-
PDF
- Split View
-
Views
-
Cite
Cite
Sing-Chien Yap, Jolien W. Roos-Hesselink, Elke S. Hoendermis, Werner Budts, Hubert W. Vliegen, Barbara J.M. Mulder, Arie P.J. van Dijk, Martin J. Schalij, Willem Drenthen, Outcome of implantable cardioverter defibrillators in adults with congenital heart disease: a multi-centre study, European Heart Journal, Volume 28, Issue 15, August 2007, Pages 1854–1861, https://doi.org/10.1093/eurheartj/ehl306
Close - Share Icon Share
Abstract
To investigate outcome and complications of implantable cardioverter defibrillators (ICDs) in adults with congenital heart disease (CHD) and to identify predictors of (in-) appropriate shocks.
Sixty-four CHD patients ≥ 18 years at first ICD implantation [63% tetralogy of Fallot (TOF) and age at implantation 37 ± 13 years] were identified using the Dutch adult CHD registry and a Belgian tertiary care centre database. Median follow-up duration was 3.7 years. Early complications included pocket haematoma (n = 3), lead failure (n = 2), and pneumothorax (n = 2). Late complications occurred in 11 (17%) patients, including lead failure (n = 6) and and electrical storm (n = 3). Overall, 30 device-related re-interventions were performed in 20 patients (31%), including four premature generator changes and seven lead replacements. Half of the patients received one or more shocks, and 46 shocks in 15 patients (23%) were classified as appropriate. One hundred and sixty shocks in 26 patients (41%) were classified as inappropriate. No predictors of (in-)appropriate shocks were identified, except TOF being associated with less appropriate shocks than patients with other CHD (HR 0.29, P = 0.02).
The ICD provided effective therapy in a quarter of adults with CHD with low complication rates. The incidence of inappropriate shocks, however, appeared to be excessive and warrants further attention.
Introduction
Sudden cardiac death (SCD) is the leading cause of mortality in adults with congenital heart disease (CHD), particularly in patients with repaired cyanotic defects and left heart obstructive lesions.1,2 The incidence of SCD has been estimated to be approximately one per 1000 patient-years, which is 25–100 times greater than in the general population.2 Several predictors of late SCD have been proposed, including the occurrence of (non-) sustained ventricular tachyarrhythmias, prolonged QRS duration, increase in QRS duration, older age at repair, and atrial arrhythmias.3,4
Implantable cardioverter defibrillators (ICDs) have emerged as the primary therapeutic option for survivors of SCD and high risk, mainly ischaemic heart disease, patients.5 Information on the outcome of ICD therapy in adults with CHD is limited.6–9 In reports that focus on paediatric CHD ICD recipients, the prior surgical interventions and the complex cardiac anatomy have been shown to complicate the procedure. Furthermore, growth has been related to long-term complications such as lead failure.6 In adults, additional long-term sequelae (i.e. arrhythmias) and residual lesions may decrease the benefit of ICD therapy. Adults with CHD, especially those with tetralogy of Fallot (TOF), are prone to develop supraventricular tachyarrhythmias, which is a well-known cause of inappropriate shocks.10 Inappropriate shocks and the need for re-interventions with their own challenges are important issues in this relatively young and active population. The impact of ICD therapy on the quality of life in this relatively young patient population may warrant specific considerations.
The objective of the present multi-centre study is to investigate complications and outcome of ICD therapy in adults with CHD, including the possible identification of predictors of (in-)appropriate shocks.
Methods
For the present study, all adults with CHD after ICD implantation were identified using the nationwide CONgenital CORvitia (CONCOR) registry in the Netherlands and a Belgian tertiary care centre adult CHD database. Crosscheck with the local ICD registries of the six participating tertiary centres implanting ICDs revealed a total of 83 ICD recipients. After exclusion of patients < 18 years at first implantation and those with additional primary electrical disease or cardiomyopathy, 64 patients with structural CHD were identified. The central medical Ethics Committee in the Netherlands and the local Belgian Ethics Board approved the protocol and informed consent was obtained from all patients.
Baseline data prior to ICD implantation were registered from the patient files including age, gender, height, weight, blood pressure, cardiac diagnosis, surgical procedures, New York Heart Association (NYHA) functional class at the time of implantation, and use of anti-arrhythmic drugs. The last echocardiogram prior to ICD implantation was used to determine pulmonary and systemic ventricular end-diastolic diameter, qualitative ventricular function, degree of right and left ventricular outflow tract obstruction, and severity of subpulmonary and systemic valve regurgitation. The QRS width and QTc interval were measured on the last ECG prior to ICD implantation. The presence of ventricular ectopy and (non-) sustained VT was identified on the last Holter monitoring. Detailed information concerning the ICD implantation were recorded, including index event for ICD, type of ICD, type of leads, defibrillation threshold, duration of intervention, and duration of postoperative stay. Early (intervention-related) and late complications (e.g. pocket haematoma, pleural effusion, lead failure, thrombo-embolic events, pneumothorax, haemothorax, T-wave oversensing, pocket, and other infections) were documented. Follow-up data were obtained from the review of medical records and stored intracardiac electrocardiograms. Appropriate shock was defined as an ICD shock, delivered in response to a ventricular arrhythmia. Inappropriate shock was an ICD shock, delivered for reasons other than ventricular arrhythmia. Outcome data included appropriate shocks, inappropriate shocks, ICD re-interventions, and cardiac and non-cardiac death. The scheduled follow-up differed between centres, but close follow-up (every 3–6 months) is standard after ICD implantation. Median follow-up time was 3.7 years (range: 0.1–13.6).
Statistical analysis
Descriptive statistics for nominal data were expressed in absolute numbers and percentages. After checking for normality, mean values and standard deviations were calculated for normally distributed continuous variables. Medians and ranges were computed for continuous variables with non-normal distribution. Comparison of continuous variables between groups was made by unpaired Student's t-tests. In the case of a skewed distribution, the Mann–Whitney U test was used. When comparing frequencies, the χ2 test or Fisher's exact test was used, where applicable. Cumulative Kaplan–Meier survival curves were constructed for each outcome variable. Patients without an inappropriate or appropriate shock, respectively, were censored at the date of most recent follow-up. Univariable Cox regression analysis was used to evaluate the prognostic significance of the following variables concerning the occurrence of appropriate or inappropriate shocks: age at ICD implantation, gender, body mass index, QRS width >180 ms, history of atrial arrhythmias, TOF, single-chamber ICD device, secondary prophylaxis, positive programmed electrical stimulation (PES), impaired subpulmonary ventricular function, and impaired systemic ventricular function. A separate analysis was performed for the subgroup of TOF patients including additional variables as age at repair, moderate/severe pulmonary regurgitation, and right ventricular dilatation. Hazard ratios (95% CI) are presented. The proportional hazard assumption was checked for each categorical variable through visual inspection of log–log survival curves. For continuous variables, the linearity assumption was checked graphically for all variables using the Martingale residuals. There was no sign of violation of the assumptions. Multi-variable analysis was not attempted because of the low number of events. All tests were two-tailed and a P-value less than 0.05 was considered statistically significant. As there was a high amount of testing, we present exact P-values to show the significance of the findings. All statistics were performed using SPSS version 12.0.
