-
PDF
- Split View
-
Views
-
Cite
Cite
Nelleke M Korteland, Jonathan R G Etnel, Bardia Arabkhani, M Mostafa Mokhles, Arezo Mohamad, Jolien W Roos-Hesselink, Ad J J C Bogers, Johanna J M Takkenberg, Mechanical aortic valve replacement in non-elderly adults: meta-analysis and microsimulation, European Heart Journal, Volume 38, Issue 45, 01 December 2017, Pages 3370–3377, https://doi.org/10.1093/eurheartj/ehx199
Close - Share Icon Share
Abstract
To support decision-making regarding prosthetic valve selection in non-elderly adults, we aim to provide a detailed overview of outcome after contemporary mechanical aortic valve replacement (AVR).
A systematic review was conducted for papers reporting clinical outcome after AVR with bileaflet mechanical valves with a mean patient age ≥18 and ≤55 years, published between 1 January 1995 and 31 December 2015. Through meta-analysis outcomes were pooled and entered into a microsimulation model to calculate (event-free) life expectancy and lifetime event risk. Twenty-nine publications, encompassing a total of 5728 patients with 32 515 patient-years of follow-up (pooled mean follow-up: 5.7 years), were included. Pooled mean age at surgery was 48.0 years. Pooled early mortality risk was 3.15% (95% confidence interval (CI):2.37–4.23), late mortality rate was 1.55%/year (95%CI:1.25–1.92); 38.7% of late deaths were valve-related. Pooled thromboembolism rate was 0.90%/year (95%CI:0.68–1.21), major bleeding 0.85%/year (95%CI:0.65–1.12), nonstructural valve dysfunction 0.39%/year (95%CI:0.21–0.76), endocarditis 0.41%/year (95%CI:0.29–0.57), valve thrombosis 0.14%/year (95%CI:0.08–0.25), structural valve deterioration 0.00%/year (zero events observed), and reintervention 0.51%/year (95%CI:0.37–0.71), mostly due to nonstructural valve dysfunction and endocarditis. For a 45-year-old, for example, this translated to an estimated life expectancy of 19 years (general population: 34 years) and lifetime risks of thromboembolism, bleeding and reintervention of 18%, 15%, and 10%, respectively.
This study demonstrates that outcome after mechanical AVR in non-elderly adults is characterized by suboptimal survival and considerable lifetime risk of anticoagulation-related complications, but also reoperation. Non-elderly adult patients who are facing prosthetic valve selection are entitled to conveyance of evidence-based estimates of the risks and benefits of both mechanical and biological valve options in a shared decision-making process.
Introduction
Aortic valve replacement (AVR) is the most widely used surgical treatment for aortic valve disease in non-elderly adults. When valve repair is not possible, two types of valve substitutes are available: mechanical and biological valves. The primary advantage of mechanical valves is their durability. They do, however, require lifelong anticoagulation due to their increased thrombogenicity, which gives rise to a substantial risk of thromboembolic and bleeding complications that may have an important impact on quality of life.1 Furthermore, patients are faced with the hassle of INR regulation, the valve sound and, in the case of a woman with pregnancy wishes, the hazards of anticoagulation during pregnancy. Biological valves do not require long-term anticoagulation unless another indication is present. However, they are subject to valve deterioration over time and young patients, in particular, may require a reoperation later in life.2
Since all currently available valve substitutes have important limitations, younger patients who require AVR are facing a difficult choice. A mechanical valve is often recommended in non-elderly adult patients due to the lower, though not absent, rate of reoperation compared with biological valves. Subsequently, most non-elderly adult patients will face a lifelong risk of bleeding and thromboembolic events after their mechanical AVR. To improve decision-making with regard to prosthetic valve selection in non-elderly adults, detailed and up-to-date information on mechanical valve-related morbidity and mortality is required. To gain insight in morbidity and mortality after contemporary mechanical AVR in non-elderly adults, we aim to provide an overview of published evidence by conducting a systematic review and meta-analysis of reported outcome. Furthermore, we aim to estimate age-specific life expectancy and lifetime risk of valve-related events with the use of a microsimulation model based on the results of our meta-analysis.
Methods
This systematic review was conducted according to the PRISMA guidelines.3 This study was approved by the institutional review board and informed consent was waived (MEC-2015-170).
Literature search
On 7 December 2015, a systematic literature search was conducted in Embase, MEDLINE, The Cochrane Collaboration and Web of Science by a biomedical information specialist (see Supplementary material online, S1). All studies were screened by two independent reviewers (NMK, JRGE). Studies reporting survival after contemporary AVR with a mechanical valve in patients with a mean age ≥18 and ≤55 years published in English after 1 January 1995 were considered for inclusion. Studies were included if >90% of the cohort received bileaflet prostheses. Studies limited to patients with pre-existing comorbidities or patients with a history of previous AVR were excluded. Studies with a study size ≤20 patients or focusing only on certain prosthetic valve sizes or multiple valve replacement were also excluded.
In case of overlapping study populations, only the most recent or most complete study was included. In case of disagreement between the reviewers, a consensus was negotiated.
In case a full text publication was not available or information was missing the author was contacted by e-mail.
Data extraction
Microsoft Office Excel (details in Supplementary material online, S5) was used for data extraction. The same pair of reviewers (NMK, JRGE) extracted the data independently. After data extraction, each reviewer verified the other reviewer’s data entries. Recorded study characteristics, baseline patient and operative characteristics and outcome events are listed in Supplementary material online, S5. Morbidity and mortality were documented according to the guidelines.4 Early outcome events were defined as occurring within the first 30 postoperative days, regardless of the patient’s location, and late outcome events were defined as occurring after the first 30 postoperative days. If the total follow-up was not reported, it was calculated by multiplying the number of patients with the mean follow-up duration of that study.
