-
PDF
- Split View
-
Views
-
Cite
Cite
Michiel Rienstra, Isabelle C. Van Gelder, Maarten P. Van den Berg, Frans Boomsma, Dirk J. Van Veldhuisen, Natriuretic peptides in patients with atrial fibrillation and advanced chronic heart failure: determinants and prognostic value of (NT-)ANP and (NT-pro)BNP, EP Europace, Volume 8, Issue 7, July 2006, Pages 482–487, https://doi.org/10.1093/europace/eul060
Close - Share Icon Share
Abstract
Aims To study the determinants of natriuretic peptides in advanced chronic heart failure (CHF) patients with and without atrial fibrillation (AF) and to evaluate the prognostic value of natriuretic peptides in AF compared with sinus rhythm patients with advanced CHF.
Methods and results The study group comprised 354 advanced CHF patients [all New York Heart Association (NYHA) III/IV], including 76 AF patients. AF patients were older (70±7 vs. 67±8; P=0.01), and non-ischaemic CHF was more common (42 vs. 19%; P=0.002) than in sinus rhythm patients, but left-ventricular ejection fraction was comparable (0.23±0.08 vs. 0.24±0.07; P=ns). At baseline, (NT-)ANP and NT-proBNP levels were significantly higher in AF patients, compared with those in sinus rhythm. By multivariate regression analysis, AF was identified as independent determinant of (NT-)ANP, but not of (NT-pro)BNP levels. After a mean follow-up of 3.2±0.9 (range 0.4−5.4) years, cardiovascular mortality was comparable (55 vs. 47%; P=ns). In both groups, AF and sinus rhythm, NT-proBNP [AF: adjusted HR 5.8 (1.3−25.4), P=0.02; sinus rhythm: adjusted HR 3.1 (1.7−5.7), P<0.001] was an independent risk indicator of cardiovascular mortality.
Conclusion In advanced CHF patients, AF affects (NT-)ANP levels, but not (NT-pro)BNP levels. NT-proBNP is an independent determinant of prognosis in advanced CHF, irrespective of the rhythm, AF, or sinus rhythm.
Introduction
Plasma natriuretic peptides play an important role in chronic heart failure (CHF). By counteracting the deleterious effects of the activation of renin–angiotensin system in CHF, they promote diuresis and vasodilatation.1 Brain natriuretic peptide (BNP) is mainly produced in the ventricles, whereas the atria are the main site of atrial natriuretic peptide (ANP) production,2 in response to myocardial stretch and increased volume. ANP, N-terminal ANP (NT-ANP), BNP, and N-terminal proBNP (NT-proBNP) are increased in CHF,3–7 the levels of natriuretic peptides being correlated with the extent of ventricular dysfunction.4 Natriuretic peptides are strong predictors of mortality in CHF.8–15 However, none of these trials focused on the underlying cardiac rhythm, atrial fibrillation (AF), or sinus rhythm. This may be of importance because the presence of AF in CHF may further increase levels of natriuretic peptides.16,17
Therefore, in the present study, we investigated whether the prognostic value of the natriuretic peptides in CHF also holds true for patients with concomitant AF. Furthermore, we investigated the determinants of natriuretic peptides in patients with advanced CHF with and without AF.
Methods
Patient selection
All patients participated in the neuroendocrine substudy of the PRIME II study, which was a randomized international survival study comparing ibopamine with placebo in moderate-to-severe (‘advanced’) CHF. The neuroendocrine substudy was performed in The Netherlands.16,18 After discontinuation of PRIME II, all patients were followed for at least 2 years. In PRIME II, patients with symptoms at rest or a recent hospital admission for CHF, according to the New York Heart Association (NYHA) functional Class III–IV, and left ventricular impairment were included. All patients were clinically stable. Left ventricular impairment was proved by one of the following: (i) left ventricular ejection fraction <0.35; (ii) left ventricular end-diastolic diameter >60 mm; (iii) left ventricular fractional shortening <20%; or (iv) cardiothoracic ratio on standard chest X-ray >0.50. Patients were on medical treatment for CHF, including angiotensin-converting enzyme-inhibitors and diuretics (median daily dose of furosemide 80 mg) and, if indicated, digitalis and beta-blockers. At baseline, 76 patients with persistent or permanent AF were identified by two consecutive electrocardiograms taken at least 7 days apart.
Natriuretic peptide measurement
Methods of natriuretic peptide measurements have been previously published.16,19 In short, at baseline, before treatment with ibopamine was started, blood was collected between 9.00 and 10.00 AM after patients had rested in supine position for >30 min. An intravenous canula was used to transfer blood into chilled 10 mL tubes containing EDTA (19 mg) and aprotinin (1000 kIU). The tubes were centrifuged within 30 min (4°C, 10 min, 2000g) and plasma was separated and stored in polyethylene tubes at −70°C. Measurement of ANP was performed after SepPak extraction with commercially available radioimmunoassay kits from the Nichols Institute, Wijchen, the Netherlands. Plasma NT-ANP was determined using a commercially available radioimmunoassay kit (Biotop, Oulu, Finland). NT-proBNP was measured using a radioimmunoassay with reagents including antibody, standards, and radiolabel. The assay uses 50 µL of unextracted plasma and has a standard range of 60–1000 pmol L−1. All samples giving results of >900 pmol L−1 were re-analysed in appropriate dilutions with physiological salt. In 12 consecutive assays, variabilities were 14, 11, 4, and 4% at concentrations of 131, 199, 293, and 901 pmol L−1, respectively. Brain natriuretic peptide was determined by a commercially available immunoradiometric assay (Shionoria, Osaka, Japan).
