-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Serletis, Sarah Rose, Aaron G. Sheldon, Robert S. Sheldon, Vasovagal syncope in medical students and their first-degree relatives, European Heart Journal, Volume 27, Issue 16, August 2006, Pages 1965–1970, https://doi.org/10.1093/eurheartj/ehl147
Close - Share Icon Share
Aims To determine the effect of family history on the likelihood of vasovagal syncope.
Methods and results Sixty-two medical students and 228 first-degree relatives were studied. Vasovagal syncope was ascertained with the Calgary syncope symptom score. The effects of the sex of the subject and parental syncope history on the likelihood of offspring fainting were described using Kaplan–Meier estimates and analysed using proportional hazards regression. The prevalence of vasovagal syncope was 32% and the median age of first faint in those who fainted was 14 years. More females than males fainted [42 vs. 31%; P=0.02; hazard ratio (HR) 1.34 (95% CI 1.07–1.68)]. An individual with two fainting parents was more likely to faint than one with no fainting parents [P<0.0001; HR 3.4 (95% CI 1.7–7.03)]. In the proportional hazards model, offspring of either sex whose mother faints are more likely to faint than those whose mother does not faint [HR 2.86 (95% CI 1.54–5.31)]. Having a father who faints significantly increases the risk of syncope in sons [HR 4.12 (95%CI 1.39–12.31)], but not in daughters [HR 1.18 (95% CI 0.56–3.34)].
Conclusion Family history and sex of subject are important predictors of vasovagal syncope in offspring.
Introduction
Vasovagal syncope is an important clinical problem whose ultimate cause is poorly understood. An exploration of the genetics underlying the trait may improve our understanding of its physiology through eventual identification of the associated gene(s). Few studies have examined the genetic basis of vasovagal syncope. Although they do suggest an association of family history and likelihood of fainting, the studies are limited by lack of controls,1,2 standardized diagnostic criteria,2–6 potential recall bias from probands,4 and a lack of consideration of the effect of its age of onset.2,3,5 The latter may be important, because syncope is usually not expressed until adolescence.5,6
To overcome these limitations, we studied the families of second-year medical students. In such a group, details of syncopal events are likely to be remembered, and they may have time to express the syncopal trait if they possess it.5,6 Medical students often remain close to their families, both geographically and socially. Thus, the direct acquisition of information from first-degree relatives is feasible, allowing us to obviate potential problems with ascertainment and second-hand recall bias. To diagnose vasovagal syncope, we used the Calgary syncope symptom score, a recently developed, brief, validated questionnaire that diagnoses vasovagal syncope with 90% accuracy.7
Methods
The Office of Medical Bioethics of the University of Calgary Faculty of Medicine approved this study. Students were eligible for inclusion if they were registered medical students in the class of 2006 of the University of Calgary. Families of consenting students were then approached to participate. Families and specific family members were excluded if either they or the relevant medical student refused to give informed consent, or if the medical student was a member of a family living overseas.
Recruitment of the medical students and their first-degree family members began on 1 October 2004 and concluded on 10 December 2004. Students were approached individually to obtain consent to contact their family members, and all necessary contact information was obtained from the student. Family members were contacted by telephone, email, and conventional mail.
A brief questionnaire was administered to the consenting subjects and first-degree relatives. Its purposes were to ensure eligibility of subject participation in the study, to determine whether the individual had ever fainted, and to ascertain a diagnosis of vasovagal syncope. We did not capture presyncope in this study, because it lacks the categorical precision of a complete loss of consciousness. The primary inclusion criterion for fainting was a positive response to the question ‘In your lifetime, have you ever fainted?’ The primary exclusion criterion was a positive response to the question ‘Do you have a history of epilepsy?’ A standardized questionnaire, the Calgary syncope symptom score, was administered directly to students and family members. This questionnaire was developed in a study of 418 patients with syncope and no apparent structural heart disease. It was derived from a comparison of prespecified historical characteristics in a population of patients in whom vasovagal syncope was diagnosed with tilt table tests and another population with other known gold standard proven causes of syncope. It has a sensitivity of 89% and a specificity of 91% for vasovagal syncope, after correction with bootstrapping for optimism error. It is part of a larger study of the diagnosis by focused histories of causes of transient loss of consciousness.8 Subjects were diagnosed with vasovagal syncope if they had fainted at least once in their life and had a score of ≥−2 on the Calgary syncope symptom scale. Returned questionnaires were checked for completion, and subjects were contacted again to obtain any missing information.
