-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos Collet, Taku Asano, Yosuke Miyazaki, Erhan Tenekecioglu, Yuki Katagiri, Yohei Sotomi, Rafael Cavalcante, Robbert J. de Winter, Takeshi Kimura, Runlin Gao, Serban Puricel, Stéphane Cook, Davide Capodanno, Yoshinobu Onuma, Patrick W. Serruys, Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials, European Heart Journal, Volume 38, Issue 33, 01 September 2017, Pages 2559–2566, https://doi.org/10.1093/eurheartj/ehx155
Close - Share Icon Share
Abstract
To compare the long-term safety and efficacy of bioresorbable vascular scaffold (BVS) with everolimus-eluting stent (EES) after percutaneous coronary interventions.
A systematic review and meta-analysis of randomized clinical trials comparing clinical outcomes of patients treated with BVS and EES with at least 24 months follow-up was performed. Adjusted random-effect model by the Knapp–Hartung method was used to compute odds ratios (OR) and 95% confidence intervals (CI). The primary safety outcome of interest was the risk of definite/probable device thrombosis (DT). The primary efficacy outcome of interest was the risk of target lesion failure (TLF). Five randomized clinical trials (n = 1730) were included. Patients treated with Absorb BVS had a higher risk of definite/probable DT compared with patients treated with EES (OR 2.93, 95%CI 1.37–6.26, P = 0.01). Very late DT (VLDT) occurred in 13 patients [12/996 (1.4%, 95%CI: 0.08–2.5) Absorb BVS vs. 1/701 (0.5%, 95%CI: 0.2–1.6) EES; OR 3.04; 95%CI 1.2–7.68, P = 0.03], 92% of the VLDT in the BVS group occurred in the absence of dual antiplatelet therapy (DAPT). Patients treated with Absorb BVS had a trend towards higher risk of TLF (OR 1.48, 95%CI 0.90–2.42, P = 0.09), driven by a higher risk of target vessel myocardial infarction and ischaemia-driven target lesion revascularization. No difference was found in the risk of cardiac death.
Compared with EES, the use of Absorb BVS was associated with a higher rate of DT and a trend towards higher risk of TLF. VLDT occurred in 1.4% of the patients, the majority of these events occurred in the absence of DAPT.
Introduction
Historically, the occurrence of stent thrombosis (ST) has jeopardized the safety of percutaneous coronary interventions (PCIs). The presence of a metallic device in the coronary artery disrupts laminar flow and creates a prothrombotic environment.1 The use of dual antiplatelet therapy (DAPT), appropriate stent implantation techniques (i.e. post-dilatation with adequate stent expansion), and the advent of drug-eluting stents (DES) have significantly reduced the rate of thrombotic complication following PCI.2–7 Furthermore, after several iterations of DES, clinical outcomes have considerably improved. In contemporary clinical trials, ST rates have been reported in <1% of cases even in all-comer population.8 The reduced strut thickness and biocompatibility and stability of the polymers are likely to be responsible for the improved performance of novel DES. However, despite the low rates of events during the first year after implantation, an unabated rate of target lesion failure (TLF) has been observed at long-term follow-up after DES implantation, thus challenging the durability of the results after PCI.9
Focusing on long-term safety and efficacy, the concept of the bioresorbable scaffold was developed. Early scaffolding and very late resorption was aimed at maintaining efficacy and returning the treated region to the natural anatomical and physiological environment; this would translate into a clinical benefit at long-term follow-up.10 The bioresorbable vascular scaffold (BVS), i.e. Absorb BVS, have been evaluated in six randomized clinical trials comprising 3708 patients.11–16 At 1-year follow-up, patients treated with Absorb BVS have shown non-inferior rates of TLF compared with the fluoropolymer everolimus-eluting stent (EES); however, a higher rate of target vessel myocardial infarction (TVMI) and ST was observed.17 , 18 The promise of the bioresorbable scaffold is to decrease very late (>1 year) device-related events. Therefore, long-term data from randomized clinical trials are awaited and has started to emerge. We sought to compare the long-term safety and efficacy of BVS vs. EES by means of a systematic review and meta-analysis.
Methods
Search strategy and selection criteria
Two independent reviewers (C.C. and T.A.) systematically searched MEDLINE/Embase/CENTRAL applying the search terms ‘bioresorbable’, ‘scaffold’ ‘everolimus-eluting stent(s)’, and ‘randomized trial’. The search was conducted in November 2016. No restrictions were applied concerning language. Data were obtained from full articles in publication and abstracts presented at the Transcatheter Cardiovascular Therapeutics and EuroPCR meetings. The principal investigator for each of the studies included was contacted and requested for additional analyses or follow-up data. We included randomized clinical trials with patients who: (i) underwent PCI for obstructive coronary artery disease; (ii) had at least 24 months clinical follow-up; and (iii) underwent PCI with implantation of Absorb BVS. In the case of multiple publications with the same population, the latest report was prioritized. Studies with inadequate data for abstraction, duplication of data, studies using other bioresorbable scaffolds (polymeric or metallic) were not included. Data were extracted by the same two investigators in agreement with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines Supplementary material online, Table S1.19 Bias assessment was performed using the Cochrane Collaboration’s tool.20
Clinical outcomes
The primary safety outcome of interest was to compare the risk of definite/probable device thrombosis (DT) after BVS and EES implantation. The primary efficacy outcome of interest was the risk of TLF [cardiac death, TVMI, and ischaemia-driven target lesion revascularization (ID-TLR)]. Patient/lesion characteristics and outcome data for TLF, cardiac death, TVMI, and ID-TLR were collected. The longest available follow-up was used for each study. Data on definite and probable DT were extracted from randomized trials according to the time of the event (i.e. acute, <24 h; subacute, 1–30 days; late, 30–365 days; very late, >365 days). Definitions of DT were according to the Academic Research Consortium criteria. Definite DT was defined as angiographic confirmation of DT or pathological confirmation of DT. Probable DT was defined any unexplained death within the first 30 days, irrespective of the time after the index procedure, any MI that is related to documented acute ischaemia in the territory of the implanted device without angiographic confirmation of DT and in the absence of any other obvious cause.21
Statistical analysis
Categorical variables are reported as percentages, and continuous variables are reported as mean ± SD or median (interquartile range) as appropriate. Binary outcomes from individual studies were combined with the random-effects model based on the DerSimonian and Laird method adjusted by the Knapp–Hartung method to compute odds ratios (ORs) with 95% confidence intervals (CIs) that were used for the comparison between BVS and EES.22 , 23 The weighted rate of each event was calculated using the random-effects model. Weighted events are reported with 95% intervals, with standard errors computed using Comprehensive Meta-Analysis Software. I 2 was calculated as a measure of statistical heterogeneity; I 2 values of 25%, 50%, and 75% represented mild, moderate, and severe inconsistency, respectively. Small study or publication bias was explored with funnel plots. All analyses were performed using Comprehensive Meta-Analysis (version 3.3, Englewood, NJ, USA) and RevMan (Review Manager [RevMan] Version 5.3, The Cochrane Collaboration, Copenhagen, Denmark).