Meta-analysis
Continuous variables are presented as mean ± standard deviation. Categorical variables are presented as counts and percentages. Linearized event occurrence rates are presented as percentages per year.
Pooled baseline patient characteristics were calculated with the use of sample size weighting. Early mortality risk and linearized occurrence rates of late mortality, reoperations and complications after AVR were calculated and pooled with the use of inverse variance weighting on a logarithmic scale, as the Shapiro–Wilk test revealed a significantly skewed distribution among the included studies in the majority of outcome measures. Inverse variance weighting was conducted according to the number of patients for early mortality and according to the number of patient-years of follow-up for late events. In case a particular event was reported not to occur in an individual study, then for the purpose of inverse variance weighting it was assumed that 0.5 patient experienced that event. A random-effects model was used to estimate pooled effects.
The Cochran Q statistic and the I2 test were used to assess heterogeneity. Potential causes of heterogeneity were explored by investigating the effect of year of first inclusion, mean follow-up duration, case mix and study design (retrospective versus prospective/randomized controlled trial) by means of univariable random-effects meta-regression. Funnel plots were used to investigate publication bias. To investigate the potential influence of publication bias on pooled outcome, sensitivity analyses were conducted by temporarily excluding the smallest quartile (by sample size) of included studies. Statistical analyses were performed in Microsoft Office Excel, IBM SPSS Statistics and R (software details are listed in Supplementary material online, S5).
Microsimulation
A microsimulation model based on the pooled outcome estimates of our meta-analysis was used to calculate age-specific life expectancy and lifetime risk of valve-related morbidity.5 , 6 The microsimulation model iteratively simulates individual patient lives after surgery, taking into account the morbidity and mortality events that the patient may experience. The simulated individual patient life histories are then aggregated to obtain estimates of population level outcome. The mortality of a patient is composed of the background mortality of the general population, operative mortality, mortality due to valve-related events and an additional excess mortality component that is not a direct result of valve-related events, but is associated with underlying valve pathology, left ventricular function and other associated pathology.
The operative mortality risk, the occurrence rate of each valve-related event and the risk of mortality and reintervention as a direct result of each of these valve-related events were obtained from our meta-analysis. The occurrence rates of all events were assumed to be linear and non-age-dependent. The hazard ratios of the additional excess mortality not directly resulting from valve-related events have been previously estimated.6 For patients aged 25, 35, 45, and 55, these hazard ratios were 5.5, 4.4, 2.9, and 1.8 for males and 7.0, 7.0, 4.2, and 2.8 for females, respectively. The background mortality of the general population was obtained from the 1996 USA Life Tables, as 1996 was the pooled median year of intervention (assuming a constant incidence rate over time in each study) and the majority of the included study population originated from, or was comparable to the US population.7
To obtain age-specific estimates of life expectancy and lifetime risk of valve-related morbidity, the microsimulation model was run for the ages 25, 35, 45, and 55 years for 10 000 iterations each and separately for males and females. The age-specific outcomes of both genders were then pooled at the male/female ratio obtained from our meta-analysis (72.0% male).
For the purposes of internal validation, the model was additionally run for 10 000 iterations at the pooled mean age (48 years) and pooled male/female ratio of the included studies (72.0% male). The actuarial survival curve obtained from this model was then plotted against the pooled overall mortality observed in our meta-analysis.
Results
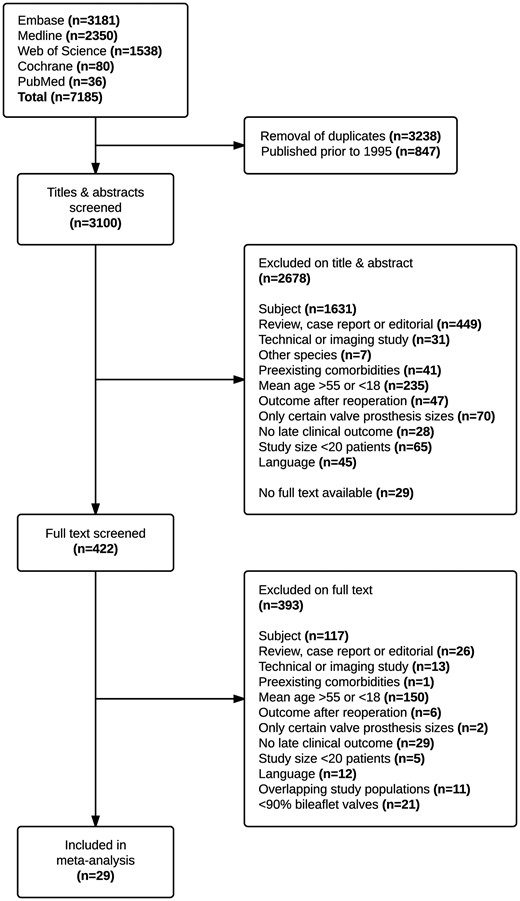
The systematic literature search identified 3100 publications, of which 29 were included in the meta-analysis, encompassing a total of 5728 patients with 32 515 patient-years of follow-up (pooled mean follow-up: 5.7 years) (Figure 1). Supplementary material online, S2 represents the characteristics of the included studies (references listed in Supplementary material online, S6). Pooled baseline patient characteristics are shown in Table 1.
