 |
| LA size (cm) | 3.6 ± 0.6 |
| Lone AF, n (%) | 6 (46) |
| Co-morbidities | |
| Hypertension, n (%) | 6 (46) |
| Hyperlipidaemia, n (%) | 2 (15) |
| Ischaemic heart disease, n (%) | 1 (8) |
| Sick sinus syndrome, n (%) | 1 (8) |
| CHADS2 score | |
| 0, n (%) | 7 (54) |
| 1, n (%) | 5 (38) |
| 2, n (%) | 1 (8) |
| Number of patients | 13 |
| Men/women | 9/4 |
| Age (years) | 54.9 ± 13.0 |
| Paroxysmal AF, n (%) | 10 (78) |
| Persistent AF, n (%) | 3 (22) |
| LVEF (%) | 66.6 ± 11.8 |
| LA size (cm) | 3.6 ± 0.6 |
| Lone AF, n (%) | 6 (46) |
| Co-morbidities | |
| Hypertension, n (%) | 6 (46) |
| Hyperlipidaemia, n (%) | 2 (15) |
| Ischaemic heart disease, n (%) | 1 (8) |
| Sick sinus syndrome, n (%) | 1 (8) |
| CHADS2 score | |
| 0, n (%) | 7 (54) |
| 1, n (%) | 5 (38) |
| 2, n (%) | 1 (8) |
AF, atrial fibrillation; LVEF, left ventricular ejection fraction; LA, left atrium; CHADS2, 1 mark for congestive heart failure, hypertension, age or diabetes mellitus, 2 marks for stroke or transient ischaemic event.
Echocardiographic characteristics
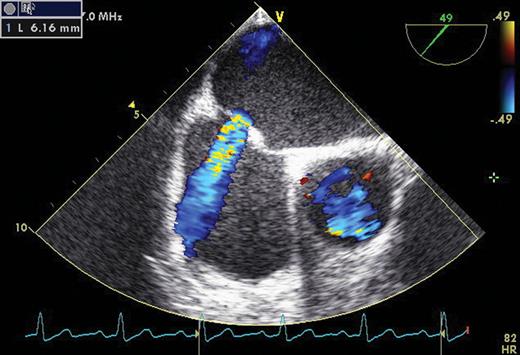
There were no pre-existing ASD in any patient during baseline TEE examination. PFO without RLS was detected in two patients. There was no echocardiographic evidence of valvular abnormalities, right ventricular dilatation or dysfunction, or elevated pulmonary artery pressure. At 6-month echocardiographic follow-up, five (38%) patients had persistent IASD with left-to-right shunt (LRS) and mean size of 5.5 ± 2.4 mm. On agitated saline contrast echocardiography, no RLS was present in patients with IASD. At 9-month echocardiographic follow-up, one of the IASDs closed. Four (31%) patients had persistent IASD with LRS and mean size of 4.6 ± 1.4 mm (Figure 2). On agitated saline contrast echocardiography, no RLS was present. No significant difference in clinical characteristics was identified between patients with and without persistent IASD (Table 2).
Comparison of clinical and echocardiographic characteristics between patients with and without persistent iatrogenic atrial septal defect
| . | With persistent IASD . | Without persistent IASD . | P value . |
|---|---|---|---|
| (n= 5) . | (n= 8) . | ||
| Age (years) | 58.6 ± 17.6 | 57 ± 10.5 | ns |
| Sex (M/F) | 3/2 | 6/2 | ns |
| Paroxysmal AF, n (%) | 4 (80) | 6 (75) | ns |
| Persistent AF, n (%) | 1 (20) | 2 (25) | ns |
| Lone AF, n (%) | 2 (40) | 4 (50) | ns |
| Hypertension, n (%) | 2 (40) | 4 (50) | ns |
| LA size (cm) | 3.4 ± 0.7 | 3.9 ± 0.4 | ns |
| LVEF (%) | 71.8 ± 9.8 | 61.4 ± 12.3 | ns |
| . | With persistent IASD . | Without persistent IASD . | P value . |
|---|---|---|---|
| (n= 5) . | (n= 8) . | ||
| Age (years) | 58.6 ± 17.6 | 57 ± 10.5 | ns |
| Sex (M/F) | 3/2 | 6/2 | ns |
| Paroxysmal AF, n (%) | 4 (80) | 6 (75) | ns |
| Persistent AF, n (%) | 1 (20) | 2 (25) | ns |
| Lone AF, n (%) | 2 (40) | 4 (50) | ns |
| Hypertension, n (%) | 2 (40) | 4 (50) | ns |
| LA size (cm) | 3.4 ± 0.7 | 3.9 ± 0.4 | ns |
| LVEF (%) | 71.8 ± 9.8 | 61.4 ± 12.3 | ns |
IASD, iatrogenic atrial septal defect; AF, atrial fibrillation; LA, left atrium; LVEF, left ventricular ejection fraction.