Results
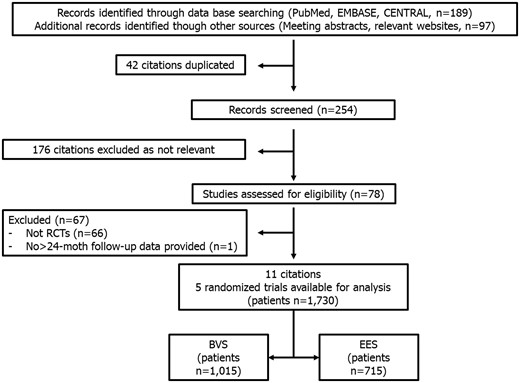
Five randomized clinical trials (n = 1730 patients) comparing outcomes of patients treated with BVS and EES were included (Figure 1).24–28 Patients were randomized to receive PCI with Absorb BVS (Abbott Vascular, Santa Clara, CA, USA; n = 1015) or fluoropolymer–EES (Xience Stent, Abbott Vascular, Santa Clara, CA, USA; n = 635) or platinum–chromium–EES (Promus Element, Boston Scientific, Natick, MA, USA; n = 80). After PCI, P2Y12 inhibitors were prescribed for a period ranging from ≥6 to 12 months, while aspirin was prescribed indefinitely. The median follow-up was 24 months (range 24–36 months). Long-term follow-up data were assessed as full-text articles in two studies and as abstract presentations in three. Bias assessment is reported in the Supplementary material online, Table S2. Baseline patient/lesion characteristics and long-term outcomes are shown in Table 1. Patients included in this analysis had a mean age of 61 ± 3 years, 77% were male, 62% had hypertension, 24% had diabetes mellitus, 33% were smokers, and acute coronary syndrome was the clinical presentation in 48% of the patients. Regarding lesion preparation, pre-dilatation, and post-dilatation were performed in 89% and 54% of the cases, respectively. Long-term follow-up was available in 95% (n = 1642) of the population [94% (950/1015) Absorb BVS vs. 97% (692/715) EES].
Clinical, procedural, and lesion characteristics and clinical outcomes of patients treated included in randomized clinical trials
| . | ABSORB II . | ABSORB Japan . | ABSORB China . | TROFI II . | EVERBIO II . | Overall . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . |
| Patients (n) | 335 | 166 | 266 | 134 | 238 | 237 | 95 | 96 | 78 | 80 | 1012 | 713 |
| Age (years) | 61.5 ± 10.0 | 60.9 ± 10.0 | 67.1 ± 9.4 | 67.3 ± 9.6 | 57.2 ± 11.4 | 57.6 ± 9.6 | 59.1 ± 10.7 | 58.2 ± 9.6 | 65 ± 11 | 65 ± 11 | 61.8 ± 4.2 | 61.4 ± 4.4 |
| Male gender, n (%) | 253 (76) | 132 (80) | 210 (78.9) | 99 (73.9) | 171 (71.8) | 172 (72.6) | 73 (76.8) | 84 (87.5) | 61 (78) | 64 (80) | 768 (75.9) | 551 (77.3) |
| Diabetes, n (%) | 80 (24) | 40 (24) | 96 (36.1) | 48 (35.8) | 60 (25.2) | 55 (23.2) | 18 (18.9) | 14 (14.7) | 17 (22) | 13 (16) | 271 (26.8) | 170 (23.8) |
| Hypertension, n (%) | 231 (69) | 119 (72) | 208 (78.2) | 107 (79.9) | 140 (58.8) | 143 (60.3) | 41 (44.1) | 35 (36.5) | 43 (55) | 51 (64) | 663 (65.5) | 455 (63.8) |
| Smoking, n (%) | 79 (24) | 36 (22) | 53 (19.9) | 29 (21.6) | 78 (32.8) | 84 (35.4) | 46 (48.4) | 47 (49.5) | 28 (36) | 30 (38) | 284 (28.1) | 226 (31.7) |
| ACS at admission, n (%) | 68 (20) | 37 (22) | 26 (9.8) | 22 (16.4) | 154 (64.7) | 152 (64.1) | 95 (100) | 96 (100) | 28 (35.9) | 38 (47.5) | 370 (36.6) | 345 (48.4) |
| ≥2 year clinical follow-up, n (%) | 313 (93.4) | 155 (93.4) | 258 (97.0) | 130 (97.0) | 236 (99.2) | 231 (97.5) | 93 (97.9) | 96 (100) | 77 (98.7) | 80 (100) | 977 (96.5) | 692 (97.1) |
| Lesions | ||||||||||||
| Number | 364 | 182 | 275 | 137 | 251 | 252 | 95 | 98 | 96 | 112 | 1080 | 781 |
| Diameter stenosis (%) | 58.90 ± 11.31 | 59.15 ± 11.42 | 64.6 ± 11.2 | 64.7 ± 10.9 | 65.3 ± 0.82 | 64.5 ± 0.82 | 89.5 ± 15.1 | 89.9 ± 15.4 | 81.3 ± 16.2 | 79.78 ± 15.3 | 71.9 ± 12.9 | 71.6 ± 12.8 |