Power calculations
The sample size was estimated from the results of two previous studies. Kleinknecht and Lenz4 reported that 66% of fainters and 41% of non-fainters had positive family histories for vasovagal syncope. Camfield and Camfield3 found that 90% of fainters and 33% of non-fainters had positive family histories. From these, we estimated that 78% of fainters and 37% of non-fainters would have positive family histories. Using a power of 90% and a confidence level of 95%, a sample size of 58 subjects was calculated.8 Assuming that 25% of medical students and separately 25% of first-degree relatives would refuse to participate, the sample size was increased by a factor of 4/3 for each drop-out estimate. The final estimate was 103, a sample size attainable by approaching the entire University of Calgary medical class of 2006.
Statistical analysis
Results are presented as mean±standard deviation, or medians with interquartile ranges (IQR), where appropriate. The significance of categorical or proportional differences was determined with the χ2 test. The time-dependence of presentation with syncope was estimated using the Kaplan–Meier method. The log-rank test was not used because of the non-independence of observations within families. Instead, a single-predictor proportional hazards model was used to estimate a robust standard error of the regression coefficients, which accounted for this non-independence.9 The proportional hazards model was fitted with the variables offspring sex, fainting mother, and fainting father. The three main effects were first included in the model, then each interaction was added one at a time, and the significance of the interaction assessed using robust Wald statistic9 and retained in the model if P≤0.10. Main effects significant at P≤0.05 were also retained. Significance values are two-sided. No formal adjustments for multiple comparisons were made, as the number of variables available for inclusion in the multivariable model is small and not independent (three main effects and three two-way interactions), and the sample size is small. The number of significance tests was taken into consideration in the interpretation of the results. The assumption of proportional hazards was examined using the test based on Schoenfeld residuals.9 The assumption of linearity of the predictor variables was not relevant, as all variables are binary. The data were analysed using Splus 6.2.
Results
Families
There were 113 medical students and 111 families screened. Of the families screened, 102 were eligible. Reasons for exclusion included a Calgary syncope symptom score of <−2 in the proband (three families) and family members living overseas (six families). Consent was denied by the medical student in 27/102 eligible families. Data from 62 of the remaining 75 families with complete parental information were obtained, and 58 of these families had complete offspring data as well. Reasons for missing family data in the 17 incomplete families included death (three families), unreachable members (seven families), refusal of informed consent by family members (seven families), and members of families with symptom scores <−2 (two families). A total of five offspring were missing. All statistical analysis is based on data from these 62 families. The mean number of individuals per family in the 62 families was 4.7±1.0.
Individuals
There were 142 males (49%) and 148 females (51%) in a total of 290 individuals. The mean age was 39±16 years, with a median age of 32 years (IQR 25–56 years). Ninety-four subjects (32%) had ≥1 faint in their lives. Females were more likely to faint than males: 59 females (40%) fainted, compared with 35 males (25%; P=0.02). The median Calgary syncope symptom score for all eligible individuals was 2 (range −2 to 6). Fainting began at a mean age of 16±9 years in the population of fainters, with a median age of 14 years (IQR 11–19 years). The median number of spells in all fainting subjects was 2 (IQR 1–3).
Parents
There were 62 males and 62 females in a total of 124 parents (Table 1). The mean age at interview was 57±5 years, with a median age of 57 years (IQR 54–59 years). Similar proportions of mothers and fathers had fainted by that age: 23 women (37%) compared with 20 men (32%; P=0.63). The median Calgary syncope symptom score for all fainting parents was 2 (range −2 to 5). The median number of spells in fainting parents was 2 (IQR 1–3 faints).