Results
Patient characteristics
The baseline characteristics of the 64 included adults with CHD are summarized in Table 1. The majority of the patients had repaired TOF (n = 40, 63%). In the remaining 24 patients, the following structural defects were present: complete transposition of great arteries after atrial switch operation (d-TGA) (n = 7), corrected double-outlet right ventricle (n = 5), repaired ventricular septal defects (n = 3), congenital aortic regurgitation with aortic valve replacement (n = 2), repaired aortic coarctation (n = 2), atrial septal defect (n = 2, 1 uncorrected), unrepaired double chambered right ventricle (n = 1), congenital pulmonary stenosis with previous balloon dilatation (n = 1), and uncorrected Ebstein malformation (n = 1).
Baseline characteristics of 64 adults with CHD ≥ 18 years at first ICD implantation
| Variable . | Tetralogy of Fallot (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Age at first implantation | 36 ± 10 | 38 ± 17 | 37 ± 13 |
| Male | 24 (60) | 19 (79) | 43 (67) |
| BMI (kg/m2) | 23.7 ± 5.5 | 23.6 ± 3.5 | 23.6 ± 4.8 |
| NYHA functional class | |||
| I | 27 (68) | 18 (75) | 45 (70) |
| II | 10 (25) | 5 (21) | 15 (23) |
| III | 3 (8) | 1 (4) | 4 (6) |
| History of atrial tachyarrhythmias | 12 (30) | 8 (33) | 20 (31) |
| Anti-arrhythmic drug therapy | |||
| Class I | 1 (3) | 2 (8) | 3 (5) |
| Class II | 11 (28) | 4 (17) | 15 (23) |
| Class III | 21 (53) | 9 (38) | 30 (47) |
| Class IV | 3 (8) | 1 (4) | 4 (6) |
| ECG | |||
| Available | 39 (98) | 22 (92) | 61 (95) |
| Sinus rhythm | 34 (87) | 18 (82) | 52 (85) |
| Paced | 4 (10) | 3 (14) | 7 (12) |
| AF | 0 (0) | 1 (3) | 1 (2) |
| Atrial rhythm | 1 (3) | 0 (0) | 1 (2) |
| QRS duration (ms)a | 172 ± 23 | 131 ± 34 | 158 ± 33 |
| QRS duration >180 msa | 10 (29) | 1 (5) | 11 (20) |
| QTc interval (ms)a | 479 ± 50 | 453 ± 34 | 470 ± 46 |
| 24 h Holter | |||
| Performed | 20 (50) | 18 (75) | 38 (59) |
| Mean heart rate (beats/min) | 71 ± 15 | 72 ± 11 | 72 ± 13 |
| Minimum heart rate (b.p.m.) | 51 ± 14 | 45 ± 10 | 48 ± 13 |
| Maximum heart rate (b.p.m.) | 120 ± 24 | 121 ± 26 | 121 ± 24 |
| Documented episodes of NSVT | 6 (30) | 8 (44) | 14 (37) |
| PES | |||
| Performed | 31 (78) | 13 (54) | 44 (69) |
| Negative | 4 (13) | 2 (15) | 6 (14) |
| NSVT | 1 ( 3) | 1 ( 7) | 2 ( 5) |
| SMVT | 21 (68) | 6 (46) | 27 (61) |
| SPVT | 4 (13) | 0 ( 0) | 4 ( 9) |
| VF | 1 ( 3) | 4 (31) | 5 (11) |
| Echocardiogram | |||
| Performed | 40 (100) | 23 (96) | 63 (98) |
| Impaired pulmonary ventricular function | 13 (33) | 7 (30) | 20 (32) |
| Impaired systemic ventricular function | 4 (10) | 7 (30) | 11 (17) |
| Pulmonary regurgitation (≥moderate) | 8 (20) | 1 ( 4) | 9 (14) |
| Variable . | Tetralogy of Fallot (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Age at first implantation | 36 ± 10 | 38 ± 17 | 37 ± 13 |
| Male | 24 (60) | 19 (79) | 43 (67) |
| BMI (kg/m2) | 23.7 ± 5.5 | 23.6 ± 3.5 | 23.6 ± 4.8 |
| NYHA functional class | |||
| I | 27 (68) | 18 (75) | 45 (70) |
| II | 10 (25) | 5 (21) | 15 (23) |
| III | 3 (8) | 1 (4) | 4 (6) |
| History of atrial tachyarrhythmias | 12 (30) | 8 (33) | 20 (31) |
| Anti-arrhythmic drug therapy | |||
| Class I | 1 (3) | 2 (8) | 3 (5) |
| Class II | 11 (28) | 4 (17) | 15 (23) |
| Class III | 21 (53) | 9 (38) | 30 (47) |
| Class IV | 3 (8) | 1 (4) | 4 (6) |
| ECG | |||
| Available | 39 (98) | 22 (92) | 61 (95) |
| Sinus rhythm | 34 (87) | 18 (82) | 52 (85) |
| Paced | 4 (10) | 3 (14) | 7 (12) |
| AF | 0 (0) | 1 (3) | 1 (2) |
| Atrial rhythm | 1 (3) | 0 (0) | 1 (2) |
| QRS duration (ms)a | 172 ± 23 | 131 ± 34 | 158 ± 33 |
| QRS duration >180 msa | 10 (29) | 1 (5) | 11 (20) |
| QTc interval (ms)a | 479 ± 50 | 453 ± 34 | 470 ± 46 |
| 24 h Holter | |||
| Performed | 20 (50) | 18 (75) | 38 (59) |
| Mean heart rate (beats/min) | 71 ± 15 | 72 ± 11 | 72 ± 13 |
| Minimum heart rate (b.p.m.) | 51 ± 14 | 45 ± 10 | 48 ± 13 |
| Maximum heart rate (b.p.m.) | 120 ± 24 | 121 ± 26 | 121 ± 24 |
| Documented episodes of NSVT | 6 (30) | 8 (44) | 14 (37) |
| PES | |||
| Performed | 31 (78) | 13 (54) | 44 (69) |
| Negative | 4 (13) | 2 (15) | 6 (14) |
| NSVT | 1 ( 3) | 1 ( 7) | 2 ( 5) |
| SMVT | 21 (68) | 6 (46) | 27 (61) |
| SPVT | 4 (13) | 0 ( 0) | 4 ( 9) |
| VF | 1 ( 3) | 4 (31) | 5 (11) |
| Echocardiogram | |||
| Performed | 40 (100) | 23 (96) | 63 (98) |
| Impaired pulmonary ventricular function | 13 (33) | 7 (30) | 20 (32) |
| Impaired systemic ventricular function | 4 (10) | 7 (30) | 11 (17) |
| Pulmonary regurgitation (≥moderate) | 8 (20) | 1 ( 4) | 9 (14) |
All data are presented as n (%), unless stated otherwise. BMI, body mass index; NYHA, New York Heart Association; NSVT, non-sustained ventricular tachycardia; SMVT, sustained monomorphic ventricular tachycardia; SPVT, sustained polymorphic ventricular tachycardia; VF, ventricular fibrillation.
aExcluding patients with pacemaker.