| Reference vessel diameter, mm | 2.59 ± 0.39 | 2.61 ± 0.40 | 2.76 ± 0.42 | 2.85 ± 0.43 | 2.81 ± 0.03 | 2.82 ± 0.03 | 2.86 ± 0.48 | 2.76 ± 0.51 | 2.77 ± 0.60 | 2.39 ± 0.70 | 2.76 ± 0.10 | 2.69 ± 0.19 |
| Length, mm | 13.94 ± 6.65 | 13.40 ± 6.01 | 13.5 ± 5.28 | 13.3 ± 5.52 | 14.1 ± 0.32 | 13.9 ± 0.30 | 12.88 ± 6.94 | 13.41 ± 7.40 | N/A | N/A | 13.61 ± 0.55 | 13.50 ± 0.27 |
| Type B2/C, n (%) | 165 (45.3) | 89 (48.9) | 209 (76) | 104 (74.9) | 188 (68.9) | 181 (71.8) | N/A | N/A | 28 (29) | 39 (35) | 575 (58.3) | 413 (60.5) |
| Pre-dilatation, n (%) | 364 (100) | 180 (99) | 275 (100) | 137 (100) | 250 (99.6) | 247 (98) | 53 (55.8) | 50 (51.0) | 93 (96.9) | 96 (85.7) | 1035 (95.8) | 710 (90.9) |
| Post-dilatation, n (%) | 221 (60.7) | 107 (58.8) | 226 (82.2) | 106 (77.4) | 162/257 (63) | 141/259 (54.4) | 48 (50.5) | 25 (25.5) | 33 (34) | 35 (31) | 690 (63.9) | 414 (53.0) |
| Clinical outcome | ||||||||||||
| Follow-up (months) | 36 | 24 | 24 | 24 | 24 | 24a | ||||||
| Target lesion failure, n (%) | 34 (10.5) | 8 (5) | 19 (7.3) | 5 (3.8) | 10 (4.3) | 11 (4.6) | 3 (3.2) | 3 (3.1) | 16 (20.5) | 13 (16.3) | 82 (8.3) | 40 (5.7) |
| Cardiac death, n (%) | 3 (0.9) | 3 (1.9) | 1 (0.4) | 0 (0) | 1 (0.4) | 3 (1.3) | 1 (1.1) | 0 (0) | 1 (1.3) | 1 (1.3) | 7 (0.7) | 7 (1.0) |
| TVMI, n (%) | 23 (7.1) | 2 (1.2) | 13 (5) | 4 (3.1) | 5 (2.2) | 2 (0.8) | 2 (2.1) | 3 (3.1) | 2 (2.6) | 0 (0) | 45 (4.5) | 11 (1.6) |
| ID-TLR, n (%) | 20 (6.2) | 3 (1.9) | 14 (5.4) | 3 (2.3) | 8 (3.5) | 6 (2.5) | 2 (2.1) | 1 (1) | 11 (14.1) | 8 (10) | 55 (5.6) | 21 (3.0) |
| DT | ||||||||||||
| Definite DT, n (%) | 8 (2.4) | 0 (0) | 8 (3.1) | 1 (0.8) | 1 (0.4) | 0 (0) | 2 (2.1) | 1 (1) | NA | NA | 19 (1.9) | 2 (0.3) |
| Definite/probable DT, n (%) | 9 (2.7) | 0 (0) | 8 (3.1) | 2 (1.5) | 2 (0.8) | 0 (0) | 2 (2.1) | 1 (1) | 1 (1.3) | 0 (0) | 22 (2.2) | 3 (0.4) |
| Early, n (%) | 2 (0.6) | 0 (0) | 3 (1.1) | 1 (0.9) | 1 (0.4) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) | 7 (0.7) | 1 (0.1) |
| Late, n (%) | 1 (0.3) | 0 (0) | 1 (0.4) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) | 3 (0.3) | 1 (0.1) |
| Very late, n (%) | 6 (1.8) | 0 (0) | 4 (1.6) | 0 (0) | 1 (0.4) | 0 (0) | 1 (1.1) | 1 (1) | 0 (0) | 0 (0) | 12 (1.2) | 1 (0.1) |
| . | ABSORB II . | ABSORB Japan . | ABSORB China . | TROFI II . | EVERBIO II . | Overall . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . |
| Patients (n) | 335 | 166 | 266 | 134 | 238 | 237 | 95 | 96 | 78 | 80 | 1012 | 713 |
| Age (years) | 61.5 ± 10.0 | 60.9 ± 10.0 | 67.1 ± 9.4 | 67.3 ± 9.6 | 57.2 ± 11.4 | 57.6 ± 9.6 | 59.1 ± 10.7 | 58.2 ± 9.6 | 65 ± 11 | 65 ± 11 | 61.8 ± 4.2 | 61.4 ± 4.4 |
| Male gender, n (%) | 253 (76) | 132 (80) | 210 (78.9) | 99 (73.9) | 171 (71.8) | 172 (72.6) | 73 (76.8) | 84 (87.5) | 61 (78) | 64 (80) | 768 (75.9) | 551 (77.3) |
| Diabetes, n (%) | 80 (24) | 40 (24) | 96 (36.1) | 48 (35.8) | 60 (25.2) | 55 (23.2) | 18 (18.9) | 14 (14.7) | 17 (22) | 13 (16) | 271 (26.8) | 170 (23.8) |
| Hypertension, n (%) | 231 (69) | 119 (72) | 208 (78.2) | 107 (79.9) | 140 (58.8) | 143 (60.3) | 41 (44.1) | 35 (36.5) | 43 (55) | 51 (64) | 663 (65.5) | 455 (63.8) |