Primary endpoint
Primary endpoint was cardiovascular mortality, which consisted of sudden cardiac death, progressive CHF, myocardial infarction, ischaemic stroke, and systemic embolus. Sudden cardiac death was defined as witnessed or unwitnessed instantaneous death in a patient who had no deterioration of CHF for 1 week before death and no chest pain. Death due to progressive CHF was defined as shock, intractable CHF that led to hospital admission, or deterioration of CHF during the month before death. Death due to myocardial infarction was defined as death during 1 week after documented diagnosis of myocardial infarction or death after suspected myocardial infarction. Death due to ischaemic stroke was confirmed by a neurologist and death due to systemic embolus was confirmed by a surgeon.
Statistical analysis
Baseline descriptive statistics are presented as the mean±SD or median (range) for continuous variables and numbers with percentages for categorical variables. Differences between variables in patients with AF vs. sinus rhythm were evaluated by Student's t-test or Mann–Whitney U test, depending on normality of the data, for continuous data and by Fisher's exact test or χ2 test for categorical data.
Linear regression analyses were performed to determine risk indicators of the natriuretic peptide levels. To realize a constant variance, natriuretic peptide values were logarithmically transformed. All patient characteristics were included. Kaplan–Meier estimates of cumulative event rates were calculated and cardiovascular mortality for the two study groups was compared using the log-rank test. Adjusted hazard ratios were calculated using Cox proportional hazards regression models. Linearity of the continuous variables with respect to the response variable was assessed by determining the quartiles of their distribution. In the case of a linear trend in the estimated risk ratios, the variable was introduced in the model as continuous. If no linearity was demonstrated, the variable was further categorized, primarily, the median value, or otherwise based on clinical relevance (first quartile for ANP, NT-ANP, BNP and NT-proBNP, and age). All patient characteristics and natriuretic peptides were tested. All univariate variables with P<0.1 were investigated in a multivariate model. In the multivariate model, a variable was excluded when P≥0.05. A stepwise approach was used and first-line interactions were investigated. In all analyses, a value of P<0.05 was considered statistically significant.
Results
Clinical characteristics
The neuroendocrine substudy of PRIME II comprised 354 patients, including 76 patients with AF. All patients had CHF NYHA Class III or IV. Clinical characteristics are shown in Table 1. In the AF patients, the total duration of AF at baseline was 21 (0–336) months. Ischaemic heart disease was less frequently present in AF patients (58 vs. 81%; P<0.001). Mean left ventricular ejection fraction was comparable in the two groups (0.23±0.08 vs. 0.24±0.07; P=ns). The duration of CHF was longer in patients with AF, compared with those in sinus rhythm [36 (3–180) vs. 22 (0–180) months; P=0.006]. The heart rate was comparable between the two groups (84±17 vs. 80±14 bpm; P=ns). Patients with AF were more often treated with digoxin, antiarrhythmic drugs, and oral anticoagulation. Beta-blocker therapy was rarely instituted. Ibopamine treatment was equally distributed in both groups (57 vs. 48%; P=ns).
Clinical characteristics comparing patients with AF and sinus rhythm
| Clinical characteristic . | AF (n=76) . | Sinus rhythm (n=278) . | P-value . |
|---|---|---|---|
| Age (years) | 70±7 | 67±8 | 0.01 |
| Male sex (%) | 80 | 76 | ns |
| Duration of CHF (months) | 36 (3–180) | 22 (2–180) | 0.006 |
| Underlying heart disease (%) | |||
| Ischaemic heart disease | 58 | 81 | <0.001 |
| Dilated cardiomyopathy | 29 | 14 | 0.002 |
| Hypertension | 5 | 3 | ns |
| Valvular disease | 5 | 2 | ns |
| Other | 3 | 0 | ns |
| Heart failure functional class (%) | ns | ||
| NYHA III | 61 | 71 | |
| NYHA III/IV | 34 | 26 | |
| NYHA IV | 5 | 3 | |
| Diabetes mellitus (%) | 21 | 23 | ns |
| LVEF | 0.23±0.08 | 0.24±0.07 | ns |
| LVEDD (mm) | 68±9 | 69±8 | ns |
| Heart rate (bpm) | 84±17 | 80±14 | ns |
| Blood pressure (mmHg) | |||
| Systolic | 119±19 | 126±18 | 0.002 |
| Diastolic | 74±10 | 77±9 | 0.02 |
| Sodium (mmol/L) | 138±4 | 139±3 | ns |
| Creatinine (µmol/L) | 119±30 | 117±40 | ns |