Baseline characteristics of 64 adults with CHD ≥ 18 years at first ICD implantation
| Variable . | Tetralogy of Fallot (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Age at first implantation | 36 ± 10 | 38 ± 17 | 37 ± 13 |
| Male | 24 (60) | 19 (79) | 43 (67) |
| BMI (kg/m2) | 23.7 ± 5.5 | 23.6 ± 3.5 | 23.6 ± 4.8 |
| NYHA functional class | |||
| I | 27 (68) | 18 (75) | 45 (70) |
| II | 10 (25) | 5 (21) | 15 (23) |
| III | 3 (8) | 1 (4) | 4 (6) |
| History of atrial tachyarrhythmias | 12 (30) | 8 (33) | 20 (31) |
| Anti-arrhythmic drug therapy | |||
| Class I | 1 (3) | 2 (8) | 3 (5) |
| Class II | 11 (28) | 4 (17) | 15 (23) |
| Class III | 21 (53) | 9 (38) | 30 (47) |
| Class IV | 3 (8) | 1 (4) | 4 (6) |
| ECG | |||
| Available | 39 (98) | 22 (92) | 61 (95) |
| Sinus rhythm | 34 (87) | 18 (82) | 52 (85) |
| Paced | 4 (10) | 3 (14) | 7 (12) |
| AF | 0 (0) | 1 (3) | 1 (2) |
| Atrial rhythm | 1 (3) | 0 (0) | 1 (2) |
| QRS duration (ms)a | 172 ± 23 | 131 ± 34 | 158 ± 33 |
| QRS duration >180 msa | 10 (29) | 1 (5) | 11 (20) |
| QTc interval (ms)a | 479 ± 50 | 453 ± 34 | 470 ± 46 |
| 24 h Holter | |||
| Performed | 20 (50) | 18 (75) | 38 (59) |
| Mean heart rate (beats/min) | 71 ± 15 | 72 ± 11 | 72 ± 13 |
| Minimum heart rate (b.p.m.) | 51 ± 14 | 45 ± 10 | 48 ± 13 |
| Maximum heart rate (b.p.m.) | 120 ± 24 | 121 ± 26 | 121 ± 24 |
| Documented episodes of NSVT | 6 (30) | 8 (44) | 14 (37) |
| PES | |||
| Performed | 31 (78) | 13 (54) | 44 (69) |
| Negative | 4 (13) | 2 (15) | 6 (14) |
| NSVT | 1 ( 3) | 1 ( 7) | 2 ( 5) |
| SMVT | 21 (68) | 6 (46) | 27 (61) |
| SPVT | 4 (13) | 0 ( 0) | 4 ( 9) |
| VF | 1 ( 3) | 4 (31) | 5 (11) |
| Echocardiogram | |||
| Performed | 40 (100) | 23 (96) | 63 (98) |
| Impaired pulmonary ventricular function | 13 (33) | 7 (30) | 20 (32) |
| Impaired systemic ventricular function | 4 (10) | 7 (30) | 11 (17) |
| Pulmonary regurgitation (≥moderate) | 8 (20) | 1 ( 4) | 9 (14) |
| Variable . | Tetralogy of Fallot (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Age at first implantation | 36 ± 10 | 38 ± 17 | 37 ± 13 |
| Male | 24 (60) | 19 (79) | 43 (67) |
| BMI (kg/m2) | 23.7 ± 5.5 | 23.6 ± 3.5 | 23.6 ± 4.8 |
| NYHA functional class | |||
| I | 27 (68) | 18 (75) | 45 (70) |
| II | 10 (25) | 5 (21) | 15 (23) |
| III | 3 (8) | 1 (4) | 4 (6) |
| History of atrial tachyarrhythmias | 12 (30) | 8 (33) | 20 (31) |
| Anti-arrhythmic drug therapy | |||
| Class I | 1 (3) | 2 (8) | 3 (5) |
| Class II | 11 (28) | 4 (17) | 15 (23) |
| Class III | 21 (53) | 9 (38) | 30 (47) |
| Class IV | 3 (8) | 1 (4) | 4 (6) |
| ECG | |||
| Available | 39 (98) | 22 (92) | 61 (95) |
| Sinus rhythm | 34 (87) | 18 (82) | 52 (85) |
| Paced | 4 (10) | 3 (14) | 7 (12) |
| AF | 0 (0) | 1 (3) | 1 (2) |
| Atrial rhythm | 1 (3) | 0 (0) | 1 (2) |
| QRS duration (ms)a | 172 ± 23 | 131 ± 34 | 158 ± 33 |
| QRS duration >180 msa | 10 (29) | 1 (5) | 11 (20) |
| QTc interval (ms)a | 479 ± 50 | 453 ± 34 | 470 ± 46 |
| 24 h Holter | |||
| Performed | 20 (50) | 18 (75) | 38 (59) |
| Mean heart rate (beats/min) | 71 ± 15 | 72 ± 11 | 72 ± 13 |
| Minimum heart rate (b.p.m.) | 51 ± 14 | 45 ± 10 | 48 ± 13 |
| Maximum heart rate (b.p.m.) | 120 ± 24 | 121 ± 26 | 121 ± 24 |
| Documented episodes of NSVT | 6 (30) | 8 (44) | 14 (37) |
| PES | |||
| Performed | 31 (78) | 13 (54) | 44 (69) |
| Negative | 4 (13) | 2 (15) | 6 (14) |
| NSVT | 1 ( 3) | 1 ( 7) | 2 ( 5) |
| SMVT | 21 (68) | 6 (46) | 27 (61) |
| SPVT | 4 (13) | 0 ( 0) | 4 ( 9) |
| VF | 1 ( 3) | 4 (31) | 5 (11) |
| Echocardiogram | |||
| Performed | 40 (100) | 23 (96) | 63 (98) |
| Impaired pulmonary ventricular function | 13 (33) | 7 (30) | 20 (32) |
| Impaired systemic ventricular function | 4 (10) | 7 (30) | 11 (17) |
| Pulmonary regurgitation (≥moderate) | 8 (20) | 1 ( 4) | 9 (14) |
All data are presented as n (%), unless stated otherwise. BMI, body mass index; NYHA, New York Heart Association; NSVT, non-sustained ventricular tachycardia; SMVT, sustained monomorphic ventricular tachycardia; SPVT, sustained polymorphic ventricular tachycardia; VF, ventricular fibrillation.
aExcluding patients with pacemaker.