Demographics of offspring and parents
| . | Offspring . | Parents . |
|---|---|---|
| Total population | ||
| Number | 166 | 124 |
| Male sex (%) | 80 (48) | 62 (50) |
| Fainter (%) | 51 (31) | 43 (35) |
| Age (year) | ||
| Mean | 26±5 | 57±5 |
| Mode | 23 | 57 |
| Median (IQR) | 25 (23–29) | 57 (54–59) |
| Fainting population | ||
| Number of faints | ||
| Mean | 4±10 | 5±12 |
| Median (IQR) | 2 (1–3) | 2 (1–3) |
| Age of fainting onset (year) | ||
| Mean | 15±6 | 18±12 |
| Median (IQR) | 14 (12–18) | 13 (10–25) |
| Syncope symptom score | ||
| Mean | 2±2 | 2±2 |
| Median (IQR) | 2 (1–4) | 2 (0–3) |
| . | Offspring . | Parents . |
|---|---|---|
| Total population | ||
| Number | 166 | 124 |
| Male sex (%) | 80 (48) | 62 (50) |
| Fainter (%) | 51 (31) | 43 (35) |
| Age (year) | ||
| Mean | 26±5 | 57±5 |
| Mode | 23 | 57 |
| Median (IQR) | 25 (23–29) | 57 (54–59) |
| Fainting population | ||
| Number of faints | ||
| Mean | 4±10 | 5±12 |
| Median (IQR) | 2 (1–3) | 2 (1–3) |
| Age of fainting onset (year) | ||
| Mean | 15±6 | 18±12 |
| Median (IQR) | 14 (12–18) | 13 (10–25) |
| Syncope symptom score | ||
| Mean | 2±2 | 2±2 |
| Median (IQR) | 2 (1–4) | 2 (0–3) |
Plus–minus values are means ± SD.
Demographics of offspring and parents
| . | Offspring . | Parents . |
|---|---|---|
| Total population | ||
| Number | 166 | 124 |
| Male sex (%) | 80 (48) | 62 (50) |
| Fainter (%) | 51 (31) | 43 (35) |
| Age (year) | ||
| Mean | 26±5 | 57±5 |
| Mode | 23 | 57 |
| Median (IQR) | 25 (23–29) | 57 (54–59) |
| Fainting population | ||
| Number of faints | ||
| Mean | 4±10 | 5±12 |
| Median (IQR) | 2 (1–3) | 2 (1–3) |
| Age of fainting onset (year) | ||
| Mean | 15±6 | 18±12 |
| Median (IQR) | 14 (12–18) | 13 (10–25) |
| Syncope symptom score | ||
| Mean | 2±2 | 2±2 |
| Median (IQR) | 2 (1–4) | 2 (0–3) |
| . | Offspring . | Parents . |
|---|---|---|
| Total population | ||
| Number | 166 | 124 |
| Male sex (%) | 80 (48) | 62 (50) |
| Fainter (%) | 51 (31) | 43 (35) |
| Age (year) | ||
| Mean | 26±5 | 57±5 |
| Mode | 23 | 57 |
| Median (IQR) | 25 (23–29) | 57 (54–59) |
| Fainting population | ||
| Number of faints | ||
| Mean | 4±10 | 5±12 |
| Median (IQR) | 2 (1–3) | 2 (1–3) |
| Age of fainting onset (year) | ||
| Mean | 15±6 | 18±12 |
| Median (IQR) | 14 (12–18) | 13 (10–25) |
| Syncope symptom score | ||
| Mean | 2±2 | 2±2 |
| Median (IQR) | 2 (1–4) | 2 (0–3) |
Plus–minus values are means ± SD.
Offspring
There were 86 females (52%) and 80 males (48%) in a total of 166 first-degree offspring (Table 1). The mean age at interview was 26±5 years, with a median age of 25 years (IQR 23–29 years). Fifty-one first-degree offspring (31%) had ≥1 fainting episodes by that age. Females were more likely to faint than males: 36 females (42%) had fainted, compared with 15 males (19%; P=0.016, unadjusted for intrafamily correlation). The median Calgary syncope symptom score for fainting offspring was 2 (range −2 to 6). The median number of spells in fainting offspring was 2 (IQR 1–3 faints).