Pooled pre-operative and peri-operative characteristics
| Variable . | . | Pooled data . | Range . | Included studies (n) . |
|---|---|---|---|---|
| Total number of patients | 5728 | 20–865 | 29 | |
| Mean age (years) | 48.0 | 33.0–54.9 | 29 | |
| Gender | Male | 72.0% | 50.0–91.0% | 23 |
| Etiology | Degenerative | 21.5% | 0.0–78.0% | 12 |
| Endocarditis | 10.0% | 0.0–100% | 19 | |
| Rheumatic | 36.4% | 0.0–77.8% | 12 | |
| Congenital | 16.5% | 0.0–57.0% | 10 | |
| Prosthetic valve dysfunction | 3.8% | 0.0–22.0% | 14 | |
| Other/unknown | 11.7% | 0.0–66.0% | 13 | |
| Aortic valve haemodynamics | Stenosis | 43.5% | 0.0–100% | 13 |
| Regurgitation | 40.4% | 0.0–70.0% | 13 | |
| Combined | 16.2% | 0.0–30.0% | 12 | |
| Bicuspid aortic valve | 24.5% | 1.4–100% | 4 | |
| Previous cardiac intervention | 8.4% | 0.0–26.0% | 13 | |
| Emergency surgery | 3.4% | 0.0–35.0% | 10 | |
| Prosthetic valve type | Bileaflet | 99.9% | 96.5–100% | 29 |
| Tilting-disc | 0.1% | 0.0–3.5% | 29 | |
| Caged-ball | 0.0% | 0.0–0.0% | 29 | |
| Concomitant procedures | 22.2% | 0.0–52.2% | 11 | |
| CABG | 7.1% | 0.0–17.5% | 21 | |
| Aortic surgery | 8.6% | 0.0–33.0% | 11 | |
| Multiple valve replacement | 2.6% | 0.0–24.6% | 17 |
| Variable . | . | Pooled data . | Range . | Included studies (n) . |
|---|---|---|---|---|
| Total number of patients | 5728 | 20–865 | 29 | |
| Mean age (years) | 48.0 | 33.0–54.9 | 29 | |
| Gender | Male | 72.0% | 50.0–91.0% | 23 |
| Etiology | Degenerative | 21.5% | 0.0–78.0% | 12 |
| Endocarditis | 10.0% | 0.0–100% | 19 | |
| Rheumatic | 36.4% | 0.0–77.8% | 12 | |
| Congenital | 16.5% | 0.0–57.0% | 10 | |
| Prosthetic valve dysfunction | 3.8% | 0.0–22.0% | 14 | |
| Other/unknown | 11.7% | 0.0–66.0% | 13 | |
| Aortic valve haemodynamics | Stenosis | 43.5% | 0.0–100% | 13 |
| Regurgitation | 40.4% | 0.0–70.0% | 13 | |
| Combined | 16.2% | 0.0–30.0% | 12 | |
| Bicuspid aortic valve | 24.5% | 1.4–100% | 4 | |
| Previous cardiac intervention | 8.4% | 0.0–26.0% | 13 | |
| Emergency surgery | 3.4% | 0.0–35.0% | 10 | |
| Prosthetic valve type | Bileaflet | 99.9% | 96.5–100% | 29 |
| Tilting-disc | 0.1% | 0.0–3.5% | 29 | |
| Caged-ball | 0.0% | 0.0–0.0% | 29 | |
| Concomitant procedures | 22.2% | 0.0–52.2% | 11 | |
| CABG | 7.1% | 0.0–17.5% | 21 | |
| Aortic surgery | 8.6% | 0.0–33.0% | 11 | |
| Multiple valve replacement | 2.6% | 0.0–24.6% | 17 |
CABG, coronary artery bypass grafting.
Pooled pre-operative and peri-operative characteristics
| Variable . | . | Pooled data . | Range . | Included studies (n) . |
|---|---|---|---|---|
| Total number of patients | 5728 | 20–865 | 29 | |
| Mean age (years) | 48.0 | 33.0–54.9 | 29 | |
| Gender | Male | 72.0% | 50.0–91.0% | 23 |
| Etiology | Degenerative | 21.5% | 0.0–78.0% | 12 |
| Endocarditis | 10.0% | 0.0–100% | 19 | |
| Rheumatic | 36.4% | 0.0–77.8% | 12 | |
| Congenital | 16.5% | 0.0–57.0% | 10 | |
| Prosthetic valve dysfunction | 3.8% | 0.0–22.0% | 14 | |
| Other/unknown | 11.7% | 0.0–66.0% | 13 | |
| Aortic valve haemodynamics | Stenosis | 43.5% | 0.0–100% | 13 |
| Regurgitation | 40.4% | 0.0–70.0% | 13 | |
| Combined | 16.2% | 0.0–30.0% | 12 | |
| Bicuspid aortic valve | 24.5% | 1.4–100% | 4 | |
| Previous cardiac intervention | 8.4% | 0.0–26.0% | 13 | |
| Emergency surgery | 3.4% | 0.0–35.0% | 10 | |
| Prosthetic valve type | Bileaflet | 99.9% | 96.5–100% | 29 |
| Tilting-disc | 0.1% | 0.0–3.5% | 29 | |
| Caged-ball | 0.0% | 0.0–0.0% | 29 | |
| Concomitant procedures | 22.2% | 0.0–52.2% | 11 | |
| CABG | 7.1% | 0.0–17.5% | 21 | |
| Aortic surgery | 8.6% | 0.0–33.0% | 11 | |
| Multiple valve replacement | 2.6% | 0.0–24.6% | 17 |
| Variable . | . | Pooled data . | Range . | Included studies (n) . |
|---|---|---|---|---|
| Total number of patients | 5728 | 20–865 | 29 | |