The index event before ICD implantation was cardiac arrest in 13 (20%), spontaneous sustained VT in 26 (41%), (pre)syncope in 14 (22%), palpitations in four (6%), and other in seven (11%). Detailed data on the sustained monomorphic VT were available in 20 of 26 patients (77%), showing a mean cycle length of 302 ± 75 ms. The sustained monomorphic VT was haemodynamically tolerated in eight of 26 patients (31%).
In the subgroup of TOF patients, the index event was cardiac arrest in six (15%), spontaneous sustained VT in 18 (45%), (pre)syncope in 11 (28%), palpitations in one (3%), and other in four patients (10%). Nine TOF patients (23%) had a relieve of residual haemodynamic lesions just before (n = 7) or after (n = 2) ICD implantation. The following interventions were performed: pulmonary valve replacement (n = 7), balloon dilatation of pulmonary valve (n = 1), and infundibulectomy (n = 1).
PES performed in 44 patients prior to ICD implantation mainly resulted in sustained monomorphic VTs. In 20 of the 25 patients (80%), who did not present with cardiac arrest or spontaneous sustained ventricular tachyarrhythmia, PES was performed. In 18 (90%) of them, sustained ventricular tachyarrhythmias were induced. In the two patients without a positive PES, one had experienced syncope and a spontaneous non-sustained VT documented on Holter registration and the other had a history of presyncope without documented VT. In the five patients who did not have PES, four patients had had spontaneous non-sustained VT [Holter monitoring (n = 3) and telemetry(n = 1)] and the remaining fifth patient had experienced two episodes of unexplained syncope.
In seven patients, at least one ablation procedure to address the monomorphic VT was performed. All were unsuccessful and the attending cardiologists subsequently decided to implant an ICD.
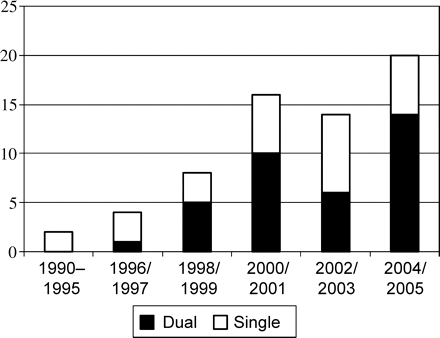
Details on the ICD implantation, complications, and outcome are summarized in Table 2. The devices were implanted between March 1990 and October 2005 (Figure 1). ICDs were manufactured by Medtronic (n = 37), Guidant (n = 19), St Jude Medical (n = 6), and ELA Medical and Biotronik (both n = 1). Almost all ICDs (97%) were implanted in the subclavicular position using standard transvenous techniques. One ICD was implanted in the abdominal position and another in the subclavicular position using epicardial patches. ICD programming was intended to avoid inappropriate therapy and tailored according to the clinical presentation. In 36 patients (56%), one tachycardia detection zone (VF zone: 314 ± 20 ms) was programmed. In 28 patients (44%), the tachycardia detection zones were programmed to recognize fibrillation and either one (n = 24) or two tachycardia zones (n = 4). The programmed fibrillation and tachycardia zones were 288 ± 24 and 346 ± 48 ms, respectively, and antitachycardia pacing (ATP) was activated in 22 of these 28 patients (79%).
Bar graph of the number of adults with CHD undergoing first implantation of ICD per calendar year. Note the steady increase in the number of ICD implantations.
Implantation-related data, complications and outcome of ICD therapy in 64 adults with CHD
| Variable . | TOF (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Median duration of follow-up (years) | 4.0 (0.1–13.6) | 2.8 (0.3–7.3) | 3.7 (0.1–13.6) |
| Type of ICD | |||
| Single chamber | 19 (48) | 9 (38) | 28 (44) |
| Dual chamber | 21 (52) | 15 (62) | 36 (56) |
| Defibrillation threshold testing | |||
| Available | 13 (33) | 21 (88) | 34 (53) |
| Defibrillation threshold (J) | 15 ± 7 | 18 ± 6 | 16 ± 6 |
| Median duration of postoperative stay (days) | 3 (1–25) | 3 (1–50) | 3 (1–50) |
| Early complications | |||
| Pocket haematoma | 3 (8) | 0 (0) | 3 (5) |
| Lead failure | 1 (3) | 1 (4) | 2 (3) |
| Pneumothorax | 2 (5) | 0 (0) | 2 (3) |
| Fever without known cause | 1 (3) | 0 (0) | 1 (2) |
| Late complications | |||
| Lead failure | 4 (10) | 2 (8) | 6 (9) |
| Thrombo-embolic event | 4 (10) | 0 (0) | 4 (6) |
| Electrical storm | 1 (3) | 2 (8) | 3 (5) |
| Infection | 0 (0) | 1 (4) | 1 (2) |
| T-wave oversensing | 1 (3) | 0 (0) | 1 (2) |
| Luxation ICD | 1 (3) | 0 (0) | 1 (2) |
| Appropriate therapy | |||
| Number of patients | 7 (18) | 8 (33) | 15 (23) |
| Median time to first shock (years) | 4.8 (0.4–6.6) | 1.5 (0.0–3.4) | 2.3 (0.0–6.6) |
| Median number of shocks | 1 (1–16) | 2 (1–9) | 1 (1–16) |
| Inappropriate therapy | |||
| Number of patients | 16 (40) | 10 (42) | 26 (41) |
| Median time to first shock (years) | 1.7 (0.0–4.4) | 0.3 (0.0–6.2) | 0.6 (0.0–6.2) |
| Median number of shocks | 4 (1–22) | 4 (1–11) | 4 (1–22) |
| Re-intervention of ICD | 17 (43) | 3 (13) | 20 (31) |
| Anti-arrhythmic drug therapy at last follow-up | |||
| Class I | 1 (3) | 1 (4) | 2 (3) |
| Class II | 17 (43) | 7 (29) | 24 (38) |
| Class III | 16 (40) | 8 (33) | 24 (38) |
| Class IV | 1 (3) | 1 (4) | 2 (3) |
| Variable . | TOF (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Median duration of follow-up (years) | 4.0 (0.1–13.6) | 2.8 (0.3–7.3) | 3.7 (0.1–13.6) |
| Type of ICD | |||
| Single chamber | 19 (48) | 9 (38) | 28 (44) |
| Dual chamber | 21 (52) | 15 (62) | 36 (56) |
| Defibrillation threshold testing | |||
| Available | 13 (33) | 21 (88) | 34 (53) |
| Defibrillation threshold (J) | 15 ± 7 | 18 ± 6 | 16 ± 6 |
| Median duration of postoperative stay (days) | 3 (1–25) | 3 (1–50) | 3 (1–50) |
| Early complications | |||
| Pocket haematoma | 3 (8) | 0 (0) | 3 (5) |
| Lead failure | 1 (3) | 1 (4) | 2 (3) |
| Pneumothorax | 2 (5) | 0 (0) | 2 (3) |
| Fever without known cause | 1 (3) | 0 (0) | 1 (2) |
| Late complications | |||
| Lead failure | 4 (10) | 2 (8) | 6 (9) |
| Thrombo-embolic event | 4 (10) | 0 (0) | 4 (6) |
| Electrical storm | 1 (3) | 2 (8) | 3 (5) |
| Infection | 0 (0) | 1 (4) | 1 (2) |
| T-wave oversensing | 1 (3) | 0 (0) | 1 (2) |
| Luxation ICD | 1 (3) | 0 (0) | 1 (2) |
| Appropriate therapy | |||
| Number of patients | 7 (18) | 8 (33) | 15 (23) |
| Median time to first shock (years) | 4.8 (0.4–6.6) | 1.5 (0.0–3.4) | 2.3 (0.0–6.6) |
| Median number of shocks | 1 (1–16) | 2 (1–9) | 1 (1–16) |
| Inappropriate therapy | |||
| Number of patients | 16 (40) | 10 (42) | 26 (41) |
| Median time to first shock (years) | 1.7 (0.0–4.4) | 0.3 (0.0–6.2) | 0.6 (0.0–6.2) |
| Median number of shocks | 4 (1–22) | 4 (1–11) | 4 (1–22) |
| Re-intervention of ICD | 17 (43) | 3 (13) | 20 (31) |
| Anti-arrhythmic drug therapy at last follow-up | |||
| Class I | 1 (3) | 1 (4) | 2 (3) |
| Class II | 17 (43) | 7 (29) | 24 (38) |
| Class III | 16 (40) | 8 (33) | 24 (38) |
| Class IV | 1 (3) | 1 (4) | 2 (3) |
All data are presented as n (%), unless stated otherwise.