Age-dependence of presentation of vasovagal syncope
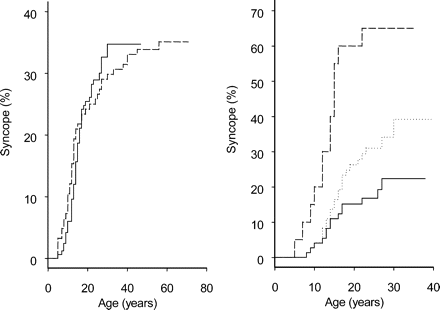
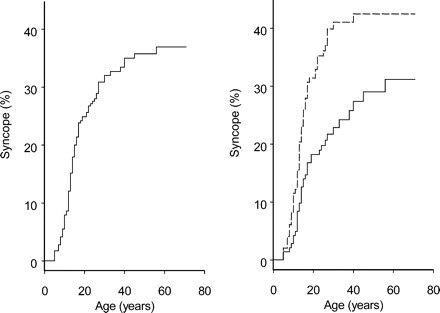
Figure 1 (left panel) shows that the proportion of subjects having a first faint begins to increase around age 7, climbs steeply in adolescence and early adulthood, and only 3/52 fainting individuals (5.8%) above the age of 40 experienced their first syncopal spell after 40 years of age. The estimated proportion of subjects with vasovagal syncope by age 60, shown in Figure 1 (right panel), is 37% in total, 42% in females and 31% in males (P=0.016; robust score test in proportional hazards regression analysis), with a hazard ratio (HR) for females vs. males of 1.34 (95% CI 1.07–1.68). The age of onset of vasovagal syncope was similar for parents and offspring in the fainting population. The parental median was 13 years (IQR 10–25 years) and that of the offspring was 14 years (IQR 12–18 years) (P=0.62). Figure 2 (left panel) shows that there was no significant difference in HRs for vasovagal syncope between parents and offspring (P=0.63).
Likelihood of syncope using Kaplan–Meier analysis. (Left panel) Age-dependent likelihood of vasovagal syncope in all eligible individuals. (Right panel) Age-dependent likelihood of vasovagal syncope in all eligible females (top dashed line) is compared with that of males (bottom solid line).
(Left panel) Age-dependent likelihood of all eligible offspring (dashed line) and parents (solid line) are compared. (Right panel) Age-dependent likelihood of vasovagal syncope in all eligible offspring with no fainting parents (bottom solid line), one fainting parent (middle dotted line), and two fainting parents (top dashed line).
Individual predictor analysis of family history
Figure 2 (right panel) depicts the effect of a parental history of syncope on the age-dependent proportion of fainting offspring. The probability of fainting by age 30 was estimated to be 34 and 10% for female and male offspring with 0 fainting parents, 48 and 28% for female and male offspring with one fainting parent, and 78 and 55% for female and male offspring with two fainting parents. Offspring with two fainting parents are more likely to faint than those with no fainting parents [HR 3.4 (95% CI 1.7–7.03); P<0.0001], but offspring with one fainting parent were not significantly more likely to faint than those with no fainting parents [HR 1.60 (95% CI 0.8–2.1); P=0.31]. The apparent range in lifetime proportions of fainting subjects is also reflected by a range in crude syncope prevalence. Offspring of zero, one, and two fainting parents had a probability of having ≥1 faint of 20, 33, and 65% (χ2, P=0.0003 for trend and P=0.0007 for association, not corrected for censoring and intrafamily correlation).
Table 2 reports the univariable HRs and point estimates of lifetime proportions of fainting subjects for all subgroups. At the extremes, a female with two fainting parents is much more likely to faint than a male with zero fainting parents [HR 18.7 (95% CI 5.7–61.1); P<0.0001] and have estimated lifetime fainting probabilities of 78 and 10%, respectively. Therefore, both the sex of the individual and parental syncope history are univariable predictors of the likelihood of syncope. The subsequent proportional hazards model showed that this parental effect was statistically significant (robust score test, P=0.018) when taking the non-independence into account.