| Mean age (years) | 48.0 | 33.0–54.9 | 29 | |
| Gender | Male | 72.0% | 50.0–91.0% | 23 |
| Etiology | Degenerative | 21.5% | 0.0–78.0% | 12 |
| Endocarditis | 10.0% | 0.0–100% | 19 | |
| Rheumatic | 36.4% | 0.0–77.8% | 12 | |
| Congenital | 16.5% | 0.0–57.0% | 10 | |
| Prosthetic valve dysfunction | 3.8% | 0.0–22.0% | 14 | |
| Other/unknown | 11.7% | 0.0–66.0% | 13 | |
| Aortic valve haemodynamics | Stenosis | 43.5% | 0.0–100% | 13 |
| Regurgitation | 40.4% | 0.0–70.0% | 13 | |
| Combined | 16.2% | 0.0–30.0% | 12 | |
| Bicuspid aortic valve | 24.5% | 1.4–100% | 4 | |
| Previous cardiac intervention | 8.4% | 0.0–26.0% | 13 | |
| Emergency surgery | 3.4% | 0.0–35.0% | 10 | |
| Prosthetic valve type | Bileaflet | 99.9% | 96.5–100% | 29 |
| Tilting-disc | 0.1% | 0.0–3.5% | 29 | |
| Caged-ball | 0.0% | 0.0–0.0% | 29 | |
| Concomitant procedures | 22.2% | 0.0–52.2% | 11 | |
| CABG | 7.1% | 0.0–17.5% | 21 | |
| Aortic surgery | 8.6% | 0.0–33.0% | 11 | |
| Multiple valve replacement | 2.6% | 0.0–24.6% | 17 |
CABG, coronary artery bypass grafting.
Pooled risks of early mortality and early complications and pooled linearized occurrence rates of late mortality and late morbid events are presented in Table 2 (individual study estimates are presented in Supplementary material online, S3).
Pooled risk of early outcome events and linearized occurrence rates of late outcome events obtained from the meta-analysis
| Outcome events . | Pooled estimate . | Heterogeneitya . | Included studies (n) . |
|---|---|---|---|
| Early(<30 days) | |||
| Early mortality(%) | 3.15(2.37–4.21) | I2 = 70%(P < 0.001) | 25 |
| Re-exploration for bleeding(%) | 5.15(2.57–11.81) | I2 = 87%(P < 0.001) | 7 |
| Pacemaker implantation(%) | 3.53(2.47–5.05) | I2 = 20%(P = 0.289) | 4 |
| Deep sternal infection/mediastinitis(%) | 2.48(1.56–3.94) | I2 = 0%(P = 0.409) | 5 |
| Endocarditis(%) | 0.43(0.16–1.13) | I2 = 0%(P = 0.853) | 7 |
| Stroke(%) | 1.55(0.98–2.46) | I2 = 15%(P = 0.312) | 8 |
| Transient ischemic attack(%) | 0.81(0.38–1.72) | I2 = 1%(P = 0.400) | 5 |
| Myocardial infarction(%) | 0.87(0.40–1.87) | I2 = 0%(P = 0.687) | 5 |
| Valve thrombosis(%) | 0.30(0.09–1.05) | I2 = 0%(P = 0.782) | 5 |
| Peripheral bleeding(%) | 0.41(0.15–1.09) | I2 = 0%(P = 0.756) | 7 |
| Late(>30 days) | |||
| Late mortality(%/year) | 1.55(1.25–1.92)c | I2 = 83%(P < 0.001) | 29 |
| Cardiac death(%/year) | 0.95(0.71–1.27) | I2 = 70%(P < 0.001) | 22 |
| Valve-related death(%/year) | 0.60(0.44–0.81) | I2 = 64%(P < 0.001) | 24 |
| SUD(%/year) | 0.37(0.26–0.54) | I2 = 47%(P = 0.011) | 19 |
| Reintervention(%/year) | 0.51(0.37–0.71) | I2 = 47%(P = 0.011) | 20 |
| Thromboembolism(%/year) | 0.90(0.68–1.21)d | I2 = 79%(P < 0.001) | 25 |
| Valve thrombosis(%/year) | 0.14(0.08–0.25) | I2 = 62%(P < 0.001) | 18 |
| Bleeding(%/year) | 0.85(0.65–1.12)d | I2 = 67%(P < 0.001) | 26 |
| SVD(%/year) | 0.00b | – | 15 |
| NSVD(%/year) | 0.39(0.21–0.76) | I2 = 83%(P < 0.001) | 17 |
| Endocarditis(%/year) | 0.41(0.29–0.57) | I2 = 34%(P = 0.072) | 19 |
| Outcome events . | Pooled estimate . | Heterogeneitya . | Included studies (n) . |
|---|---|---|---|
| Early(<30 days) | |||
| Early mortality(%) | 3.15(2.37–4.21) | I2 = 70%(P < 0.001) | 25 |
| Re-exploration for bleeding(%) | 5.15(2.57–11.81) | I2 = 87%(P < 0.001) | 7 |
| Pacemaker implantation(%) | 3.53(2.47–5.05) | I2 = 20%(P = 0.289) | 4 |
| Deep sternal infection/mediastinitis(%) | 2.48(1.56–3.94) | I2 = 0%(P = 0.409) | 5 |
| Endocarditis(%) | 0.43(0.16–1.13) | I2 = 0%(P = 0.853) | 7 |
| Stroke(%) | 1.55(0.98–2.46) | I2 = 15%(P = 0.312) | 8 |
| Transient ischemic attack(%) | 0.81(0.38–1.72) | I2 = 1%(P = 0.400) | 5 |
| Myocardial infarction(%) | 0.87(0.40–1.87) | I2 = 0%(P = 0.687) | 5 |