| Smoking, n (%) | 79 (24) | 36 (22) | 53 (19.9) | 29 (21.6) | 78 (32.8) | 84 (35.4) | 46 (48.4) | 47 (49.5) | 28 (36) | 30 (38) | 284 (28.1) | 226 (31.7) |
| ACS at admission, n (%) | 68 (20) | 37 (22) | 26 (9.8) | 22 (16.4) | 154 (64.7) | 152 (64.1) | 95 (100) | 96 (100) | 28 (35.9) | 38 (47.5) | 370 (36.6) | 345 (48.4) |
| ≥2 year clinical follow-up, n (%) | 313 (93.4) | 155 (93.4) | 258 (97.0) | 130 (97.0) | 236 (99.2) | 231 (97.5) | 93 (97.9) | 96 (100) | 77 (98.7) | 80 (100) | 977 (96.5) | 692 (97.1) |
| Lesions | ||||||||||||
| Number | 364 | 182 | 275 | 137 | 251 | 252 | 95 | 98 | 96 | 112 | 1080 | 781 |
| Diameter stenosis (%) | 58.90 ± 11.31 | 59.15 ± 11.42 | 64.6 ± 11.2 | 64.7 ± 10.9 | 65.3 ± 0.82 | 64.5 ± 0.82 | 89.5 ± 15.1 | 89.9 ± 15.4 | 81.3 ± 16.2 | 79.78 ± 15.3 | 71.9 ± 12.9 | 71.6 ± 12.8 |
| Reference vessel diameter, mm | 2.59 ± 0.39 | 2.61 ± 0.40 | 2.76 ± 0.42 | 2.85 ± 0.43 | 2.81 ± 0.03 | 2.82 ± 0.03 | 2.86 ± 0.48 | 2.76 ± 0.51 | 2.77 ± 0.60 | 2.39 ± 0.70 | 2.76 ± 0.10 | 2.69 ± 0.19 |
| Length, mm | 13.94 ± 6.65 | 13.40 ± 6.01 | 13.5 ± 5.28 | 13.3 ± 5.52 | 14.1 ± 0.32 | 13.9 ± 0.30 | 12.88 ± 6.94 | 13.41 ± 7.40 | N/A | N/A | 13.61 ± 0.55 | 13.50 ± 0.27 |
| Type B2/C, n (%) | 165 (45.3) | 89 (48.9) | 209 (76) | 104 (74.9) | 188 (68.9) | 181 (71.8) | N/A | N/A | 28 (29) | 39 (35) | 575 (58.3) | 413 (60.5) |
| Pre-dilatation, n (%) | 364 (100) | 180 (99) | 275 (100) | 137 (100) | 250 (99.6) | 247 (98) | 53 (55.8) | 50 (51.0) | 93 (96.9) | 96 (85.7) | 1035 (95.8) | 710 (90.9) |
| Post-dilatation, n (%) | 221 (60.7) | 107 (58.8) | 226 (82.2) | 106 (77.4) | 162/257 (63) | 141/259 (54.4) | 48 (50.5) | 25 (25.5) | 33 (34) | 35 (31) | 690 (63.9) | 414 (53.0) |
| Clinical outcome | ||||||||||||
| Follow-up (months) | 36 | 24 | 24 | 24 | 24 | 24a | ||||||
| Target lesion failure, n (%) | 34 (10.5) | 8 (5) | 19 (7.3) | 5 (3.8) | 10 (4.3) | 11 (4.6) | 3 (3.2) | 3 (3.1) | 16 (20.5) | 13 (16.3) | 82 (8.3) | 40 (5.7) |
| Cardiac death, n (%) | 3 (0.9) | 3 (1.9) | 1 (0.4) | 0 (0) | 1 (0.4) | 3 (1.3) | 1 (1.1) | 0 (0) | 1 (1.3) | 1 (1.3) | 7 (0.7) | 7 (1.0) |
| TVMI, n (%) | 23 (7.1) | 2 (1.2) | 13 (5) | 4 (3.1) | 5 (2.2) | 2 (0.8) | 2 (2.1) | 3 (3.1) | 2 (2.6) | 0 (0) | 45 (4.5) | 11 (1.6) |
| ID-TLR, n (%) | 20 (6.2) | 3 (1.9) | 14 (5.4) | 3 (2.3) | 8 (3.5) | 6 (2.5) | 2 (2.1) | 1 (1) | 11 (14.1) | 8 (10) | 55 (5.6) | 21 (3.0) |
| DT | ||||||||||||
| Definite DT, n (%) | 8 (2.4) | 0 (0) | 8 (3.1) | 1 (0.8) | 1 (0.4) | 0 (0) | 2 (2.1) | 1 (1) | NA | NA | 19 (1.9) | 2 (0.3) |
| Definite/probable DT, n (%) | 9 (2.7) | 0 (0) | 8 (3.1) | 2 (1.5) | 2 (0.8) | 0 (0) | 2 (2.1) | 1 (1) | 1 (1.3) | 0 (0) | 22 (2.2) | 3 (0.4) |
| Early, n (%) | 2 (0.6) | 0 (0) | 3 (1.1) | 1 (0.9) | 1 (0.4) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) | 7 (0.7) | 1 (0.1) |
| Late, n (%) | 1 (0.3) | 0 (0) | 1 (0.4) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) | 3 (0.3) | 1 (0.1) |
| Very late, n (%) | 6 (1.8) | 0 (0) | 4 (1.6) | 0 (0) | 1 (0.4) | 0 (0) | 1 (1.1) | 1 (1) | 0 (0) | 0 (0) | 12 (1.2) | 1 (0.1) |
Median follow-up.
ACS, Acute coronary syndromes; EES, everolimus-eluting stent; DT, device thrombosis; ID-TLR, ischaemia-driven target lesion revascularization; N/A, not available; TVMI, target vessel myocardial Infarction.