Point estimates of proportion of populations who faint, relative to males with no fainting parents, using a univariable proportional hazards model to adjust for intrafamily correlations
| . | Proportion fainting by age 60 (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|
| Female with two fainting parents | 78 | 18.7 (5.7–61.1) | <0.0001 |
| Female with one fainting parent | 48 | 6.6 (2.3–19.1) | 0.0005 |
| Female with zero fainting parents | 34 | 4.4 (1.6–11.8) | 0.003 |
| Male with two fainting parents | 55 | 9.1 (2.6–31.6) | 0.0006 |
| Male with one fainting parent | 29 | 2.7 (0.8–9.2) | 0.12 |
| Male with zero fainting parents | 10 | 1.00 | — |
| . | Proportion fainting by age 60 (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|
| Female with two fainting parents | 78 | 18.7 (5.7–61.1) | <0.0001 |
| Female with one fainting parent | 48 | 6.6 (2.3–19.1) | 0.0005 |
| Female with zero fainting parents | 34 | 4.4 (1.6–11.8) | 0.003 |
| Male with two fainting parents | 55 | 9.1 (2.6–31.6) | 0.0006 |
| Male with one fainting parent | 29 | 2.7 (0.8–9.2) | 0.12 |
| Male with zero fainting parents | 10 | 1.00 | — |
Data are expressed as HRs with 95% CIs.
Point estimates of proportion of populations who faint, relative to males with no fainting parents, using a univariable proportional hazards model to adjust for intrafamily correlations
| . | Proportion fainting by age 60 (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|
| Female with two fainting parents | 78 | 18.7 (5.7–61.1) | <0.0001 |
| Female with one fainting parent | 48 | 6.6 (2.3–19.1) | 0.0005 |
| Female with zero fainting parents | 34 | 4.4 (1.6–11.8) | 0.003 |
| Male with two fainting parents | 55 | 9.1 (2.6–31.6) | 0.0006 |
| Male with one fainting parent | 29 | 2.7 (0.8–9.2) | 0.12 |
| Male with zero fainting parents | 10 | 1.00 | — |
| . | Proportion fainting by age 60 (%) . | HR (95% CI) . | P-value . |
|---|---|---|---|
| Female with two fainting parents | 78 | 18.7 (5.7–61.1) | <0.0001 |
| Female with one fainting parent | 48 | 6.6 (2.3–19.1) | 0.0005 |
| Female with zero fainting parents | 34 | 4.4 (1.6–11.8) | 0.003 |
| Male with two fainting parents | 55 | 9.1 (2.6–31.6) | 0.0006 |
| Male with one fainting parent | 29 | 2.7 (0.8–9.2) | 0.12 |
| Male with zero fainting parents | 10 | 1.00 | — |
Data are expressed as HRs with 95% CIs.
Multivariable model
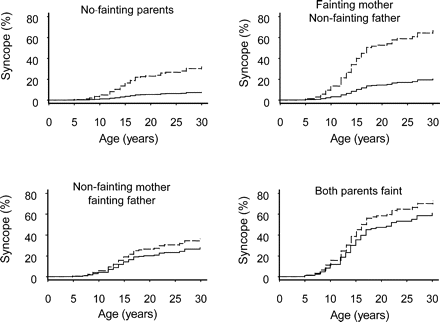
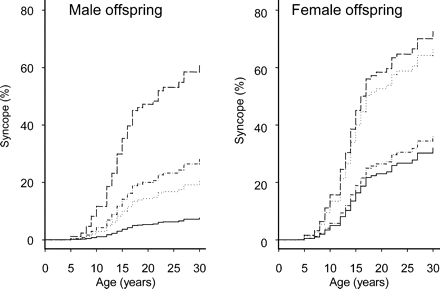
There was no evidence against the assumption of proportional hazards for all variables in the model. The model is summarized in Table 3 and displayed in Figures 3 and 4. If neither parent has vasovagal syncope, female offspring are more likely to faint than males [HR 4.82 (95% CI 1.99–11.66); Figure 3]. This sex-specific risk ratio is the same if the mother has syncope (Figure 3). The risk of syncope in offspring of either sex if the mother has syncope is increased compared with offspring whose mother does not faint [HR 2.86 (95% CI 1.54–5.31)]. In contrast, a history of vasovagal syncope in the father increased the risk of syncope only in male children (for interaction with offspring sex, Z=−1.92; P=0.055). In male offspring, a history of vasovagal syncope in the father significantly increased the risk of syncope [HR 4.12 (95% CI 1.39–12.31)]. The risk of syncope for females when the father fainted was not significantly different compared with when neither parent fainted [HR 1.17 (95% CI 0.56–3.34)]. The risk of syncope in children of fainting fathers was not sex-dependent [HR 1.36 (95% CI 0.58–3.33); Figure 3)].