| Valve thrombosis(%) | 0.30(0.09–1.05) | I2 = 0%(P = 0.782) | 5 |
| Peripheral bleeding(%) | 0.41(0.15–1.09) | I2 = 0%(P = 0.756) | 7 |
| Late(>30 days) | |||
| Late mortality(%/year) | 1.55(1.25–1.92)c | I2 = 83%(P < 0.001) | 29 |
| Cardiac death(%/year) | 0.95(0.71–1.27) | I2 = 70%(P < 0.001) | 22 |
| Valve-related death(%/year) | 0.60(0.44–0.81) | I2 = 64%(P < 0.001) | 24 |
| SUD(%/year) | 0.37(0.26–0.54) | I2 = 47%(P = 0.011) | 19 |
| Reintervention(%/year) | 0.51(0.37–0.71) | I2 = 47%(P = 0.011) | 20 |
| Thromboembolism(%/year) | 0.90(0.68–1.21)d | I2 = 79%(P < 0.001) | 25 |
| Valve thrombosis(%/year) | 0.14(0.08–0.25) | I2 = 62%(P < 0.001) | 18 |
| Bleeding(%/year) | 0.85(0.65–1.12)d | I2 = 67%(P < 0.001) | 26 |
| SVD(%/year) | 0.00b | – | 15 |
| NSVD(%/year) | 0.39(0.21–0.76) | I2 = 83%(P < 0.001) | 17 |
| Endocarditis(%/year) | 0.41(0.29–0.57) | I2 = 34%(P = 0.072) | 19 |
Pooled estimates presented as ‘percentage (95% confidence interval)’.
SUD, sudden unexplained death; SVD, structural valve deterioration; NSVD, nonstructural valve dysfunction.
The reported P-values are the P-values of Cochran’s Q test for heterogeneity.
There were zero events of SVD in the 15 studies that reported this outcome.
The background mortality rate in the age- and gender-matched United States general population for the pooled year of surgery and length of follow-up of our cohort was 0.55%/year.
The background rates of thromboembolism and bleeding events in the age- and gender-matched general population were 0.12%/year and 0.03%/year, respectively (based on the Oxford Vascular Study8).
Pooled risk of early outcome events and linearized occurrence rates of late outcome events obtained from the meta-analysis
| Outcome events . | Pooled estimate . | Heterogeneitya . | Included studies (n) . |
|---|---|---|---|
| Early(<30 days) | |||
| Early mortality(%) | 3.15(2.37–4.21) | I2 = 70%(P < 0.001) | 25 |
| Re-exploration for bleeding(%) | 5.15(2.57–11.81) | I2 = 87%(P < 0.001) | 7 |
| Pacemaker implantation(%) | 3.53(2.47–5.05) | I2 = 20%(P = 0.289) | 4 |
| Deep sternal infection/mediastinitis(%) | 2.48(1.56–3.94) | I2 = 0%(P = 0.409) | 5 |
| Endocarditis(%) | 0.43(0.16–1.13) | I2 = 0%(P = 0.853) | 7 |
| Stroke(%) | 1.55(0.98–2.46) | I2 = 15%(P = 0.312) | 8 |
| Transient ischemic attack(%) | 0.81(0.38–1.72) | I2 = 1%(P = 0.400) | 5 |
| Myocardial infarction(%) | 0.87(0.40–1.87) | I2 = 0%(P = 0.687) | 5 |
| Valve thrombosis(%) | 0.30(0.09–1.05) | I2 = 0%(P = 0.782) | 5 |
| Peripheral bleeding(%) | 0.41(0.15–1.09) | I2 = 0%(P = 0.756) | 7 |
| Late(>30 days) | |||
| Late mortality(%/year) | 1.55(1.25–1.92)c | I2 = 83%(P < 0.001) | 29 |
| Cardiac death(%/year) | 0.95(0.71–1.27) | I2 = 70%(P < 0.001) | 22 |
| Valve-related death(%/year) | 0.60(0.44–0.81) | I2 = 64%(P < 0.001) | 24 |
| SUD(%/year) | 0.37(0.26–0.54) | I2 = 47%(P = 0.011) | 19 |
| Reintervention(%/year) | 0.51(0.37–0.71) | I2 = 47%(P = 0.011) | 20 |
| Thromboembolism(%/year) | 0.90(0.68–1.21)d | I2 = 79%(P < 0.001) | 25 |
| Valve thrombosis(%/year) | 0.14(0.08–0.25) | I2 = 62%(P < 0.001) | 18 |
| Bleeding(%/year) | 0.85(0.65–1.12)d | I2 = 67%(P < 0.001) | 26 |
| SVD(%/year) | 0.00b | – | 15 |
| NSVD(%/year) | 0.39(0.21–0.76) | I2 = 83%(P < 0.001) | 17 |
| Endocarditis(%/year) | 0.41(0.29–0.57) | I2 = 34%(P = 0.072) | 19 |
| Outcome events . | Pooled estimate . | Heterogeneitya . | Included studies (n) . |
|---|---|---|---|
| Early(<30 days) | |||
| Early mortality(%) | 3.15(2.37–4.21) | I2 = 70%(P < 0.001) | 25 |
| Re-exploration for bleeding(%) | 5.15(2.57–11.81) | I2 = 87%(P < 0.001) | 7 |
| Pacemaker implantation(%) | 3.53(2.47–5.05) | I2 = 20%(P = 0.289) | 4 |
| Deep sternal infection/mediastinitis(%) | 2.48(1.56–3.94) | I2 = 0%(P = 0.409) | 5 |