| GFR (mL/min) | 56±20 | 61±23 | ns |
| Ibopamine (%) | 57 | 48 | ns |
| Concomitant medication (%) | |||
| ACE-inhibitor | 92 | 96 | ns |
| Antiarrhythmic druga | 28 | 16 | 0.02 |
| Beta blocker | 8 | 10 | ns |
| Calcium antagonist | 5 | 7 | ns |
| Digoxin | 90 | 51 | <0.001 |
| Diuretic | 100 | 99 | ns |
| Oral anticoagulant | 82 | 67 | 0.02 |
| Clinical characteristic . | AF (n=76) . | Sinus rhythm (n=278) . | P-value . |
|---|---|---|---|
| Age (years) | 70±7 | 67±8 | 0.01 |
| Male sex (%) | 80 | 76 | ns |
| Duration of CHF (months) | 36 (3–180) | 22 (2–180) | 0.006 |
| Underlying heart disease (%) | |||
| Ischaemic heart disease | 58 | 81 | <0.001 |
| Dilated cardiomyopathy | 29 | 14 | 0.002 |
| Hypertension | 5 | 3 | ns |
| Valvular disease | 5 | 2 | ns |
| Other | 3 | 0 | ns |
| Heart failure functional class (%) | ns | ||
| NYHA III | 61 | 71 | |
| NYHA III/IV | 34 | 26 | |
| NYHA IV | 5 | 3 | |
| Diabetes mellitus (%) | 21 | 23 | ns |
| LVEF | 0.23±0.08 | 0.24±0.07 | ns |
| LVEDD (mm) | 68±9 | 69±8 | ns |
| Heart rate (bpm) | 84±17 | 80±14 | ns |
| Blood pressure (mmHg) | |||
| Systolic | 119±19 | 126±18 | 0.002 |
| Diastolic | 74±10 | 77±9 | 0.02 |
| Sodium (mmol/L) | 138±4 | 139±3 | ns |
| Creatinine (µmol/L) | 119±30 | 117±40 | ns |
| GFR (mL/min) | 56±20 | 61±23 | ns |
| Ibopamine (%) | 57 | 48 | ns |
| Concomitant medication (%) | |||
| ACE-inhibitor | 92 | 96 | ns |
| Antiarrhythmic druga | 28 | 16 | 0.02 |
| Beta blocker | 8 | 10 | ns |
| Calcium antagonist | 5 | 7 | ns |
| Digoxin | 90 | 51 | <0.001 |
| Diuretic | 100 | 99 | ns |
| Oral anticoagulant | 82 | 67 | 0.02 |
GFR, glomerular filtration rate; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
a90% amiodarone.
Clinical characteristics comparing patients with AF and sinus rhythm
| Clinical characteristic . | AF (n=76) . | Sinus rhythm (n=278) . | P-value . |
|---|---|---|---|
| Age (years) | 70±7 | 67±8 | 0.01 |
| Male sex (%) | 80 | 76 | ns |
| Duration of CHF (months) | 36 (3–180) | 22 (2–180) | 0.006 |
| Underlying heart disease (%) | |||
| Ischaemic heart disease | 58 | 81 | <0.001 |
| Dilated cardiomyopathy | 29 | 14 | 0.002 |
| Hypertension | 5 | 3 | ns |
| Valvular disease | 5 | 2 | ns |
| Other | 3 | 0 | ns |
| Heart failure functional class (%) | ns | ||
| NYHA III | 61 | 71 | |
| NYHA III/IV | 34 | 26 | |
| NYHA IV | 5 | 3 | |
| Diabetes mellitus (%) | 21 | 23 | ns |
| LVEF | 0.23±0.08 | 0.24±0.07 | ns |
| LVEDD (mm) | 68±9 | 69±8 | ns |
| Heart rate (bpm) | 84±17 | 80±14 | ns |
| Blood pressure (mmHg) | |||
| Systolic | 119±19 | 126±18 | 0.002 |
| Diastolic | 74±10 | 77±9 | 0.02 |
| Sodium (mmol/L) | 138±4 | 139±3 | ns |
| Creatinine (µmol/L) | 119±30 | 117±40 | ns |
| GFR (mL/min) | 56±20 | 61±23 | ns |
| Ibopamine (%) | 57 | 48 | ns |
| Concomitant medication (%) | |||
| ACE-inhibitor | 92 | 96 | ns |
| Antiarrhythmic druga | 28 | 16 | 0.02 |
| Beta blocker | 8 | 10 | ns |
| Calcium antagonist | 5 | 7 | ns |
| Digoxin | 90 | 51 | <0.001 |
| Diuretic | 100 | 99 | ns |
| Oral anticoagulant | 82 | 67 | 0.02 |
| Clinical characteristic . | AF (n=76) . | Sinus rhythm (n=278) . | P-value . |
|---|---|---|---|
| Age (years) | 70±7 | 67±8 | 0.01 |
| Male sex (%) | 80 | 76 | ns |
| Duration of CHF (months) | 36 (3–180) | 22 (2–180) | 0.006 |
| Underlying heart disease (%) | |||
| Ischaemic heart disease | 58 | 81 | <0.001 |
| Dilated cardiomyopathy | 29 | 14 | 0.002 |
| Hypertension | 5 | 3 | ns |
| Valvular disease | 5 | 2 | ns |
| Other | 3 | 0 | ns |
| Heart failure functional class (%) | ns | ||
| NYHA III | 61 | 71 | |
| NYHA III/IV | 34 | 26 | |
| NYHA IV | 5 | 3 | |
| Diabetes mellitus (%) | 21 | 23 | ns |
| LVEF | 0.23±0.08 | 0.24±0.07 | ns |
| LVEDD (mm) | 68±9 | 69±8 | ns |
| Heart rate (bpm) | 84±17 | 80±14 | ns |
| Blood pressure (mmHg) | |||
| Systolic | 119±19 | 126±18 | 0.002 |
| Diastolic | 74±10 | 77±9 | 0.02 |
| Sodium (mmol/L) | 138±4 | 139±3 | ns |
| Creatinine (µmol/L) | 119±30 | 117±40 | ns |
| GFR (mL/min) | 56±20 | 61±23 | ns |
| Ibopamine (%) | 57 | 48 | ns |
| Concomitant medication (%) | |||
| ACE-inhibitor | 92 | 96 | ns |
| Antiarrhythmic druga | 28 | 16 | 0.02 |
| Beta blocker | 8 | 10 | ns |
| Calcium antagonist | 5 | 7 | ns |
| Digoxin | 90 | 51 | <0.001 |
| Diuretic | 100 | 99 | ns |
| Oral anticoagulant | 82 | 67 | 0.02 |
GFR, glomerular filtration rate; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
a90% amiodarone.