Comparison of clinical and echocardiographic characteristics between patients with and without persistent iatrogenic atrial septal defect
| . | With persistent IASD . | Without persistent IASD . | P value . |
|---|---|---|---|
| (n= 5) . | (n= 8) . | ||
| Age (years) | 58.6 ± 17.6 | 57 ± 10.5 | ns |
| Sex (M/F) | 3/2 | 6/2 | ns |
| Paroxysmal AF, n (%) | 4 (80) | 6 (75) | ns |
| Persistent AF, n (%) | 1 (20) | 2 (25) | ns |
| Lone AF, n (%) | 2 (40) | 4 (50) | ns |
| Hypertension, n (%) | 2 (40) | 4 (50) | ns |
| LA size (cm) | 3.4 ± 0.7 | 3.9 ± 0.4 | ns |
| LVEF (%) | 71.8 ± 9.8 | 61.4 ± 12.3 | ns |
| . | With persistent IASD . | Without persistent IASD . | P value . |
|---|---|---|---|
| (n= 5) . | (n= 8) . | ||
| Age (years) | 58.6 ± 17.6 | 57 ± 10.5 | ns |
| Sex (M/F) | 3/2 | 6/2 | ns |
| Paroxysmal AF, n (%) | 4 (80) | 6 (75) | ns |
| Persistent AF, n (%) | 1 (20) | 2 (25) | ns |
| Lone AF, n (%) | 2 (40) | 4 (50) | ns |
| Hypertension, n (%) | 2 (40) | 4 (50) | ns |
| LA size (cm) | 3.4 ± 0.7 | 3.9 ± 0.4 | ns |
| LVEF (%) | 71.8 ± 9.8 | 61.4 ± 12.3 | ns |
IASD, iatrogenic atrial septal defect; AF, atrial fibrillation; LA, left atrium; LVEF, left ventricular ejection fraction.
Persistent iatrogenic atrial septal defect with left-to-right shunt in a patient 9 months after a cryoballoon procedure as shown by transoesophageal echocardiography.
Procedural characteristics
A total of 49 out of 55 PVs (89%) could be successfully isolated with a single 28 mm cryoballoon catheter. The remaining six PVs (two left superior PVs, two left inferior PVs, and two right inferior PVs) could be successfully isolated with additional ablation by 8 mm tip cryoablation catheter. The average number of freezing cycles per PV was 2.6. One patient developed right PNP during ablation of the right middle PV and the diaphragmatic function normalized on fluoroscopic screening at 1 week. There was one minor guidewire dissection of the right inferior PV and the patient remained asymptomatic. The mean procedural time and fluoroscopic time was 231 ± 32.3 and 61.6 ± 18.2 min, respectively.
Follow-up
With a median follow-up of 14 months, nine (69%) patients were free of recurrence of AF without antiarrhythmic drugs. During the follow-up period, no patients died or suffered from cerebral, cardiac, or peripheral embolism. No patients complained of any migraine-like headache. One patient with CHADS2 score of 2 was maintained on long-term warfarin. Five patients with CHADS2 score of 1 and 7 patients with CHADS2 score of 0 were given long-term aspirin.