| Endocarditis(%) | 0.43(0.16–1.13) | I2 = 0%(P = 0.853) | 7 |
| Stroke(%) | 1.55(0.98–2.46) | I2 = 15%(P = 0.312) | 8 |
| Transient ischemic attack(%) | 0.81(0.38–1.72) | I2 = 1%(P = 0.400) | 5 |
| Myocardial infarction(%) | 0.87(0.40–1.87) | I2 = 0%(P = 0.687) | 5 |
| Valve thrombosis(%) | 0.30(0.09–1.05) | I2 = 0%(P = 0.782) | 5 |
| Peripheral bleeding(%) | 0.41(0.15–1.09) | I2 = 0%(P = 0.756) | 7 |
| Late(>30 days) | |||
| Late mortality(%/year) | 1.55(1.25–1.92)c | I2 = 83%(P < 0.001) | 29 |
| Cardiac death(%/year) | 0.95(0.71–1.27) | I2 = 70%(P < 0.001) | 22 |
| Valve-related death(%/year) | 0.60(0.44–0.81) | I2 = 64%(P < 0.001) | 24 |
| SUD(%/year) | 0.37(0.26–0.54) | I2 = 47%(P = 0.011) | 19 |
| Reintervention(%/year) | 0.51(0.37–0.71) | I2 = 47%(P = 0.011) | 20 |
| Thromboembolism(%/year) | 0.90(0.68–1.21)d | I2 = 79%(P < 0.001) | 25 |
| Valve thrombosis(%/year) | 0.14(0.08–0.25) | I2 = 62%(P < 0.001) | 18 |
| Bleeding(%/year) | 0.85(0.65–1.12)d | I2 = 67%(P < 0.001) | 26 |
| SVD(%/year) | 0.00b | – | 15 |
| NSVD(%/year) | 0.39(0.21–0.76) | I2 = 83%(P < 0.001) | 17 |
| Endocarditis(%/year) | 0.41(0.29–0.57) | I2 = 34%(P = 0.072) | 19 |
Pooled estimates presented as ‘percentage (95% confidence interval)’.
SUD, sudden unexplained death; SVD, structural valve deterioration; NSVD, nonstructural valve dysfunction.
The reported P-values are the P-values of Cochran’s Q test for heterogeneity.
There were zero events of SVD in the 15 studies that reported this outcome.
The background mortality rate in the age- and gender-matched United States general population for the pooled year of surgery and length of follow-up of our cohort was 0.55%/year.
The background rates of thromboembolism and bleeding events in the age- and gender-matched general population were 0.12%/year and 0.03%/year, respectively (based on the Oxford Vascular Study8).
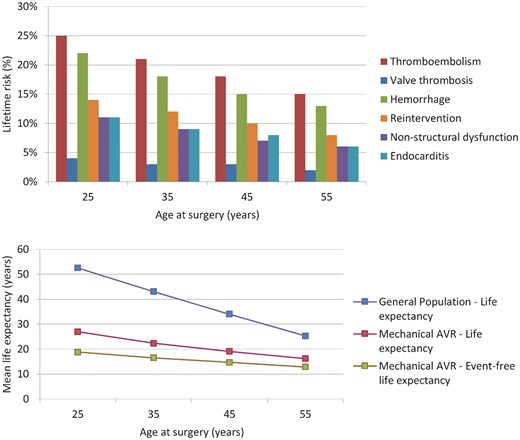
Microsimulation-based age-specific estimates of (event-free) life expectancy and lifetime risk of valve-related morbidity are shown in Figure 2. The microsimulation model calibrated well with the pooled mortality observed in our meta-analysis over the first postoperative decade (see Supplementary material online, S7). For a 45-year-old, for example, microsimulation-based estimated life expectancy was 19 years (general population: 34 years) and lifetime risks of thrombo-embolism, bleeding and reintervention were 18%, 15%, and 10%, respectively.
Microsimulation-based age-specific life expectancy and lifetime risk of valve-related morbidity. AVR, aortic valve replacement.
The funnel plots showed evidence of possible publication bias in early mortality, late mortality, thromboembolism, and bleeding (Supplementary material online, S8). Sensitivity analyses showed that this potential publication bias did not substantially influence our pooled outcomes, as pooled outcomes remained largely unchanged after temporary exclusion of the smallest quartile of studies (before vs. after exclusion: early mortality [3.15% vs. 3.03%], late mortality [1.55%/year vs. 1.55%/year], thromboembolism [0.90%/year vs. 0.88%/year], bleeding rates [0.85%/year vs. 0.87%/year]).
Heterogeneity
There was substantial heterogeneity in early mortality, re-exploration for bleeding and all late outcome measures with the exception of structural valve deterioration (SVD) and endocarditis. Univariable random-effects meta-regression (Supplementary material online, S4) showed that studies with a longer mean follow-up reported lower early mortality (P < 0.001), lower reintervention rates (P = 0.010) and lower bleeding rates (P = 0.042), although follow-up duration was moderately negatively correlated with concomitant CABG (r = −0.37) and earlier year of first inclusion (r = −0.31).