Implantation-related data, complications and outcome of ICD therapy in 64 adults with CHD
| Variable . | TOF (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Median duration of follow-up (years) | 4.0 (0.1–13.6) | 2.8 (0.3–7.3) | 3.7 (0.1–13.6) |
| Type of ICD | |||
| Single chamber | 19 (48) | 9 (38) | 28 (44) |
| Dual chamber | 21 (52) | 15 (62) | 36 (56) |
| Defibrillation threshold testing | |||
| Available | 13 (33) | 21 (88) | 34 (53) |
| Defibrillation threshold (J) | 15 ± 7 | 18 ± 6 | 16 ± 6 |
| Median duration of postoperative stay (days) | 3 (1–25) | 3 (1–50) | 3 (1–50) |
| Early complications | |||
| Pocket haematoma | 3 (8) | 0 (0) | 3 (5) |
| Lead failure | 1 (3) | 1 (4) | 2 (3) |
| Pneumothorax | 2 (5) | 0 (0) | 2 (3) |
| Fever without known cause | 1 (3) | 0 (0) | 1 (2) |
| Late complications | |||
| Lead failure | 4 (10) | 2 (8) | 6 (9) |
| Thrombo-embolic event | 4 (10) | 0 (0) | 4 (6) |
| Electrical storm | 1 (3) | 2 (8) | 3 (5) |
| Infection | 0 (0) | 1 (4) | 1 (2) |
| T-wave oversensing | 1 (3) | 0 (0) | 1 (2) |
| Luxation ICD | 1 (3) | 0 (0) | 1 (2) |
| Appropriate therapy | |||
| Number of patients | 7 (18) | 8 (33) | 15 (23) |
| Median time to first shock (years) | 4.8 (0.4–6.6) | 1.5 (0.0–3.4) | 2.3 (0.0–6.6) |
| Median number of shocks | 1 (1–16) | 2 (1–9) | 1 (1–16) |
| Inappropriate therapy | |||
| Number of patients | 16 (40) | 10 (42) | 26 (41) |
| Median time to first shock (years) | 1.7 (0.0–4.4) | 0.3 (0.0–6.2) | 0.6 (0.0–6.2) |
| Median number of shocks | 4 (1–22) | 4 (1–11) | 4 (1–22) |
| Re-intervention of ICD | 17 (43) | 3 (13) | 20 (31) |
| Anti-arrhythmic drug therapy at last follow-up | |||
| Class I | 1 (3) | 1 (4) | 2 (3) |
| Class II | 17 (43) | 7 (29) | 24 (38) |
| Class III | 16 (40) | 8 (33) | 24 (38) |
| Class IV | 1 (3) | 1 (4) | 2 (3) |
| Variable . | TOF (n = 40) . | Other CHD (n = 24) . | Total (n = 64) . |
|---|---|---|---|
| Median duration of follow-up (years) | 4.0 (0.1–13.6) | 2.8 (0.3–7.3) | 3.7 (0.1–13.6) |
| Type of ICD | |||
| Single chamber | 19 (48) | 9 (38) | 28 (44) |
| Dual chamber | 21 (52) | 15 (62) | 36 (56) |
| Defibrillation threshold testing | |||
| Available | 13 (33) | 21 (88) | 34 (53) |
| Defibrillation threshold (J) | 15 ± 7 | 18 ± 6 | 16 ± 6 |
| Median duration of postoperative stay (days) | 3 (1–25) | 3 (1–50) | 3 (1–50) |
| Early complications | |||
| Pocket haematoma | 3 (8) | 0 (0) | 3 (5) |
| Lead failure | 1 (3) | 1 (4) | 2 (3) |
| Pneumothorax | 2 (5) | 0 (0) | 2 (3) |
| Fever without known cause | 1 (3) | 0 (0) | 1 (2) |
| Late complications | |||
| Lead failure | 4 (10) | 2 (8) | 6 (9) |
| Thrombo-embolic event | 4 (10) | 0 (0) | 4 (6) |
| Electrical storm | 1 (3) | 2 (8) | 3 (5) |
| Infection | 0 (0) | 1 (4) | 1 (2) |
| T-wave oversensing | 1 (3) | 0 (0) | 1 (2) |
| Luxation ICD | 1 (3) | 0 (0) | 1 (2) |
| Appropriate therapy | |||
| Number of patients | 7 (18) | 8 (33) | 15 (23) |
| Median time to first shock (years) | 4.8 (0.4–6.6) | 1.5 (0.0–3.4) | 2.3 (0.0–6.6) |
| Median number of shocks | 1 (1–16) | 2 (1–9) | 1 (1–16) |
| Inappropriate therapy | |||
| Number of patients | 16 (40) | 10 (42) | 26 (41) |
| Median time to first shock (years) | 1.7 (0.0–4.4) | 0.3 (0.0–6.2) | 0.6 (0.0–6.2) |
| Median number of shocks | 4 (1–22) | 4 (1–11) | 4 (1–22) |
| Re-intervention of ICD | 17 (43) | 3 (13) | 20 (31) |
| Anti-arrhythmic drug therapy at last follow-up | |||
| Class I | 1 (3) | 1 (4) | 2 (3) |
| Class II | 17 (43) | 7 (29) | 24 (38) |
| Class III | 16 (40) | 8 (33) | 24 (38) |
| Class IV | 1 (3) | 1 (4) | 2 (3) |
All data are presented as n (%), unless stated otherwise.