Determinants of natriuretic peptide levels
All natriuretic peptides exceeded the normal range, irrespective of the rhythm (Table 2). (NT-)ANP and NT-proBNP levels were significantly higher in patients with AF, compared with those in sinus rhythm. There was a trend for higher BNP levels in patients with AF, although not statistically significant. There were no differences in natriuretic peptide levels between patients with ischaemic CHF and those with non-ischaemic CHF: ANP [103 (12–815) vs. 98 (12–720); P=ns], NT-ANP [1.10 (0.12–4.21) vs. 0.97 (0.13–3.08); P=ns], BNP [53 (3–460) vs. 40 (1–502); P=ns], and NT-proBNP [580 (0–5295) vs. 554 (3–3600); P=ns]. In addition, in both the AF and the sinus rhythm groups, natriuretic peptide levels were comparable between patients with ischaemic CHF and those with non-ischaemic CHF (data not given). Linear multivariate regression analyses were performed to determine the determinants of natriuretic peptide levels (Table 3). AF was an independent determinant of both higher ANP and NT-ANP levels, but not of higher BNP and NT-proBNP levels. Impaired left-ventricular function and higher creatinine plasma levels were independent determinants of higher levels of all four natriuretic peptides. Both AF duration and CHF duration were not determinants of the levels of the natriuretic peptides. Lower systolic blood pressure was associated with higher BNP levels, whereas lower diastolic blood pressure was associated with the elevation of NT-ANP and NT-proBNP levels. Diabetes was a determinant of higher BNP levels.
Natriuretic peptide plasma levels in patients with AF vs. sinus rhythm
| Natriuretic peptide . | Normal range . | AF . | Sinus rhythm . | P-value . |
|---|---|---|---|---|
| ANP (pmol/L) | 15–35 | 116 (18–500) | 95 (12–815) | 0.007 |
| NT-ANP (nmol/L) | 0.15–0.50 | 1.37 (0.31–3.08) | 0.96 (0.13–4.21) | <0.001 |
| BNP (pmol/L) | <5 | 71 (3–285) | 47 (1–502) | ns |
| NT-proBNP (pmol/L) | 25–200 | 774 (3–3600) | 509 (0–5295) | 0.008 |
| Natriuretic peptide . | Normal range . | AF . | Sinus rhythm . | P-value . |
|---|---|---|---|---|
| ANP (pmol/L) | 15–35 | 116 (18–500) | 95 (12–815) | 0.007 |
| NT-ANP (nmol/L) | 0.15–0.50 | 1.37 (0.31–3.08) | 0.96 (0.13–4.21) | <0.001 |
| BNP (pmol/L) | <5 | 71 (3–285) | 47 (1–502) | ns |
| NT-proBNP (pmol/L) | 25–200 | 774 (3–3600) | 509 (0–5295) | 0.008 |
Natriuretic peptide plasma levels in patients with AF vs. sinus rhythm
| Natriuretic peptide . | Normal range . | AF . | Sinus rhythm . | P-value . |
|---|---|---|---|---|
| ANP (pmol/L) | 15–35 | 116 (18–500) | 95 (12–815) | 0.007 |
| NT-ANP (nmol/L) | 0.15–0.50 | 1.37 (0.31–3.08) | 0.96 (0.13–4.21) | <0.001 |
| BNP (pmol/L) | <5 | 71 (3–285) | 47 (1–502) | ns |
| NT-proBNP (pmol/L) | 25–200 | 774 (3–3600) | 509 (0–5295) | 0.008 |