Clinical, procedural, and lesion characteristics and clinical outcomes of patients treated included in randomized clinical trials
| . | ABSORB II . | ABSORB Japan . | ABSORB China . | TROFI II . | EVERBIO II . | Overall . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . |
| Patients (n) | 335 | 166 | 266 | 134 | 238 | 237 | 95 | 96 | 78 | 80 | 1012 | 713 |
| Age (years) | 61.5 ± 10.0 | 60.9 ± 10.0 | 67.1 ± 9.4 | 67.3 ± 9.6 | 57.2 ± 11.4 | 57.6 ± 9.6 | 59.1 ± 10.7 | 58.2 ± 9.6 | 65 ± 11 | 65 ± 11 | 61.8 ± 4.2 | 61.4 ± 4.4 |
| Male gender, n (%) | 253 (76) | 132 (80) | 210 (78.9) | 99 (73.9) | 171 (71.8) | 172 (72.6) | 73 (76.8) | 84 (87.5) | 61 (78) | 64 (80) | 768 (75.9) | 551 (77.3) |
| Diabetes, n (%) | 80 (24) | 40 (24) | 96 (36.1) | 48 (35.8) | 60 (25.2) | 55 (23.2) | 18 (18.9) | 14 (14.7) | 17 (22) | 13 (16) | 271 (26.8) | 170 (23.8) |
| Hypertension, n (%) | 231 (69) | 119 (72) | 208 (78.2) | 107 (79.9) | 140 (58.8) | 143 (60.3) | 41 (44.1) | 35 (36.5) | 43 (55) | 51 (64) | 663 (65.5) | 455 (63.8) |
| Smoking, n (%) | 79 (24) | 36 (22) | 53 (19.9) | 29 (21.6) | 78 (32.8) | 84 (35.4) | 46 (48.4) | 47 (49.5) | 28 (36) | 30 (38) | 284 (28.1) | 226 (31.7) |
| ACS at admission, n (%) | 68 (20) | 37 (22) | 26 (9.8) | 22 (16.4) | 154 (64.7) | 152 (64.1) | 95 (100) | 96 (100) | 28 (35.9) | 38 (47.5) | 370 (36.6) | 345 (48.4) |
| ≥2 year clinical follow-up, n (%) | 313 (93.4) | 155 (93.4) | 258 (97.0) | 130 (97.0) | 236 (99.2) | 231 (97.5) | 93 (97.9) | 96 (100) | 77 (98.7) | 80 (100) | 977 (96.5) | 692 (97.1) |
| Lesions | ||||||||||||
| Number | 364 | 182 | 275 | 137 | 251 | 252 | 95 | 98 | 96 | 112 | 1080 | 781 |
| Diameter stenosis (%) | 58.90 ± 11.31 | 59.15 ± 11.42 | 64.6 ± 11.2 | 64.7 ± 10.9 | 65.3 ± 0.82 | 64.5 ± 0.82 | 89.5 ± 15.1 | 89.9 ± 15.4 | 81.3 ± 16.2 | 79.78 ± 15.3 | 71.9 ± 12.9 | 71.6 ± 12.8 |
| Reference vessel diameter, mm | 2.59 ± 0.39 | 2.61 ± 0.40 | 2.76 ± 0.42 | 2.85 ± 0.43 | 2.81 ± 0.03 | 2.82 ± 0.03 | 2.86 ± 0.48 | 2.76 ± 0.51 | 2.77 ± 0.60 | 2.39 ± 0.70 | 2.76 ± 0.10 | 2.69 ± 0.19 |
| Length, mm | 13.94 ± 6.65 | 13.40 ± 6.01 | 13.5 ± 5.28 | 13.3 ± 5.52 | 14.1 ± 0.32 | 13.9 ± 0.30 | 12.88 ± 6.94 | 13.41 ± 7.40 | N/A | N/A | 13.61 ± 0.55 | 13.50 ± 0.27 |
| Type B2/C, n (%) | 165 (45.3) | 89 (48.9) | 209 (76) | 104 (74.9) | 188 (68.9) | 181 (71.8) | N/A | N/A | 28 (29) | 39 (35) | 575 (58.3) | 413 (60.5) |
| Pre-dilatation, n (%) | 364 (100) | 180 (99) | 275 (100) | 137 (100) | 250 (99.6) | 247 (98) | 53 (55.8) | 50 (51.0) | 93 (96.9) | 96 (85.7) | 1035 (95.8) | 710 (90.9) |
| Post-dilatation, n (%) | 221 (60.7) | 107 (58.8) | 226 (82.2) | 106 (77.4) | 162/257 (63) | 141/259 (54.4) | 48 (50.5) | 25 (25.5) | 33 (34) | 35 (31) | 690 (63.9) | 414 (53.0) |
| Clinical outcome | ||||||||||||
| Follow-up (months) | 36 | 24 | 24 | 24 | 24 | 24a | ||||||
| Target lesion failure, n (%) | 34 (10.5) | 8 (5) | 19 (7.3) | 5 (3.8) | 10 (4.3) | 11 (4.6) | 3 (3.2) | 3 (3.1) | 16 (20.5) | 13 (16.3) | 82 (8.3) | 40 (5.7) |
| Cardiac death, n (%) | 3 (0.9) | 3 (1.9) | 1 (0.4) | 0 (0) | 1 (0.4) | 3 (1.3) | 1 (1.1) | 0 (0) | 1 (1.3) | 1 (1.3) | 7 (0.7) | 7 (1.0) |
| TVMI, n (%) | 23 (7.1) | 2 (1.2) | 13 (5) | 4 (3.1) | 5 (2.2) | 2 (0.8) | 2 (2.1) | 3 (3.1) | 2 (2.6) | 0 (0) | 45 (4.5) | 11 (1.6) |
| ID-TLR, n (%) | 20 (6.2) | 3 (1.9) | 14 (5.4) | 3 (2.3) | 8 (3.5) | 6 (2.5) | 2 (2.1) | 1 (1) | 11 (14.1) | 8 (10) | 55 (5.6) | 21 (3.0) |
| DT | ||||||||||||
| Definite DT, n (%) | 8 (2.4) | 0 (0) | 8 (3.1) | 1 (0.8) | 1 (0.4) | 0 (0) | 2 (2.1) | 1 (1) | NA | NA | 19 (1.9) | 2 (0.3) |
| Definite/probable DT, n (%) | 9 (2.7) | 0 (0) | 8 (3.1) | 2 (1.5) | 2 (0.8) | 0 (0) | 2 (2.1) | 1 (1) | 1 (1.3) | 0 (0) | 22 (2.2) | 3 (0.4) |
| Early, n (%) | 2 (0.6) | 0 (0) | 3 (1.1) | 1 (0.9) | 1 (0.4) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) | 7 (0.7) | 1 (0.1) |
| Late, n (%) | 1 (0.3) | 0 (0) | 1 (0.4) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) | 3 (0.3) | 1 (0.1) |
| Very late, n (%) | 6 (1.8) | 0 (0) | 4 (1.6) | 0 (0) | 1 (0.4) | 0 (0) | 1 (1.1) | 1 (1) | 0 (0) | 0 (0) | 12 (1.2) | 1 (0.1) |