Complications and re-interventions
Eight early complications occurred in eight different patients (13%), leading to extended hospital stay or re-hospitalization. The most common early complication was pocket haematoma (n = 3, two patients were on anticoagulation). Other early complications were micro-dislodgement requiring lead repositioning (n = 2), pneumothorax (n = 2), and fever without known cause (n = 1). There was no peri-procedural mortality.
Late complications, excluding inappropriate shocks, occurred in 11 patients (17%). The cumulative rate of late complications was 3, 10, and 28% at 1, 3, and 5 years, respectively. Lead failure, the most prominent complication, was observed in six patients (9%), with a median time to failure of 3.9 years (range 2.1–6.5). No inappropriate therapies were caused by these lead dysfunctions. Thrombo-embolic events occurred in four TOF patients, including two cerebrovascular accidents, one transient ischaemic attack, and one venous occlusion (discussed subsequently). No new-onset atrial fibrillation (AF) was documented in these patients. One patient had a persistent left superior venous caval system connecting to the left atrium, who developed a stroke 4 months after ICD implantation shortly after an attempted closure with an Amplatzer device. Another patient with a history of Wallenberg's syndrome developed a stroke 2 years after implantation. The third patient developed a transient ischaemic attack 4 years after implantation. In the last two patients, no right-left shunting was identified and the cerebrovascular accidents were considered not to be ICD-related.
Thirty device-related re-interventions were performed in 20 patients (31%). The cumulative rate of re-interventions was 0, 14, and 51% at 1, 3, and 5 years, respectively. Four patients had two re-interventions and two patients had four re-interventions. Twenty-five of the 30 re-interventions were ICD replacements performed in 19 patients, including 19 uncomplicated ICD replacements, three upgrades to dual-chamber devices, one upgrade of abdominal device to transvenous single-chamber device, one downgrade to a single-chamber device due to venous occlusion, and one downgrade to a pacemaker (on patient's request). Reasons for ICD replacement were early end of life (<3 years after implantation) in four, late end of life in 16, and device recall in five. In addition, seven lead replacements were necessary in six patients and reposition of the ICD in one patient. Three patients had an ICD replacement and lead replacement during the same procedure. During follow-up, the following complications of re-interventions were observed: pocket haematoma (n = 2) and a high defibrillation threshold needing a high-energy device (n = 1).
Appropriate and inappropriate ICD shocks
During the study interval, 32 of the 64 patients (50%) had at least one shock (88 episodes and 206 shocks). Of the 206 shocks, 46 (22%) in 15 patients (23%) were appropriate, with a median time to first appropriate shock of 2.3 years (range 0.0–6.6) (Table 2). The number of appropriate shocks for the 15 patients ranged from 1 to 16 (median 1). Forty-three shocks were given for spontaneous monomorphic VT (cycle length 291 ± 56 ms), two for VF, and one for spontaneous polymorphic VT. The index events of the subgroup of patients with an appropriate shock during follow-up were cardiac arrest in three, spontaneous sustained VT in five (four haemodynamically not well tolerated), (pre)syncope in four, palpitations in two, and spontaneous non-sustained VT in one. Eleven of these 15 patients (73%) had a PES, which were all positive. Furthermore, 11 of 36 patients (31%) with a positive pre-implant PES experienced an appropriate shock for ventricular arrhythmia.
The majority of shocks, 160 shocks (78%) in 26 patients (41%), was classified as inappropriate with a median time to first inappropriate shock of 0.6 years (range 0.0–6.2). Patients who had inappropriate shocks experienced a median of four inappropriate shocks, with a range of 1–22 shocks. All inappropriate shocks were triggered by supraventricular (including sinus) tachycardias. In response to inappropriate shocks, anti-arrhythmic drugs (mainly beta-blockers) were instituted or increased in 19 of 26 patients (73%). VT/VF detection zones were reset in 10 patients, and algorithms for SVT discrimination and ATP were changed in three and two patients, respectively. In seven of the 64 patients (11%), radio frequency isthmus ablation after ICD implantation was performed, of which five patients had experienced inappropriate shocks. Additional His-bundle ablation during the same hospital stay and redo-isthmus ablation were necessary in two of the seven patients. After ablations, no inappropriate shocks were recorded.
Predictors for appropriate and inappropriate ICD shocks
None of the recorded baseline characteristics, besides the diagnosis of TOF, including well-known risk factors of SCD appeared to predict (in-)appropriate therapies in our patient population (Table 3). The presence of TOF was associated with less appropriate shocks, compared with patients with other congenital heart defects (HR 0.29, 0.10–0.84, P = 0.02). A separate analysis was performed for the subgroup of TOF patients, including additional variables as age at repair, moderate/severe pulmonary regurgitation, and right ventricular dilatation. No predictors of inappropriate or appropriate shocks were detected.
Predictors of appropriate and inappropriate therapies in adults with CHD (n = 64)
| Variable . | Appropriate shock therapy . | Inappropriate shock therapy . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age at ICD implantation | 0.96 (0.92–1.01) | 0.12 | 0.98 (0.95–1.02) | 0.29 |
| Male gender | 1.09 (0.37–3.20) | 0.88 | 1.49 (0.62–3.57) | 0.38 |
| BMI > 25 | 0.43 (0.14–1.34) | 0.14 | 1.23 (0.55–2.75) | 0.61 |
| QRS width >180 ms | 2.00 (0.60–6.70) | 0.26 | 0.77 (0.26–2.34) | 0.65 |
| History of atrial arrhythmias | 0.97 (0.33–2.84) | 0.95 | 1.29 (0.57–2.88) | 0.54 |
| TOF | 0.29 (0.10–0.84) | 0.02 | 0.77 (0.34–1.76) | 0.53 |
| Single-chamber ICD device | 1.68 (0.55–5.14) | 0.36 | 1.90 (0.85–4.25) | 0.12 |
| Secondary prophylaxisa | 0.47 (0.16–1.38) | 0.17 | 1.75 (0.73–4.21) | 0.21 |
| Positive PESb | 1.96 (0.25–15.4) | 0.52 | 0.69 (0.19–2.48) | 0.57 |
| Impaired subpulmonary ventricular function | 1.28 (0.42–3.84) | 0.66 | 1.19 (0.50–2.84) | 0.70 |
| Impaired systemic ventricular function | 2.34 (0.62–8.87) | 0.21 | 1.22 (0.36–4.15) | 0.76 |
| Variable . | Appropriate shock therapy . | Inappropriate shock therapy . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age at ICD implantation | 0.96 (0.92–1.01) | 0.12 | 0.98 (0.95–1.02) | 0.29 |
| Male gender | 1.09 (0.37–3.20) | 0.88 | 1.49 (0.62–3.57) | 0.38 |
| BMI > 25 | 0.43 (0.14–1.34) | 0.14 | 1.23 (0.55–2.75) | 0.61 |
| QRS width >180 ms | 2.00 (0.60–6.70) | 0.26 | 0.77 (0.26–2.34) | 0.65 |
| History of atrial arrhythmias | 0.97 (0.33–2.84) | 0.95 | 1.29 (0.57–2.88) | 0.54 |
| TOF | 0.29 (0.10–0.84) | 0.02 | 0.77 (0.34–1.76) | 0.53 |
| Single-chamber ICD device | 1.68 (0.55–5.14) | 0.36 | 1.90 (0.85–4.25) | 0.12 |
| Secondary prophylaxisa | 0.47 (0.16–1.38) | 0.17 | 1.75 (0.73–4.21) | 0.21 |
| Positive PESb | 1.96 (0.25–15.4) | 0.52 | 0.69 (0.19–2.48) | 0.57 |
| Impaired subpulmonary ventricular function | 1.28 (0.42–3.84) | 0.66 | 1.19 (0.50–2.84) | 0.70 |
| Impaired systemic ventricular function | 2.34 (0.62–8.87) | 0.21 | 1.22 (0.36–4.15) | 0.76 |
aIndex event: cardiac arrest or spontaneous sustained VT.
bCompared with patients with a negative PES.