| Natriuretic peptide . | Normal range . | AF . | Sinus rhythm . | P-value . |
|---|---|---|---|---|
| ANP (pmol/L) | 15–35 | 116 (18–500) | 95 (12–815) | 0.007 |
| NT-ANP (nmol/L) | 0.15–0.50 | 1.37 (0.31–3.08) | 0.96 (0.13–4.21) | <0.001 |
| BNP (pmol/L) | <5 | 71 (3–285) | 47 (1–502) | ns |
| NT-proBNP (pmol/L) | 25–200 | 774 (3–3600) | 509 (0–5295) | 0.008 |
Determinants of natriuretic peptide levels in multivariate linear regression analysis
| Variable . | ANP . | NT-ANP . | BNP . | NT-proBNP . |
|---|---|---|---|---|
| AF | 0.007 | <0.001 | — | — |
| LVEF <0.23 | <0.001 | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure <120 mmHg | — | — | 0.002 | — |
| Diastolic blood pressure <80 mmHg | — | 0.03 | — | 0.008 |
| Creatinine >114 µmol/L | <0.001 | <0.001 | <0.001 | <0.001 |
| Diabetes mellitus | — | — | 0.008 | — |
| Variable . | ANP . | NT-ANP . | BNP . | NT-proBNP . |
|---|---|---|---|---|
| AF | 0.007 | <0.001 | — | — |
| LVEF <0.23 | <0.001 | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure <120 mmHg | — | — | 0.002 | — |
| Diastolic blood pressure <80 mmHg | — | 0.03 | — | 0.008 |
| Creatinine >114 µmol/L | <0.001 | <0.001 | <0.001 | <0.001 |
| Diabetes mellitus | — | — | 0.008 | — |
Determinants of natriuretic peptide levels in multivariate linear regression analysis
| Variable . | ANP . | NT-ANP . | BNP . | NT-proBNP . |
|---|---|---|---|---|
| AF | 0.007 | <0.001 | — | — |
| LVEF <0.23 | <0.001 | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure <120 mmHg | — | — | 0.002 | — |
| Diastolic blood pressure <80 mmHg | — | 0.03 | — | 0.008 |
| Creatinine >114 µmol/L | <0.001 | <0.001 | <0.001 | <0.001 |
| Diabetes mellitus | — | — | 0.008 | — |
| Variable . | ANP . | NT-ANP . | BNP . | NT-proBNP . |
|---|---|---|---|---|
| AF | 0.007 | <0.001 | — | — |
| LVEF <0.23 | <0.001 | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure <120 mmHg | — | — | 0.002 | — |
| Diastolic blood pressure <80 mmHg | — | 0.03 | — | 0.008 |
| Creatinine >114 µmol/L | <0.001 | <0.001 | <0.001 | <0.001 |
| Diabetes mellitus | — | — | 0.008 | — |
Prognostic value of natriuretic peptides
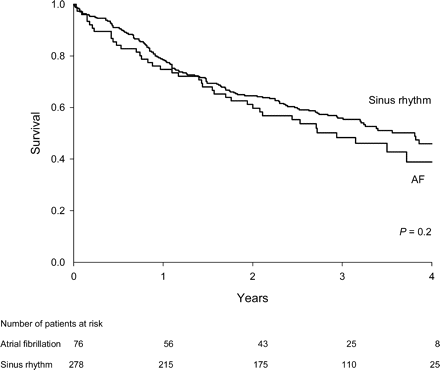
Mean follow-up was 3.2±0.9 (range 0.4–5.4) years. Cardiovascular mortality occurred in 55% of the AF patients and in 47% of the sinus rhythm patients (P=0.2; Figure 1). The majority of patients, with either AF or sinus rhythm, died owing to progressive CHF (32 vs. 26%). Sudden cardiac death was the second major cause of death (21 vs. 13%) (Table 4). Survival was comparable between patients with ischaemic CHF and those with non-ischaemic CHF (53 vs. 49%; P=ns) and in patients treated with ibopamine and those treated with placebo (P=ns), irrespective of the rhythm.
Kaplan–Meier curve for the cardiovascular mortality in the AF and sinus rhythm patients with advanced CHF.