Discussion
Main findings
Cryoballoon catheter is a novel device introduced recently to reduce the complexity and shorten the procedural time of PVI procedures. It has been shown to be safe and effective in the treatment of paroxysmal AF by preliminary clinical stuides.9,10 However, the potential complication of persistent IASD with the use of a large transseptal sheath in cryoballoon procedures was not systematically investigated in all these studies. In our study, a high incidence of persistent IASD was observed after PVI procedures by cryoballoon. At 9-month follow-up, around 30% of patients had persistent IASD with LRS but not RLS. The size of the IASD was relatively small with a mean diameter of 4.6 mm. Hammerstingl et al.1 has shown that the occurrence of persistent IASD was related to TsP technique in PVI procedures. All the persistent IASD occurred in patients with single TsP accommodating an 8 F transseptal sheath and a 7 F radiofrequency ablation catheter. In contrast, no persistent IASD was observed in patients with double TsP. Interestingly, a similar incidence of 30% of persistent IASD was observed in the patient group with single TsP in his study. In addition, the mean diameter of the IASD, which was 4 mm, is also comparable with what we found in our study. Although the TsP wound was elliptic in Hammerstingl's study and circular in ours, the same largest diameter of the TsP wound, which was 15 F (4.95 mm), probably explains the similar incidence and size of the persistent IASD in both studies. Interestingly, in our study, the mean size of the IASD was 5.5 mm at 6 months. The larger size compared with the 15 F transseptal sheath may suggest some degree of tearing of the interatrial septum during manipulation of the transseptal sheath in a long ablation procedure. In Hammerstingl's study, six out of eight patients with IASD had RLS and pre-procedural pulmonary artery pressure was significantly higher in these patients compared with patients with IASD but without RLS. In contrast, all four patients with persistent IASD did not have RLS in our study. Possible reasons explaining the discrepancy of the findings include small sample size of our study, lower baseline pulmonary artery pressure and inadequate effort in performing the Valsalva manoeuvre by the patients.
Clinical implications
Atrial septal defect and PFO with interatrial shunting has been shown to be risk factors for paradoxical embolism and stroke.13–15 High-risk features for occurrence of paradoxical embolism and stroke includes the presence of RLS with large volume, atrial septal aneurysm, and a PFO size of >4 mm.16,17 Closure of ASD either percutaneously or surgically is also recommended when there is right atrial and right ventricular enlargement even in the absence of symptoms.18 In our study, there was no RLS for all the IASD and there was also no clinically observed paradoxical embolism. However, the mean size of the persistent IASD was 4.6 mm which puts our patients in the high-risk category if IASD and PFO behave in a similar mechanism in causing paradoxical embolism and stroke.
Interatrial shunt may also be a cause of migraine.19 Recently, a 0.5% incidence rate of migraine aura after TsP for various catheter ablation procedures was reported.20 We have not observed any complaints of migraine-like headache or aura from our patients.
Paradoxical arterial gas embolism has been shown to occur in scuba divers with ASD.21 Despite the absence of RLS at rest, use of Valsalva manoeuvre, increase in the partial pressure of oxygen, and increase in cardiac filling pressures during immersion and exposure to cold can cause RLS through an ASD and as a result paradoxical arterial gas embolism during scuba diving. Whether patients with persistent IASD after cryoballoon procedures are exposed to the same risk during scuba diving is unknown. It may be advisable for these patients to refrain from this activity until spontaneous closure of the IASD has been observed.
Although the clinical significance of persistent IASD after cryoballoon procedures remains unclear at this juncture, the high incidence of this under-recognized complication of an increasingly performed procedure for AF warrants serious attention. Patients who have undergone cryoballoon procedures should be screened for the presence of persistent IASD. For those who have this complication, regular echocardiographic reassessment should be performed. Either TEE or TTE with agitated saline contrast echocardiography may be used to detect RLS. An alternative tool for reassessment of this complication is transcranial Doppler ultrasonography.22 To elucidate its clinical significance, persistent IASD and its clinical outcomes should be systemically investigated in future studies involving larger number of patients and longer follow-up. On the other hand, the large transseptal sheath for cryoballoon procedures should preferably be down-sized to reduce the incidence of this complication.
Study limitations
The number of patients in this study is small and the patient population is heterogeneous with recruitment of patients having paroxysmal or persistent AF. All patients were on either warfarin or aspirin during the follow-up period. This may potentially reduce the incidence of paradoxical embolism. On the other hand, no imaging investigation was performed to look for clinically silent cerebral infarct. Lastly, the further changes of the persistent IASD in the study patients beyond 9 months are not known.
Conclusions
Persistent IASD is a common but under-recognized complication after PVI by cryoballoon catheter. Only left-to-right, but not right-to-left interatrial shunting occurred as a result of the IASD. There was no clinical occurrence of paradoxical embolism.
Conflict of interest: none declared.
Funding
No financial support to be declared.