| . | ABSORB II . | ABSORB Japan . | ABSORB China . | TROFI II . | EVERBIO II . | Overall . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . | BVS . | EES . |
| Patients (n) | 335 | 166 | 266 | 134 | 238 | 237 | 95 | 96 | 78 | 80 | 1012 | 713 |
| Age (years) | 61.5 ± 10.0 | 60.9 ± 10.0 | 67.1 ± 9.4 | 67.3 ± 9.6 | 57.2 ± 11.4 | 57.6 ± 9.6 | 59.1 ± 10.7 | 58.2 ± 9.6 | 65 ± 11 | 65 ± 11 | 61.8 ± 4.2 | 61.4 ± 4.4 |
| Male gender, n (%) | 253 (76) | 132 (80) | 210 (78.9) | 99 (73.9) | 171 (71.8) | 172 (72.6) | 73 (76.8) | 84 (87.5) | 61 (78) | 64 (80) | 768 (75.9) | 551 (77.3) |
| Diabetes, n (%) | 80 (24) | 40 (24) | 96 (36.1) | 48 (35.8) | 60 (25.2) | 55 (23.2) | 18 (18.9) | 14 (14.7) | 17 (22) | 13 (16) | 271 (26.8) | 170 (23.8) |
| Hypertension, n (%) | 231 (69) | 119 (72) | 208 (78.2) | 107 (79.9) | 140 (58.8) | 143 (60.3) | 41 (44.1) | 35 (36.5) | 43 (55) | 51 (64) | 663 (65.5) | 455 (63.8) |
| Smoking, n (%) | 79 (24) | 36 (22) | 53 (19.9) | 29 (21.6) | 78 (32.8) | 84 (35.4) | 46 (48.4) | 47 (49.5) | 28 (36) | 30 (38) | 284 (28.1) | 226 (31.7) |
| ACS at admission, n (%) | 68 (20) | 37 (22) | 26 (9.8) | 22 (16.4) | 154 (64.7) | 152 (64.1) | 95 (100) | 96 (100) | 28 (35.9) | 38 (47.5) | 370 (36.6) | 345 (48.4) |
| ≥2 year clinical follow-up, n (%) | 313 (93.4) | 155 (93.4) | 258 (97.0) | 130 (97.0) | 236 (99.2) | 231 (97.5) | 93 (97.9) | 96 (100) | 77 (98.7) | 80 (100) | 977 (96.5) | 692 (97.1) |
| Lesions | ||||||||||||
| Number | 364 | 182 | 275 | 137 | 251 | 252 | 95 | 98 | 96 | 112 | 1080 | 781 |
| Diameter stenosis (%) | 58.90 ± 11.31 | 59.15 ± 11.42 | 64.6 ± 11.2 | 64.7 ± 10.9 | 65.3 ± 0.82 | 64.5 ± 0.82 | 89.5 ± 15.1 | 89.9 ± 15.4 | 81.3 ± 16.2 | 79.78 ± 15.3 | 71.9 ± 12.9 | 71.6 ± 12.8 |
| Reference vessel diameter, mm | 2.59 ± 0.39 | 2.61 ± 0.40 | 2.76 ± 0.42 | 2.85 ± 0.43 | 2.81 ± 0.03 | 2.82 ± 0.03 | 2.86 ± 0.48 | 2.76 ± 0.51 | 2.77 ± 0.60 | 2.39 ± 0.70 | 2.76 ± 0.10 | 2.69 ± 0.19 |
| Length, mm | 13.94 ± 6.65 | 13.40 ± 6.01 | 13.5 ± 5.28 | 13.3 ± 5.52 | 14.1 ± 0.32 | 13.9 ± 0.30 | 12.88 ± 6.94 | 13.41 ± 7.40 | N/A | N/A | 13.61 ± 0.55 | 13.50 ± 0.27 |
| Type B2/C, n (%) | 165 (45.3) | 89 (48.9) | 209 (76) | 104 (74.9) | 188 (68.9) | 181 (71.8) | N/A | N/A | 28 (29) | 39 (35) | 575 (58.3) | 413 (60.5) |
| Pre-dilatation, n (%) | 364 (100) | 180 (99) | 275 (100) | 137 (100) | 250 (99.6) | 247 (98) | 53 (55.8) | 50 (51.0) | 93 (96.9) | 96 (85.7) | 1035 (95.8) | 710 (90.9) |
| Post-dilatation, n (%) | 221 (60.7) | 107 (58.8) | 226 (82.2) | 106 (77.4) | 162/257 (63) | 141/259 (54.4) | 48 (50.5) | 25 (25.5) | 33 (34) | 35 (31) | 690 (63.9) | 414 (53.0) |
| Clinical outcome | ||||||||||||
| Follow-up (months) | 36 | 24 | 24 | 24 | 24 | 24a | ||||||
| Target lesion failure, n (%) | 34 (10.5) | 8 (5) | 19 (7.3) | 5 (3.8) | 10 (4.3) | 11 (4.6) | 3 (3.2) | 3 (3.1) | 16 (20.5) | 13 (16.3) | 82 (8.3) | 40 (5.7) |
| Cardiac death, n (%) | 3 (0.9) | 3 (1.9) | 1 (0.4) | 0 (0) | 1 (0.4) | 3 (1.3) | 1 (1.1) | 0 (0) | 1 (1.3) | 1 (1.3) | 7 (0.7) | 7 (1.0) |
| TVMI, n (%) | 23 (7.1) | 2 (1.2) | 13 (5) | 4 (3.1) | 5 (2.2) | 2 (0.8) | 2 (2.1) | 3 (3.1) | 2 (2.6) | 0 (0) | 45 (4.5) | 11 (1.6) |
| ID-TLR, n (%) | 20 (6.2) | 3 (1.9) | 14 (5.4) | 3 (2.3) | 8 (3.5) | 6 (2.5) | 2 (2.1) | 1 (1) | 11 (14.1) | 8 (10) | 55 (5.6) | 21 (3.0) |
| DT | ||||||||||||
| Definite DT, n (%) | 8 (2.4) | 0 (0) | 8 (3.1) | 1 (0.8) | 1 (0.4) | 0 (0) | 2 (2.1) | 1 (1) | NA | NA | 19 (1.9) | 2 (0.3) |
| Definite/probable DT, n (%) | 9 (2.7) | 0 (0) | 8 (3.1) | 2 (1.5) | 2 (0.8) | 0 (0) | 2 (2.1) | 1 (1) | 1 (1.3) | 0 (0) | 22 (2.2) | 3 (0.4) |
| Early, n (%) | 2 (0.6) | 0 (0) | 3 (1.1) | 1 (0.9) | 1 (0.4) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) | 0 (0) | 7 (0.7) | 1 (0.1) |
| Late, n (%) | 1 (0.3) | 0 (0) | 1 (0.4) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) | 3 (0.3) | 1 (0.1) |
| Very late, n (%) | 6 (1.8) | 0 (0) | 4 (1.6) | 0 (0) | 1 (0.4) | 0 (0) | 1 (1.1) | 1 (1) | 0 (0) | 0 (0) | 12 (1.2) | 1 (0.1) |
Median follow-up.