Components of cardiovascular mortality in patients with AF vs. sinus rhythm
| . | AF (n=76) . | Sinus rhythm (n=278) . |
|---|---|---|
| Cardiovascular mortality (%) | 42 (55) | 130 (47) |
| Progressive heart failure | 24 (32) | 73 (26) |
| Sudden death | 16 (21) | 37 (13) |
| Myocardial infarction | 1 (1) | 12 (4) |
| Ischaemic stroke | 1 (1) | 5 (2) |
| Systemic embolus | — | 3 (1) |
| . | AF (n=76) . | Sinus rhythm (n=278) . |
|---|---|---|
| Cardiovascular mortality (%) | 42 (55) | 130 (47) |
| Progressive heart failure | 24 (32) | 73 (26) |
| Sudden death | 16 (21) | 37 (13) |
| Myocardial infarction | 1 (1) | 12 (4) |
| Ischaemic stroke | 1 (1) | 5 (2) |
| Systemic embolus | — | 3 (1) |
Components of cardiovascular mortality in patients with AF vs. sinus rhythm
| . | AF (n=76) . | Sinus rhythm (n=278) . |
|---|---|---|
| Cardiovascular mortality (%) | 42 (55) | 130 (47) |
| Progressive heart failure | 24 (32) | 73 (26) |
| Sudden death | 16 (21) | 37 (13) |
| Myocardial infarction | 1 (1) | 12 (4) |
| Ischaemic stroke | 1 (1) | 5 (2) |
| Systemic embolus | — | 3 (1) |
| . | AF (n=76) . | Sinus rhythm (n=278) . |
|---|---|---|
| Cardiovascular mortality (%) | 42 (55) | 130 (47) |
| Progressive heart failure | 24 (32) | 73 (26) |
| Sudden death | 16 (21) | 37 (13) |
| Myocardial infarction | 1 (1) | 12 (4) |
| Ischaemic stroke | 1 (1) | 5 (2) |
| Systemic embolus | — | 3 (1) |
In both patient groups, we evaluated which factors were related to cardiovascular mortality (Table 5). Patient characteristics and natriuretic peptide levels were included in the multivariate models. In patients with AF, both NT-proBNP and ANP emerged as independent determinants of cardiovascular mortality. In patients with sinus rhythm, NT-proBNP was related to cardiovascular mortality, next to high creatinine plasma levels, digoxin use, and low diastolic blood pressure.
Determinants of cardiovascular mortality in patients with advanced CHF
| Variable . | Hazard ratio (95% CI) . | P-value . | |
|---|---|---|---|
| . | Univariate . | Multivariate . | . |
| AF patients (n=76) | |||
| NT-proBNP >449 pmol/L | 10.3 (2.4–43.1) | 5.8 (1.3–25.4) | 0.02 |
| ANP >87 pmol/L | 5.4 (1.9–15.3) | 4.0 (1.2–13.7) | 0.03 |
| Hypertension | 0.3 (0.1–0.9) | ||
| LVEF <0.29 | 2.0 (0.9–4.3) | ||
| NT-ANP >0.92 nmol/L | 4.7 (1.7–13.1) | ||
| BNP >24 pmol/L | 7.2 (1.7–30.2) | ||
| Sinus rhythm patients (n=278) | |||
| NT-proBNP >250 pmol/L | 4.0 (2.2–7.2) | 3.1 (1.7–5.7) | <0.001 |
| Creatinine >110 µmol/L | 2.1 (1.4–3.0) | 1.8 (1.2–2.7) | 0.003 |
| Digoxin | 2.0 (1.4–2.8) | 1.7 (1.2–2.5) | 0.004 |
| Diastolic blood pressure >80 mmHg | 0.5 (0.4–0.8) | 0.6 (0.4–0.9) | 0.013 |
| Age >61 years | 2.0 (1.3–3.2) | ||
| Diabetes mellitus | 0.5 (0.4–0.8) | ||
| Heart failure NYHA Class IV | 3.7 (1.6–8.4) | ||
| Systolic blood pressure >125 mmHg | 0.7 (0.5–0.9) | ||
| Heart rate >90 bpm | 1.6 (1.1–2.3) | ||
| LVEF <0.22 | 1.8 (1.3–2.6) | ||
| Glomerular filtration rate <73 mL/min | 2.7 (1.6–4.5) | ||
| ANP >58 pmol/L | 3.0 (1.8–5.1) | ||
| NT-ANP >0.59 nmol/L | 2.4 (1.5–4.0) | ||
| BNP >16 pmol/L | 3.9 (2.0–7.8) | ||
| Variable . | Hazard ratio (95% CI) . | P-value . | |
|---|---|---|---|
| . | Univariate . | Multivariate . | . |
| AF patients (n=76) | |||
| NT-proBNP >449 pmol/L | 10.3 (2.4–43.1) | 5.8 (1.3–25.4) | 0.02 |
| ANP >87 pmol/L | 5.4 (1.9–15.3) | 4.0 (1.2–13.7) | 0.03 |
| Hypertension | 0.3 (0.1–0.9) | ||
| LVEF <0.29 | 2.0 (0.9–4.3) | ||
| NT-ANP >0.92 nmol/L | 4.7 (1.7–13.1) | ||