Predictors of appropriate and inappropriate therapies in adults with CHD (n = 64)
| Variable . | Appropriate shock therapy . | Inappropriate shock therapy . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age at ICD implantation | 0.96 (0.92–1.01) | 0.12 | 0.98 (0.95–1.02) | 0.29 |
| Male gender | 1.09 (0.37–3.20) | 0.88 | 1.49 (0.62–3.57) | 0.38 |
| BMI > 25 | 0.43 (0.14–1.34) | 0.14 | 1.23 (0.55–2.75) | 0.61 |
| QRS width >180 ms | 2.00 (0.60–6.70) | 0.26 | 0.77 (0.26–2.34) | 0.65 |
| History of atrial arrhythmias | 0.97 (0.33–2.84) | 0.95 | 1.29 (0.57–2.88) | 0.54 |
| TOF | 0.29 (0.10–0.84) | 0.02 | 0.77 (0.34–1.76) | 0.53 |
| Single-chamber ICD device | 1.68 (0.55–5.14) | 0.36 | 1.90 (0.85–4.25) | 0.12 |
| Secondary prophylaxisa | 0.47 (0.16–1.38) | 0.17 | 1.75 (0.73–4.21) | 0.21 |
| Positive PESb | 1.96 (0.25–15.4) | 0.52 | 0.69 (0.19–2.48) | 0.57 |
| Impaired subpulmonary ventricular function | 1.28 (0.42–3.84) | 0.66 | 1.19 (0.50–2.84) | 0.70 |
| Impaired systemic ventricular function | 2.34 (0.62–8.87) | 0.21 | 1.22 (0.36–4.15) | 0.76 |
| Variable . | Appropriate shock therapy . | Inappropriate shock therapy . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Age at ICD implantation | 0.96 (0.92–1.01) | 0.12 | 0.98 (0.95–1.02) | 0.29 |
| Male gender | 1.09 (0.37–3.20) | 0.88 | 1.49 (0.62–3.57) | 0.38 |
| BMI > 25 | 0.43 (0.14–1.34) | 0.14 | 1.23 (0.55–2.75) | 0.61 |
| QRS width >180 ms | 2.00 (0.60–6.70) | 0.26 | 0.77 (0.26–2.34) | 0.65 |
| History of atrial arrhythmias | 0.97 (0.33–2.84) | 0.95 | 1.29 (0.57–2.88) | 0.54 |
| TOF | 0.29 (0.10–0.84) | 0.02 | 0.77 (0.34–1.76) | 0.53 |
| Single-chamber ICD device | 1.68 (0.55–5.14) | 0.36 | 1.90 (0.85–4.25) | 0.12 |
| Secondary prophylaxisa | 0.47 (0.16–1.38) | 0.17 | 1.75 (0.73–4.21) | 0.21 |
| Positive PESb | 1.96 (0.25–15.4) | 0.52 | 0.69 (0.19–2.48) | 0.57 |
| Impaired subpulmonary ventricular function | 1.28 (0.42–3.84) | 0.66 | 1.19 (0.50–2.84) | 0.70 |
| Impaired systemic ventricular function | 2.34 (0.62–8.87) | 0.21 | 1.22 (0.36–4.15) | 0.76 |
aIndex event: cardiac arrest or spontaneous sustained VT.
bCompared with patients with a negative PES.
Freedom of (in-)appropriate shocks and overall survival
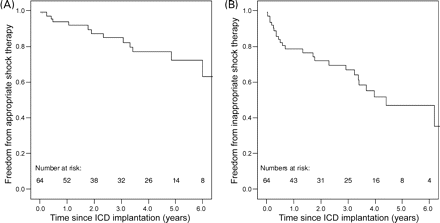
In an attempt to estimate the risk of recurrent SCD, and thus potential impact of ICD on survival, freedom from appropriate shock therapy was estimated (Figure 2). Freedom was 94, 85, 72% at 1, 3, and 5 years, respectively. Freedom from inappropriate shocks, however, was 77, 67, 47% at 1, 3, and 5 years, respectively.
Cumulative Kaplan–Meier survival depicting freedom from appropriate (A) and inappropriate shocks (B) after first ICD implantation. Freedom from appropriate shocks was 94% at 1 year, 87% at 2 years, and 72% at 5 years after ICD implantation. Freedom from inappropriate shocks was 77% at 1 year, 71% at 2 years, and 47% at 5 years after ICD implantation.
Overall survival was 98% at 5 years. One patient with d-TGA and Mustard correction died 6 months after ICD implantation because of septic shock, associated with endocarditis. He received the ICD as a bridge to heart transplantation after experiencing spontaneous non-sustained VT and severe dysfunction of the systemic ventricle.
Discussion
The present, to our knowledge, largest, and first multi-centre study that analysed the outcome of ICD therapy in adults with CHD suggests that this therapy is effective in a quarter of the patients (23%) with low early and late complication rates during a median follow-up of 3.7 years. The number of inappropriate shocks (41%), however, is relatively high, caused by supraventricular tachycardias.