Etiology was another important factor associated with heterogeneity as a higher proportion of pre-operative endocarditis appeared to be correlated with higher rates of late mortality (P = 0.008) and NSVD (P = 0.002), while a higher proportion of rheumatic etiology was associated with lower rates of NSVD (P = 0.004). Bleeding and nonstructural valve dysfunction (NSVD) rates were higher in cohorts with a higher proportion of aortic stenosis (bleeding P = 0.026; NSVD P < 0.001) and, consequently, a lower proportion of aortic regurgitation (bleeding P = 0.003; NSVD P < 0.001), although there was a moderate-to-strong negative correlation between preoperative aortic valve stenosis (as opposed to regurgitation) and etiology (endocarditis r = −0.71; rheumatic r = −0.37). Lastly, higher proportions of emergency surgeries (P = 0.007) and concomitant CABG (P = 0.046) were associated with higher rates of NSVD and a higher proportion of concomitant procedures was associated with higher reported early mortality risk (P = 0.045). We were unable to find any explanatory variables for the heterogeneity in thromboembolism and valve thrombosis rates. Differences in study design, year of first inclusion and previous cardiac interventions were not associated with heterogeneity in any of the outcome measures. Meta-regression was not conducted for re-exploration for bleeding due to limited sample size.
Discussion
This study offers an overview of reported mortality and morbidity after mechanical AVR in non-elderly adult patients and microsimulation-based age-specific estimates of expected lifetime outcome. It confirms the excellent long-term durability of mechanical valves in these patients, but also underlines the substantial late cardiovascular death and anticoagulation-related complication hazards after mechanical AVR. Although no cases of SVD were observed after contemporary AVR with currently available mechanical valves, microsimulation revealed a considerable lifetime risk of reintervention in this subgroup that ranged from 15% for patients aged 25 years at surgery to 8% for 55-year-olds, mostly due to NSVD and endocarditis. Most notably however, the combined lifetime risk of thromboembolism, valve thrombosis and bleeding ranged from 53% for patients aged 25 years at surgery to 30% for 55-year-olds. Life expectancy is substantially impaired in these patients compared with the general population and about 40% of deaths are valve-related.
Mortality
Elective, isolated mechanical AVR has been previously shown to be associated with significant excess mortality when compared with the general age-matched population.9 In our meta-analysis we found a 3.15% early mortality risk and a substantial late mortality rate of 1.55%/year in patients with a pooled mean age of 48.0 years at the time of surgery. Microsimulation-based mean life expectancy after contemporary mechanical AVR ranged from 28 years for patients aged 25 years at surgery to 16 years for 55-year-olds, which is little over half the life expectancy of the age-matched general population. When taking the absent risk of SVD and subsequent reintervention associated with contemporary mechanical AVR into account, this mortality rate appears to be relatively high in comparison with other valve substitutes in non-elderly adults, such as the Ross procedure, which has been reported to be associated with lower late mortality in non-elderly adults compared with our pooled results after contemporary mechanical AVR (0.64%/year vs. 1.55%/year), while early mortality risk was comparable (3.24% vs. 3.15%).10 Prosthetic valve-associated hemodynamic factors, such as prosthesis-patient mismatch, may play a role in this observed excess mortality.11 , 12 Furthermore, the higher mortality after mechanical AVR may be attributable in part to the required anticoagulation treatment. In this regard, optimization of the anticoagulation therapy after mechanical AVR may offer a survival benefit in these patients. This is supported by a recent study by Mokhles et al., which found that, with optimal self-management anticoagulation, mechanical AVR offers excellent late survival, comparable to the general age-matched population and also comparable to patients undergoing the Ross procedure.13
The survival differences between mechanical valves and other valve substitutes may be further explained by possible differences in patient characteristics, surgical technique and concomitant procedures performed at the time of AVR. Rheumatic valve disease being the most common etiology in present study (34% of our patients) may represent evidence of this possible selection bias.
Thromboembolism and bleeding
Present study underlines the burden of thromboembolism and bleeding after mechanical AVR in non-elderly patients as approximately half of patients aged 25 and 1 out of 3 patients aged 55 at the time of surgery are estimated to experience thromboembolism, valve thrombosis or bleeding events during their lifetime. This is most likely an underestimate as the included studies were largely retrospective in design, which may have given rise to recall bias. Anticoagulation-related complications remain an important limitation of mechanical valve prostheses, especially in the young patients in which they are generally used, as there are serious implications for life-, career- and pregnancy-planning in these patients. However, optimizations of the required anticoagulation therapy such as self-management and lower dosing may be promising methods of reducing complication rates after mechanical AVR. There is increasing evidence that patients with contemporary mechanical valves and no comorbidities may be safely managed at a lower INR than currently recommended, subsequently reducing bleeding complications without increasing the risk of thromboembolic events.14–16 Furthermore, advances in the design of mechanical valves may lead to reduced thrombogenicity. Mechanical valves specifically designed with this in mind have emerged, one of which has recently received FDA-approval for anticoagulation management at a lower INR than recommended by the guidelines.16 Nevertheless, we did not find any evidence in this systematic review that thromboembolism and bleeding hazard has decreased in more recent years.
Pharmacological advances that provide more stable INR management may further reduce complication rates as studies have shown that, in patients treated with currently available anticoagulants, 25% of periodically measured INR values lie outside of the target range.14
Reintervention, nonstructuralc valve dysfunction, and endocarditis
Our results underline excellent long-term durability as the main advantage of mechanical valves, with negligible SVD rates. Although SVD remains a rare complication in mechanical valve recipients, depending on age at surgery, approximately 8–15% of patients require reintervention during their lifetime, mostly due to NSVD (pannus formation, paravalvular leakage, etc.), valve thrombosis or prosthetic valve endocarditis. Although this risk of reintervention is very low compared with other valve substitutes in non-elderly adults, it is not absent and should always be taken into consideration and discussed with the patient when prosthetic valve selection is addressed.