| BNP >24 pmol/L | 7.2 (1.7–30.2) | ||
| Sinus rhythm patients (n=278) | |||
| NT-proBNP >250 pmol/L | 4.0 (2.2–7.2) | 3.1 (1.7–5.7) | <0.001 |
| Creatinine >110 µmol/L | 2.1 (1.4–3.0) | 1.8 (1.2–2.7) | 0.003 |
| Digoxin | 2.0 (1.4–2.8) | 1.7 (1.2–2.5) | 0.004 |
| Diastolic blood pressure >80 mmHg | 0.5 (0.4–0.8) | 0.6 (0.4–0.9) | 0.013 |
| Age >61 years | 2.0 (1.3–3.2) | ||
| Diabetes mellitus | 0.5 (0.4–0.8) | ||
| Heart failure NYHA Class IV | 3.7 (1.6–8.4) | ||
| Systolic blood pressure >125 mmHg | 0.7 (0.5–0.9) | ||
| Heart rate >90 bpm | 1.6 (1.1–2.3) | ||
| LVEF <0.22 | 1.8 (1.3–2.6) | ||
| Glomerular filtration rate <73 mL/min | 2.7 (1.6–4.5) | ||
| ANP >58 pmol/L | 3.0 (1.8–5.1) | ||
| NT-ANP >0.59 nmol/L | 2.4 (1.5–4.0) | ||
| BNP >16 pmol/L | 3.9 (2.0–7.8) | ||
Determinants of cardiovascular mortality in patients with advanced CHF
| Variable . | Hazard ratio (95% CI) . | P-value . | |
|---|---|---|---|
| . | Univariate . | Multivariate . | . |
| AF patients (n=76) | |||
| NT-proBNP >449 pmol/L | 10.3 (2.4–43.1) | 5.8 (1.3–25.4) | 0.02 |
| ANP >87 pmol/L | 5.4 (1.9–15.3) | 4.0 (1.2–13.7) | 0.03 |
| Hypertension | 0.3 (0.1–0.9) | ||
| LVEF <0.29 | 2.0 (0.9–4.3) | ||
| NT-ANP >0.92 nmol/L | 4.7 (1.7–13.1) | ||
| BNP >24 pmol/L | 7.2 (1.7–30.2) | ||
| Sinus rhythm patients (n=278) | |||
| NT-proBNP >250 pmol/L | 4.0 (2.2–7.2) | 3.1 (1.7–5.7) | <0.001 |
| Creatinine >110 µmol/L | 2.1 (1.4–3.0) | 1.8 (1.2–2.7) | 0.003 |
| Digoxin | 2.0 (1.4–2.8) | 1.7 (1.2–2.5) | 0.004 |
| Diastolic blood pressure >80 mmHg | 0.5 (0.4–0.8) | 0.6 (0.4–0.9) | 0.013 |
| Age >61 years | 2.0 (1.3–3.2) | ||
| Diabetes mellitus | 0.5 (0.4–0.8) | ||
| Heart failure NYHA Class IV | 3.7 (1.6–8.4) | ||
| Systolic blood pressure >125 mmHg | 0.7 (0.5–0.9) | ||
| Heart rate >90 bpm | 1.6 (1.1–2.3) | ||
| LVEF <0.22 | 1.8 (1.3–2.6) | ||
| Glomerular filtration rate <73 mL/min | 2.7 (1.6–4.5) | ||
| ANP >58 pmol/L | 3.0 (1.8–5.1) | ||
| NT-ANP >0.59 nmol/L | 2.4 (1.5–4.0) | ||
| BNP >16 pmol/L | 3.9 (2.0–7.8) | ||
| Variable . | Hazard ratio (95% CI) . | P-value . | |
|---|---|---|---|
| . | Univariate . | Multivariate . | . |
| AF patients (n=76) | |||
| NT-proBNP >449 pmol/L | 10.3 (2.4–43.1) | 5.8 (1.3–25.4) | 0.02 |
| ANP >87 pmol/L | 5.4 (1.9–15.3) | 4.0 (1.2–13.7) | 0.03 |
| Hypertension | 0.3 (0.1–0.9) | ||
| LVEF <0.29 | 2.0 (0.9–4.3) | ||
| NT-ANP >0.92 nmol/L | 4.7 (1.7–13.1) | ||
| BNP >24 pmol/L | 7.2 (1.7–30.2) | ||
| Sinus rhythm patients (n=278) | |||
| NT-proBNP >250 pmol/L | 4.0 (2.2–7.2) | 3.1 (1.7–5.7) | <0.001 |
| Creatinine >110 µmol/L | 2.1 (1.4–3.0) | 1.8 (1.2–2.7) | 0.003 |
| Digoxin | 2.0 (1.4–2.8) | 1.7 (1.2–2.5) | 0.004 |
| Diastolic blood pressure >80 mmHg | 0.5 (0.4–0.8) | 0.6 (0.4–0.9) | 0.013 |
| Age >61 years | 2.0 (1.3–3.2) | ||
| Diabetes mellitus | 0.5 (0.4–0.8) | ||
| Heart failure NYHA Class IV | 3.7 (1.6–8.4) | ||
| Systolic blood pressure >125 mmHg | 0.7 (0.5–0.9) | ||
| Heart rate >90 bpm | 1.6 (1.1–2.3) | ||
| LVEF <0.22 | 1.8 (1.3–2.6) | ||
| Glomerular filtration rate <73 mL/min | 2.7 (1.6–4.5) | ||
| ANP >58 pmol/L | 3.0 (1.8–5.1) | ||
| NT-ANP >0.59 nmol/L | 2.4 (1.5–4.0) | ||
| BNP >16 pmol/L | 3.9 (2.0–7.8) | ||
Discussion
The present study shows that NT-proBNP is an independent determinant of prognosis in patients with advanced CHF and also in patients with AF. Furthermore, in patients with advanced heart failure, AF is an independent determinant of ANP and NT-ANP, but not of BNP and NT-proBNP.