Age-dependent likelihoods of fainting estimated from the multivariable proportional hazards model, grouped according to parental history of fainting. The four combinations of parental fainting history are depicted in separate panels. The offspring are females (top dashed lines) and males (bottom solid lines).
Age-dependent likelihoods of fainting estimated from the multivariable proportional hazards model, grouped according to offspring sex on fainting likelihood in separate panels. The parental histories are both parents faint (top dashed lines), neither parent faints (bottom solid lines), mother only faints (broken lines), and father only faints (dotted lines).
Results of the proportional hazards regression analysis showing the effect of offspring sex and parental fainting history on the risk of vasovagal syncope
| Variable . | Coefficient (β̂) . | Robust standard error . | Wald statistic . | Proportional hazards assumption . | ||
|---|---|---|---|---|---|---|
| . | . | . | Z . | P-value . | χ12 . | P-value . |
| Sex (female) | 1.57 | 0.45 | 3.49 | 0.0005 | 0.25 | 0.62 |
| Fainting mother | 1.05 | 0.32 | 3.32 | 0.0009 | 0.56 | 0.45 |
| Fainting father | 1.42 | 0.56 | 2.54 | 0.0110 | 0.72 | 0.39 |
| Sex-specific risk of offspring fainting if fainting father | −1.26 | 0.66 | −1.92 | 0.0550 | 0.12 | 0.73 |
| Variable . | Coefficient (β̂) . | Robust standard error . | Wald statistic . | Proportional hazards assumption . | ||
|---|---|---|---|---|---|---|
| . | . | . | Z . | P-value . | χ12 . | P-value . |
| Sex (female) | 1.57 | 0.45 | 3.49 | 0.0005 | 0.25 | 0.62 |
| Fainting mother | 1.05 | 0.32 | 3.32 | 0.0009 | 0.56 | 0.45 |
| Fainting father | 1.42 | 0.56 | 2.54 | 0.0110 | 0.72 | 0.39 |
| Sex-specific risk of offspring fainting if fainting father | −1.26 | 0.66 | −1.92 | 0.0550 | 0.12 | 0.73 |
The risk ratios are not presented in the table because of the interaction term, and are reported in the text.
Results of the proportional hazards regression analysis showing the effect of offspring sex and parental fainting history on the risk of vasovagal syncope
| Variable . | Coefficient (β̂) . | Robust standard error . | Wald statistic . | Proportional hazards assumption . | ||
|---|---|---|---|---|---|---|
| . | . | . | Z . | P-value . | χ12 . | P-value . |
| Sex (female) | 1.57 | 0.45 | 3.49 | 0.0005 | 0.25 | 0.62 |
| Fainting mother | 1.05 | 0.32 | 3.32 | 0.0009 | 0.56 | 0.45 |
| Fainting father | 1.42 | 0.56 | 2.54 | 0.0110 | 0.72 | 0.39 |
| Sex-specific risk of offspring fainting if fainting father | −1.26 | 0.66 | −1.92 | 0.0550 | 0.12 | 0.73 |
| Variable . | Coefficient (β̂) . | Robust standard error . | Wald statistic . | Proportional hazards assumption . | ||
|---|---|---|---|---|---|---|
| . | . | . | Z . | P-value . | χ12 . | P-value . |
| Sex (female) | 1.57 | 0.45 | 3.49 | 0.0005 | 0.25 | 0.62 |
| Fainting mother | 1.05 | 0.32 | 3.32 | 0.0009 | 0.56 | 0.45 |
| Fainting father | 1.42 | 0.56 | 2.54 | 0.0110 | 0.72 | 0.39 |
| Sex-specific risk of offspring fainting if fainting father | −1.26 | 0.66 | −1.92 | 0.0550 | 0.12 | 0.73 |
The risk ratios are not presented in the table because of the interaction term, and are reported in the text.