ACS, Acute coronary syndromes; EES, everolimus-eluting stent; DT, device thrombosis; ID-TLR, ischaemia-driven target lesion revascularization; N/A, not available; TVMI, target vessel myocardial Infarction.
Flow chart for the trial selection process. RCTs, randomized controlled trials; BVS, bioresorbable vascular scaffold; EES, everolimus-eluting stent.
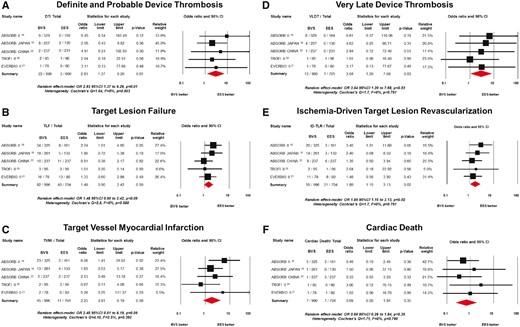
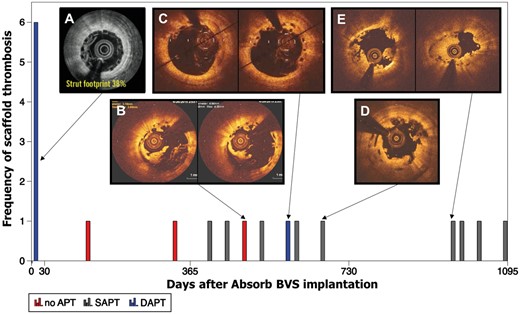
The primary safety outcome of interest of definite/probable DT had occurred in 25 patients [22/996 (2.4%, 95% CI: 1.6–3.7) Absorb BVS vs. 3/701 (0.9%, 95% CI: 0.3–2.1) EES]. Patients treated with Absorb BVS had a higher risk of definite/probable DT compared with patients treated with EES (OR 2.93, 95% CI: 1.37–6.26, P = 0.01; I 2 = 0%; Figure 2A). Twelve thrombotic events occurred during the first year (i.e. acute, subacute, and late DT), whereas very late device thrombosis (VLDT) occurred in 13 patients [12/996 (1.4%, 95% CI: 0.08–2.5) Absorb BVS vs. 1/701 (0.5%, 95% CI: 0.2–1.6) EES; OR 3.04, 95% CI: 1.20–7.68, P = 0.03; Figure 2B]. Clinical, procedural, and outcomes of patients presenting with DT is presented in Supplementary material online, Table S3. From the 22 cases presenting with definite/probable DT in the Absorb arm, the DAPT status was known in 19 cases; seven (37%) of which were on DAPT at the time of the event, whereas nine (47%) were on single antiplatelet therapy with acetyl salicylic acid and three (16%) had interrupted the antiplatelet therapy. Noteworthy, from the 12 patients presenting with VLDT in the Absorb BVS arm, 1 patient was on DAPT. Figure 3 shows the time course and frequency of the definite/probable DT stratified by DAPT status.
Risk estimates of primary outcome of interest at long-term follow-up. Odds ratio (OR) for (A) definite/probable device thrombosis associated with BVS vs. EES, (B) Target lesion failure, (C) target vessel myocardial infarction (D) target lesion failure, (E) ischaemia-driven target lesion revascularization, and (F) cardiac death. The red diamonds indicate the point estimate and the left and the right ends of the lines the 95% confidence interval. BVS, bioresorbable vascular scaffold; EES, everolimus-eluting stent.
Time course and frequency of scaffold thrombosis in randomized clinical trials. The red bars in the histogram represent the patients who had interrupted DAPT, whereas blue bar represents patients on DAPT. The grey pattern represents the patients on acetyl salicylic acid as single-antiplatelet therapy. A corresponding optical coherence tomography imaging finding shows the mechanism underlying DT. In Panel A, a post-implantation coronary optical frequency domain imaging image shows a representative example of under-deployment with a footprint of 38%. Panel B depicts a VLDT case with scaffold discontinuity and fragments protruding to the lumen, malapposition, and uncovered struts. Panel C shows a case of VLDT on DAPT with scaffold discontinuity, malapposition, and uncovered struts. Panel D shows another case of scaffold discontinuity with struts overhanging to the lumen and uncovered struts. Panel E shows a VLDT at 967 days with eccentricity (eccentricity index 0.67) and uncovered struts. Three cases of definite/probable DT were not plotted due to absence of data regarding the time of the event. DAPT, Dual antiplatelet therapy. DT, device thrombosis; VLDT, very late device thrombosis. Reproduced with permission from Onuma et al. 24
The primary efficacy outcome of interest for TLF occurred in 122 patients [82/996 (9.3%, 95% CI: 7.5 to 11.4) Absorb BVS vs. 40/704 (6.6%, 95% CI: 4.8 to 8.8) EES]. Patients treated with Absorb BVS showed a trend towards higher risk of TLF compared with patients treated with EES (OR 1.48, 95% CI: 0.90–2.42, P = 0.09, I 2 = 0%; Figure 2C). In the Absorb BVS group, 55% (45/82) of the TLF events occurred during the early period, whereas 45% (37/82) occurred at late follow-up. This was in sharp contrast to the EES group, where 80% (32/40) occurred in the early period and 20% (8/40) occurred at late follow-up. In patients treated with Absorb BVS, ID-TLR occurred more frequently when compared with EES ID-TLR (OR 1.89, 95% CI: 1.15–3.13, P = 0.02, I 2 = 0%; Figure 2D). No difference was found in the risk of TVMI and cardiac death between Absorb BVS and EES (TVMI OR 2.25, 95% CI 0.81–6.19, P = 0.09, I 2 = 2.5% and cardiac death OR 0.69, 95% CI 0.26–1.84, P = 0.35, I 2 = 0%; Figure 2 E and F).
Discussion
The main findings of this systematic review and meta-analysis can be summarized as follows: (i) a significantly higher risk of definite/probable DT was observed in patients treated with Absorb BVS compared with EES; (ii) very late scaffold thrombosis occurred in 1.4% of the patients treated with Absorb BVS, of which the majority of these events occurred in the absence of DAPT; and (3) compared with EES, Absorb BVS implantation was associated with a higher risk of ID-TLR and a trend towards higher risk of TVMI and TLF.