In the face of the increased risk of SCD in CHD patients, the search for predictors of SCD and risk reducing therapy is rational. Silka et al.2 showed in a large CHD cohort (n = 3589) that primarily patients with repaired cyanotic (e.g. TOF and TGA) and left heart obstructive lesions (e.g. corrected aortic stenosis and aortic coarctation) are at risk. The majority of our study patients (72%) had TOF or TGA; however, only 3% had left heart obstructive lesions. No clear explanation for this discrepancy was found. Perhaps, these patients receive ICD therapy at a younger age. This is supported by the survey of the Paediatric Electrophysiology Society reporting left heart obstructive lesions in five of the 22 paediatric patients (23%) with CHD receiving an ICD.11
Most adult SCD research has also focused on TOF and TGA patients. Gatzoulis et al.3 stressed the importance of QRS duration and the annual progression of QRS duration as independent risk factors for the development of sustained VT and sudden death. The early lengthening of the QRS duration after TOF repair results from surgical injury to the right bundle branch and myocardium, whereas later widening reflects right ventricular dilation often due to chronic pulmonary regurgitation.12,13 Other important risk factors are older age at repair, transventricular approach of repair, RV dilatation, and pulmonary regurgitation.3,13–15 Recently, an impaired LV function was identified as an important risk factor.16
After identification of the patients at risk, interventions to reduce the risks need to be searched. Catheter ablation and surgical repair of underlying haemodynamic abnormalities are sound treatment options. Therrien et al.17 have shown that pulmonary valve replacement for pulmonary regurgitation, a well-known long-term sequel in patients with previous TOF repair, leads to stabilization of QRS duration. In conjunction with intra-operative cryo-ablation, it decreased the incidence of pre-existing atrial and ventricular tachyarrhythmia. van Huysduynen et al.18 showed that pulmonary valve replacement even reduces QRS duration and that the amount of QRS reduction is related to the success of the operation, as expressed by the reduction in RV end-diastolic volume.
ICD therapy has proved a welcome addition in the therapeutic arsenal against SCD. Many randomized controlled trials investigating the use of ICDs for primary or secondary prevention have shown to improve survival in a spectrum of patients with ischaemic heart disease. Even without the evidence of randomized controlled trials, there is consensus that SCD survivors with CHD are also candidates for ICD implantation.5 However, no consensus exists regarding prophylactic therapy for adults with CHD who are at lower risk for SCD, such as non-sustained VT, induced VT, or unexplained syncope. In spite of the diagnostic tools and abundance of risk factors, no single predictor of SCD in adults with CHD has been found.
In the present study, we tried to identify potential predictors of appropriate shocks. However, the known risk factors of SCD, including a positive PES, did not predict the occurrence of appropriate shocks. PES was performed in 69% of the patients, with induced sustained ventricular tachyarrhythmias in 82%. In contrast, Khairy et al.19 demonstrated that a positive PES in patients after TOF repair did predict future clinical VT and SCD. It is important to realize that our study population was a selected population at high risk of SCD. Known risk factors for SCD are already weighted in the clinical decision process. This could potentially influence our ability to identify predictors of appropriate shocks.
Our finding that the diagnosis of TOF was associated with less appropriate shocks might imply that the abundance of risk factors described for this subgroup has decreased the threshold to consider ICD therapy in these patients. A decreased threshold could mean that more TOF patients had an ICD as primary prevention. However, this was not the case as the percentage of primary prevention was identical in both groups (TOF vs. other CHD, 40 vs. 38%, P = 1.0). Another explanation could be that the other CHD group might consist of patients at higher risk of SCD. To identify adults with CHD who are at high risk of SCD and profit from ICD therapy, more large-scale, international, cohort studies with long-term follow-up are required for sufficient statistical power to sort out the independent risk factors.4
Despite the complexity of the cardiac anatomy, the associated extra-cardiac malformations, and underdeveloped or obstructed vascular access that may exist in this population, only few early complications (13%) occurred after ICD implantation. Alexander et al.6 reported a similar incidence of early complications. Pocket haematoma was the most frequent (5%) implantation-related complication. In most reports on ICD placement in CHD patients, mainly paediatric patients, early lead dysfunction was a more prominent complication.6,20,21
Lead dysfunction and electrical storms were the most important late complications. A new finding was the relatively frequent occurrence of cerebral vascular events during follow-up. The presence of a right to left shunt could have played a role in one of the three events. To what extent the cerebral vascular events could be attributed to the ICD implantation remains to be elucidated.
Although most re-interventions could be attributed to expected end of life, two of the four patients needing re-intervention for early replacement had received multiple inappropriate shocks (six and eight shocks, respectively). Prevention of inappropriate shocks and improvement of technical capability of the battery may reduce the need for re-interventions, which subsequently may decrease the accompanying complications.
The percentage of patients receiving inappropriate therapy in this study is high (41%), in comparison with other types of cardiovascular disease and even compared with other reports on CHD patients; the majority of articles reports a rate below 25%.6,7,21–23 Most inappropriate shocks were due to atrial arrhythmias, which are known long-term complications in TOF and TGA patients.10,24 This implies that ICD therapy should be combined with therapy directing atrial arrhythmias. Radio frequency ablation has been successfully employed for supraventricular tachycardia and may be of value in selected patients.25 For patients with atrial arrhythmias requiring re-operation, a modified maze procedure should be considered. Furthermore, patients need to be educated that compliance with rate control anti-arrhythmic drugs is essential when they need an ICD. The management of inappropriate shocks in CHD patients poses a unique challenge,9 and every effort should be taken to avoid inappropriate shocks. Moreover, the PainFREE trial has clearly shown that activating ATP for fast VT is effective in 81% of the episodes, thereby significantly reducing the number of appropriate shocks.26 The majority of appropriate shocks in our study cohort was given for VT. Several appropriate shocks could have been prevented by using two tachycardia detection zones with activation of ATP.
ICD therapy was effective, delivering one or more potential lifesaving shock, in 23% of the patients during an average of 3.8 years of follow-up. Interestingly, no difference in the percentage of appropriate shocks was found between patients receiving ICD implantation for primary or secondary prevention (28 vs. 21%, P = 0.49). The percentage of patients receiving appropriate shocks equalled to an average of ∼6% per year of follow-up. This rate is approximately the same as found in patients with hypertrophic obstructive cardiomyopathy (7% per year), where the indication for ICD implantation is more clear.27 Other CHD studies report higher appropriate shock rates ranging from 7.5 up to 22.4% per year of follow-up in a more secondary preventive setting. Appropriate shocks, however, cannot be used as a surrogate mortality endpoint because VT is not invariably fatal and mortality due to bradyarrhythmias is also prevented by an ICD; therefore, care must be taken not to extrapolate the results to a survival benefit.
Study limitations
This study has limitations inherent to any retrospective study. Moreover, this report has the limitations of a limited cohort size and lack of a control group. The small numbers limited our ability to identify predictors of (in-)appropriate therapy. Together with the possible effects of multi-testing, e.g. inflation of type I error, all conclusions of the present study must be drawn with caution.
Conclusions
In summary, ICD therapy proved to be effective in 23% of adults with CHD and was associated with low complication rates. The high incidence of atrial arrhythmias in CHD patients, especially in TOF and d-TGA patients, caused an excessive incidence of inappropriate shocks, with probably high impact on the mental state of this vulnerable patient population, warranting a careful decision process for ICD implantation. A prospective, controlled study with long-term follow-up will be required to definitively establish the benefit of ICD therapy in adults with CHD. The present study does not support a more liberal use of ICDs in CHD patients without covering the problem of inappropriate shocks.
Acknowledgements
The authors would like to thank E. Boersma for his statistical input in the preparation of the manuscript and D.A.M.J. Theuns for his critical review of the manuscript.
Conflict of interest: none declared.
References
Author notes
This paper was guest edited by Prof. Michael A. Gatzoulis, Royal Brompton Hospital, London, UK