Prosthetic valve selection
In prosthetic valve selection, mechanical valve-associated thromboembolism and bleeding risk is generally weighed against the risk of SVD and subsequent reintervention associated with biological valve substitutes. In non-elderly patients a mechanical valve is often recommended due to the limited durability of biological alternatives. However, the durability of modern bioprostheses is improving. These improvements as well as improved outcomes in reoperative aortic valve surgery and the prospect of transcatheter valve-in-valve replacement of failing bioprostheses has led to an increase in their use in younger patients.17–22 Additionally, the Ross procedure represents another valuable option in these patients that avoids the need for long-term anticoagulation and provides superior long-term survival, excellent hemodynamic performance and a low risk of endocarditis in selected patients when performed in centres of expertise. Due to the continued improvements in bioprosthetic AVR and the option of the Ross procedure, the substantial risk of mechanical valve-related complications, as delineated by our results, will become more prominent in the process of prosthetic valve selection. Furthermore, although the risk of reintervention after mechanical AVR is low, it is certainly not absent and should also be taken into consideration in the process of prosthetic valve selection. This also applies to the risk of thromboembolism and bleeding after AVR with biological alternatives. Besides clinical factors, the benefits and limitations of each option have substantial implications for life-, career- and pregnancy planning in these patients. Therefore, conveyance of patient-tailored evidence-based risks and benefits of both mechanical and biological valve options in a shared decision-making process is of great importance.2 , 23 Innovative solutions such as patient information portals and decision aids may prove useful in this setting.24
Heterogeneity
Although heterogeneity was considerable in our meta-analysis and may have potentially influenced the results, we pursued a thorough examination of possible sources of heterogeneity. Etiology and concomitant procedures appear to be important factors of influence on the reported outcomes, which is in line with expectations based on the literature.25 , 26 Furthermore, we found aortic regurgitation vs. stenosis to be associated with more favourable reported outcome with regard to bleeding and NSVD rates, while regurgitation has been previously described to be associated with less favourable outcome.25 This discrepancy may be explained by the strong correlation we found in our meta-regression between aortic valve haemodynamics and etiology (studies with a higher proportion of stenosis had lower proportions of endocarditis and rheumatic etiology), which may have confounded the results.
Lastly, although there was no consistent evidence thereof in our analyses, the year of operation, ranging from 1977–2014 among the included studies, may still have affected the results, as case-mix may have changed over the years and evolution of operative techniques may have led to lower operative risk.
Although this observed heterogeneity might have introduced uncertainty in our meta-analysis, with the use of a random-effects model, this uncertainty is incorporated in the reported pooled outcome estimates.
Publication bias
The asymmetry we found in our funnel plots may represent evidence of possible publication bias. However, assessment of publication bias in absolute risk outcomes, as were all of our outcomes, is associated with substantial methodological limitations which may in itself give rise to funnel plot asymmetry.27 Our funnel plots should therefore be interpreted with caution. Although a conclusive investigation of publication bias may not be possible, our sensitivity analyses show that any potential publication bias did not substantially influence our pooled outcomes.
Limitations
The present study is a systematic review and meta-analysis of observational studies, most of which are retrospective in design. As such, the inherent limitations of meta-analyses and combining data from retrospective observational studies should be taken into consideration.28 Selection bias may have affected the observed outcomes, as unpublished data, abstracts and presentations were not included. Among the included studies, baseline and surgical characteristics were not reported in sufficient detail and consistently enough for us to fully account for all baseline covariates in our meta-analyses. Direct comparisons with alternative valve prostheses are hampered by the lack of published comparative data. Setting a time limit to systematic literature searches may introduce potential bias, but we chose to do so in our aim to provide an overview of contemporary outcome. Finally, there are some limitations to the microsimulation model that should be taken into account. The relationship of the occurrence rates of valve-related events after mechanical AVR with age, follow-up duration and history of previous valve-related events remains poorly defined and could, thus, not be incorporated into our microsimulation model. Uncertainty in the parameters within the model (second order uncertainty) was also not incorporated in our microsimulation model. The model requires assumptions to be made about the evolution of event occurrence rates beyond the observed follow-up period, which may have introduced uncertainty. Our United States general population-based background mortality estimate should be regarded as merely a reference point, as it may not be an ideal reflection of the general population mortality of the different countries that are represented in the individual studies in the review.
Conclusions
This review shows that the use of mechanical valves in non-elderly adult patients is associated with substantial excess mortality over time and considerable lifetime risk of anticoagulation-related complications, but also reoperation. This confirms the fact that non-elderly adult patients who require AVR are facing a difficult choice between mechanical and biological valves and, therefore, conveyance of patient-tailored evidence-based risks and benefits of both mechanical and biological valve options in a shared decision-making process is of great importance in the setting of prosthetic valve selection.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
We would like to thank Gerdien de Jonge (biomedical information specialist, Erasmus University Medical Center) for her assistance with the literature search.
Funding
This work was supported by the Dutch Heart Foundation (2013T093).
Conflict of interest: none declared.
References
Author notes
Presented at the Scientific Meeting of the Heart Valve Society, March 17–19, 2016, New York City, New York, USA.
† Nelleke M. Korteland and Jonathan R.G. Etnel contributed equally to the study.
See page 3378 for the editorial comment on this article (doi: 10.1093/eurheartj/ehx367)
- anticoagulation
- heart valve prosthesis
- endocarditis
- thrombosis
- thromboembolism
- hemorrhage
- aortic valve replacement
- heterogeneity
- adult
- decision making
- follow-up
- life expectancy
- repeat surgery
- surgical procedures, operative
- mortality
- treatment outcome
- older adult
- evidence-based practice
- shared decision making
- mechanical prosthetic aortic valve replacement
- lifetime risk