The risk of syncope in male offspring when both parents had syncope was 11.82 (95% CI 3.29–42.44) compared with those for whom neither parent fainted (Figure 4). The risk of syncope in female offspring when both parents had a history of syncope was 3.35 times that when neither parents had a history of fainting (95% CI 1.09–10.28).
Discussion
This population-based study showed that the sex of the individual and parental history of vasovagal syncope are significant predictors of syncope in offspring. It also described the age dependence of the age of onset of symptoms and the syncope burden in a non-clinical population and demonstrated that females have a higher prevalence of syncope than males. The study had three strengths: time-dependent analyses, a validated diagnostic score that relied only on the subject's history, and acquisition of the data from the subject rather than family members.
Age-dependent analysis
Two previous studies suggested the need for time-dependent analyses. Ganzeboom et al.6 reported that 39% of 394 medical students had fainted at least once, with a mean age of onset of syncope of 15 years. Driscoll et al.5 found the incidence of the first syncopal episode, in individuals under age 22, to peak between 15 and 19 years of age. Although no standardized, validated method of diagnosis of vasovagal syncope was used in either study, and the populations were limited to adolescents and young adults, they did indicate the need to capture syncope as a time-dependent event. We found a raw prevalence of syncope of 32%, and a lifetime risk of syncope was estimated at 37%. There was a steep increase in the probability of a first faint in the adolescent and young adult years, with a median age of onset of 14 years in those who had fainted by the interview date. The onset of syncope in adolescence reported here is mirrored by our recent report that syncope in a referral population first presents in adolescence.10 Fortunately, vasovagal syncope carries a benign prognosis, with no reported excess mortality.11
Syncope score
Our study used the Calgary syncope symptom score7 as the diagnostic tool for vasovagal syncope. It was developed in a multicentre, multinational study that enrolled 671 subjects with different causes of seizures and syncope. The diagnoses were based on gold standard criteria. The study has derived two questionnaires: the first distinguished between syncope and seizures8 and the second distinguished vasovagal syncope from other causes of syncope in patients with structurally normal hearts.7 The latter was validated in a prospective comparison of two populations consisting of patients with vasovagal syncope and a positive tilt test and patients with other known gold standard causes of syncope. Features against the diagnosis of vasovagal syncope included complete heart block, bifascicular block, diabetes, observed cyanosis, age of onset ≥35 years, and a memory of being unconsciousness. Features favouring the diagnosis of vasovagal syncope included presyncope or syncope with prolonged sitting or standing, sweating or warm feeling before a spell, and presyncope or syncope with pain or exposure to a medical procedure. Points associated with these features were summed in a diagnostic point score. The score has a sensitivity and specificity for vasovagal syncope of 89 and 91%, respectively. Other studies have either not prespecified diagnostic criteria3,4 or have relied on tilt table tests.1,2 Ours uniquely uses prespecified diagnostic criteria based on a point score that was validated against tilt table tests.
The limitations of tilt testing were reviewed recently.12 These include numerous protocols with little cross-validation, numerous methodological variables, variable sensitivity and specificity, limited reproducibility, and inability to select patients who might benefit from either pacing or beta-blocker therapy. Patients with syncope of unknown aetiology have the same clinical outcome regardless of whether they have positive or negative tilt tests.13 The Calgary syncope symptom score identified a large subpopulation of patients with syncope of unknown aetiology and a negative tilt test who were indistinguishable from patients with a positive tilt test, using the criteria of quantitative history and symptom burden. Diagnostic questionnaires for syncope have met with the approval of the European Society of Cardiology.14 Finally, tilt table testing poses significant logistical limitations for population-based studies. We therefore used a quantitative questionnaire rather than tilt testing as an inclusion criterion.
Direct data acquisition
The possibility of recall bias was considered during study design, because subjects have been found to report incorrect family histories of syncope. In one study, only 21% of fainting mothers and 28% of fainting fathers were correctly identified by their children, and only daughters reported accurate family histories.4 With the exception of young children, we collected the data directly from the subjects rather than from family members.