Four randomized clinical trials with non-complex stable coronary artery disease and two studies including patients with ST-elevation myocardial infarction have been performed to evaluate the safety and effectiveness of Absorb BVS.11–16 The ABSORB III trial, which is powered for clinical events had shown non-inferiority between BVS and EES in the risk of TLF at 1-year follow-up.16 Several meta-analyses performed at 1-year follow-up have suggested an increased risk of TVMI and DT after Absorb BVS implantation.29 , 17 The present meta-analysis extended the period of follow-up and confirmed a significant increase in the risk of TVMI and DT. Moreover, despite an expected long-term benefit with Absorb BVS, an opposite finding of an increased late hazard was found, challenging the concept of long-term benefit accredited to the bioresorbable scaffold. Also, the efficacy of Absorb BVS was inferior to EES, reflected by a higher risk of ID-TLR (OR 1.89, 95% CI 1.15–3.13, P = 0.02).
Scaffold thrombosis appears to have a bimodal distribution over time with one peak at the early period (<30 days) and another after the first year. Following implantation, the thick non-embedded strut may disrupt the laminar flow, create eddies with areas of reversal of the flow behind the struts that have shown to predispose to fibrin deposition and potentially DT.30 , 31 These rheological alterations might be exacerbated by a relative high footprint of the scaffold seen in cases with device/vessel mismatch and under deployment. Several registries have shown that image-guided scaffold implantation is associated with good outcomes.32 , 33 Furthermore, the use of optical coherence tomography during scaffold thrombosis have identified mechanical factors, such as underexpansion, undersizing, and geographical miss as predictors of early DT.32 , 33 Also, post-procedural minimal lumen diameter has been described as the hallmark for DT; therefore, an aggressive implantation strategy with high-pressure post-dilatation with non-compliance balloon and imaging guidance has been advocated to optimize scaffold expansion and reduce early events.34
The occurrence of VLDT was an unexpected finding.35 In a recent meta-analysis of randomized and observational registries including 16 830 patients treated with BVS across the whole spectrum of coronary artery disease, the computed weighted rate of VLDT was 1.0% (95% CI 0.6–1.5%). Within the low-risk population included in randomized trials, 12 cases of VLDT occurred. The incidence of VLDT was 1.4% (95% CI 0.8–2.5%), which represents a three-fold increase in the risk of VLDT compared with EES. The VLDT rate found in the EES groups was 0.5%, which is consistent with previous reports.36 , 37 Several authors have reported scaffold fragment protruding into the lumen (i.e. scaffold discontinuities or dismantling) associated with VLDT.38 However, in the first-in-man study, in almost half of the patients, discontinuities in the scaffold structure were observed without any clinical repercussion.39 In the ABSORB II study, six patients presented with VLDT, in one case optical coherence tomography (OCT) assessment was performed at the time of the event. No structural discontinuities or malaposition was found.25 In the ABSORB Japan trial, OCT was performed in three cases at the time of the VLDT, scaffold discontinuities, malapposition and/or uncovered struts were observed in all cases.24 Also, the presence of neoatheresclerosis, malaposition, late device recoil, and late restenosis have been reported as findings in cases presenting with VLDT.33 , 40 , 41 The increased risk of VLDT observed with Absorb BVS requires careful observation of the long-term outcomes in the ongoing studies and might anticipate the unblinding of the ABSORB III trial.16
The absence of DAPT is the single most important predictor of DT in the first year after PCI.42 , 43 In the population included in this meta-analysis, 10 cases presented with definite/probable DT during the first year, 6 of which were on DAPT, 2 interrupted the antiplatelet therapy, and in 2 cases no data on DAPT were available. Although in some cases, thrombosis may be related to DAPT cessation or absence, events also occurred while being on DAPT during the early period pointing at other factors as the substrate for DT. It remains unclear whether a prolonged course of DAPT would protect the patients treated with Absorb BVS from very late thrombotic events. Notably, 92% of the VLDT occurred in the absence of DAPT. It can therefore be hypothesized that a prolonged DAPT might benefit patients during the bioresorbtion period. Nonetheless, this question requires further investigation and should be addressed in the ongoing clinical trials.
The long-term advantage of avoiding a permanent implant in the coronary artery is still the most reasonable approach to improve late outcomes after PCI. The first generation of Absorb BVS has been found to be associated with an increased risk of DT compared with best-in-class DES. DT was shown to be the driving mechanism for the increased risk of TVMI and ID-TLR assessed in a hierarchical manner in randomized trials. In the next generation of bioresorbable scaffolds, the resorption process should be faster, and in particular, the strut thickness must be reduced. Also, the mechanical properties should be enhanced by improving material tensile strength, stiffness, and ductability, which could be achieved by controlling the composition, crystallinity, and orientation of the polymer. The refinements in the technology in combination with image-guided procedures, might ameliorate clinical outcomes during the first year after implantation where half of the thrombotic events occurred. Nonetheless, the occurrence of very late events, and VLDT, in particular, warrant further investigation to delineate the improvements required in future iterations.
Limitations
The main limitation of the meta-analysis is the lack of individual patient-level data. For that reason, further analysis to identify individual factors associated with DT could not be investigated. The impact of intravascular imaging-guided PCI could not be assessed in this cohort, given the limited number of patients undergoing imaging-guided BVS implantation and the lack of pre-specific intravascular ultrasound (IVUS)/OCT protocol for PCI guidance. The absence of the DAPT status in 3 of 22 patients with ST precluded a complete assessment of the relationship between antiplatelet therapy and thrombotic events. Also, the DAPT status of patients without clinical events was not available. Even though we included all the studies available with long-term follow-up, the sample size of 1730 patients is still underpowered to detect differences in infrequent events such as DT. In addition, due to the small number of randomized controlled trials included in this meta-analysis, no publication bias assessment was performed.
Conclusion
Compared with EES, the use of Absorb BVS was associated with a higher rate of DT and a trend towards higher risk of TLF driven by a higher risk of ID-TLR. Very late scaffold thrombosis occurred in 1.4% of the patients, the majority of these events occurred in the absence of DAPT.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: Y.O. and P.W.S. are members of the International Advisory Board for Abbott Vascular. The other authors have no conflict of interest related to this manuscript.
References