Prognostic value of natriuretic peptides in AF and CHF
The present analysis evaluates the prognostic value of natriuretic peptides in patients with AF and advanced CHF. We found that in patients with AF and advanced CHF, NT-proBNP remains an important and independent risk indicator of cardiovascular mortality. This implies that NT-proBNP can be used as marker of prognosis in patients with advanced CHF, irrespective of the presence or absence of AF.
The results of a large number of studies have shown that BNP is a strong prognostic indicator for both asymptomatic patients20,21 and for patients with heart failure at all stages of disease.9–15 However, up to now, only one retrospective study has focused on the cardiac rhythm, AF, or sinus rhythm and studied the prognostic value of (NT-pro)BNP in patients with AF and CHF.22 In that study, patients hospitalized for an exacerbation of CHF between 1996 and 2002 were included and stratified according to the cardiac rhythm at baseline, AF, or sinus rhythm. They found, as we did, that the prognostic value of BNP remains present, regardless of the underlying rhythm. However, the cut-off values of BNP predischarge as predictor of subsequent heart failure events (death or rehospitalization) were different in patients with and without AF (165 vs. 125 pg/mL).
There is a close relation between BNP, the biologically active component, and NT-proBNP levels, the inactive fragment.23 NT-proBNP has a longer halftime than BNP and seems therefore more stable. However, during an acute exacerbation of heart failure, BNP levels are more elevated.23 Despite this, studies have not revealed any difference regarding clinical utility so far.24,25 There are no studies directly comparing the prognostic value of BNP and NT-proBNP.
Determinants of natriuretic peptides in AF and CHF
AF was an independent determinant of ANP and NT-ANP in advanced CHF. This is in accordance with previous studies, which have shown that the increase in ANP due to AF adds to the effect of CHF on ANP.16,17 Loss of atrial contraction due to AF, causing volume and pressure overload, leads to atrial stretch, which leads to an increase in ANP levels, which serves to normalize haemodynamics through natriuresis and vasodilation.26 Several studies have shown that plasma BNP levels increase significantly with the occurrence or presence of AF,27–32 although Rossi et al.17 showed the contrary. In their study, BNP was not independently associated with AF, but was strongly determined by left ventricular function. In our study, AF was not an independent determinant of BNP and NT-proBNP, in contrast to left ventricular dysfunction and higher plasma creatinine levels. There may be several mechanisms as to why BNP levels increase in patients with AF. First, BNP may be secreted not only by the ventricles, but also by the atria,27,33,34 partly through elevated atrial pressure or dilatation.33 Also, the loss of atrial systole and the irregular ventricular rhythm during AF may decrease cardiac output,35 in turn elevating BNP levels. However, in contrast to mild-to-moderate CHF, severe CHF is often characterized by restrictive physiology, implying that the atrial contribution is rather limited due to severe diastolic dysfunction.36 Loss of the atrial kick seems, therefore, of limited haemodynamic importance in patients with severe CHF. Also, the influence of the irregularity of the ventricular response in AF may be limited.35 Previous studies have indicated that the degree of irregularity decreases with worsening of CHF owing to progressive neuroendocrine activation.37,38 As such, the haemodynamic consequences of AF may be less important in patients with severe CHF. Therefore, AF may not influence BNP levels. Consequently, in advanced CHF, as is the case in our study, BNP activation reflects primarily ventricular dysfunction rather than the presence of AF.
The secretion and clearance of BNP are complex and incompletely characterized, but are clearly influenced by the renal function.39 We also found that higher creatinine plasma levels determined the level of the natriuretic peptides. Furthermore, we found that lower systolic and diastolic blood pressures, as markers of more severe CHF, were also related to the level of the natriuretic peptides. In addition, we found that diabetes was an independent determinant of BNP. Accordingly, another study showed that the presence of nephropathy in patients with diabetes leads to elevated (NT-pro)BNP levels.40
Limitations
The present analysis was observational in design and therefore has important limitations. Cause–effect relationship cannot be demonstrated and the present data are thus only hypothesis generating. Half of the patients were treated with ibopamine (on average 347 days), which may have influenced the overall mortality rate. However, after premature discontinuation of the PRIME study, follow-up was extended for at least 2 years. Survival in patients treated with ibopamine was comparable with those treated with placebo. It is known that digoxin may increase the plasma levels of ANP and BNP in patients with CHF. Owing to the higher rate of digoxin use in AF patients, this may have influenced outcome. We did not observe, however, any independent impact of digoxin on the natriuretic peptide levels. It is known that beta blocker use affects the level of natriuretic peptides.41 However, at the time the PRIME study was conducted, beta blocker therapy was only rarely instituted in patients with heart failure. In the present study, mainly ischaemic CHF patients (76%), and males (78%) were included. Rhythm (AF or sinus rhythm) during follow-up is unknown.
Conclusion
In advanced CHF patients with AF, AF affects (NT-)ANP levels, but not (NT-pro)BNP levels, in contrast to impaired left-ventricular function and higher creatinine plasma levels, which were independent determinants of higher levels of all four natriuretic peptides. NT-proBNP remains an independent determinant of prognosis in patients with advanced CHF, irrespective of the rhythm, AF, or sinus rhythm. Thus, in advanced CHF patients, NT-proBNP can be used as marker of prognosis, irrespective of the presence or absence of AF.