Previous studies
Previous studies have suggested that fainting offspring are more likely to have a positive family history for vasovagal syncope, compared with non-fainting offspring. Mathias et al.2 studied 119 syncope patients of various ages. A positive family history of vasovagal fainting was present for 51% of 47 confirmed vasovagal patients and in 28% of probable vasovagal patients. The authors did not study families of non-fainters, and the diagnosis of vasovagal syncope was determined by history, with tilt table tests used in some subjects. There was no adjustment for age of onset, and family history was obtained from the patients alone.
Camfield and Camfield3 reported that 90% of 30 children with syncope had at least one affected first-degree relative, compared with only 33% of 24 controls. The diagnosis of vasovagal syncope was established by neurologists on the basis of the history, but without a validated diagnostic tool. The ages ranged from 2 to 15 years old, and therefore some subjects did not have time for the fainting phenotype to be expressed. Newton et al.1 showed that 19% of 441 vasovagal fainters had a positive family history, using tilt table tests for diagnosis. A control group was not used.
In contrast, we used a validated questionnaire to establish the diagnosis, collected information individually from each subject, and accounted for the age of onset of symptoms. We found that the likelihood of syncope in offspring could be predicted in large part by the sex of the offspring and a history of parental fainting. The probabilities of a first faint were highly dependent on age and ranged from 10–79%, depending on the sex and family history of the subject.
Genetic basis for vasovagal syncope
The pattern of parental influence suggested by the data in this study is not straightforward. Indeed, appealing alternative explanations are that there is a true genetic effect whose apparently complex effect is simply due to sampling error, or that there is no true genetic effect. It is possible that cultural, social, environmental, nutritional effects all play a major role.
There appear to be several major effects. First, there is a baseline risk of syncope, which is independent of a parental history of fainting. Second, females are more likely to faint than males. Third, fainting mothers increase the risk of syncope equally in offspring of either sex. Fourth, fainting fathers appear to increase the risk of syncope only in their sons. Finally, there is an approximately equal gene dosage effect from either parent in male offspring. Although there appears to be a major gene effect, the data do not directly establish whether the gene(s) are dominant or recessive. They also do not establish the mechanism of the sex-specific effect. A straightforward sex-linked effect seems unlikely, because we have evidence of father–son transmission. It may reflect other sex-specific effects on vascular tone. Finally, the lineage-dependent expression of the trait raises the possibility of an epigenetic effect.
Interestingly, Newton et al.15 recently reported an extended family in which syncope occurred in three successive generations of males, all of whom had positive tilt tests. Mathias et al.16 reported a similar multigenerational family with syncope in all three generations. Marquez et al.17 reported two sets of monozygotic twins who all fainted. These combined results are compatible with either a high community prevalence or an autosomal dominant pattern. Newton et al.18 also tested but could not confirm the hypothesis that vasovagal syncope was related to common insertion/deletion polymorphisms of the structural gene for the angiotensin-converting enzyme.
Limitations
There are potential limitations to this study. Inclusion in the study depended on the presence of at least one medical student in a Canadian family, creating a possible selection bias of medically oriented Canadian individuals. However, our prevalence estimate is very close to that of Dutch medical students, and the statistical weighting of a family history is very close to the estimate derived from earlier studies. Third, our conclusion that part of the genetic effect is autosomal dominant, based on this prospective and population-based study, agrees with published family histories.
There may be recall error regarding the age of onset of syncope, particularly in older individuals who began fainting at a very young age. The study was powered to test a simple hypothesis concerning family history, and the secondary analyses may be underpowered. Patients with epilepsy, heart disease, genetic arrhythmias, or a close family history of unexpected death were not excluded directly from the study, relying on the Calgary syncope symptom score to accurately distinguish vasovagal syncope from other causes of syncope and exclude these subjects. The study was a retrospective survey, and it would be of some interest to determine whether the predictive factors could be confirmed in a prospective study of asymptomatic young people. Finally and most importantly, we have not shown that the effect of family history is due to genetic factors; indeed, at this point, it remains possible that it is due to sociocultural factors or learned behaviour.
Conclusion
The data suggest that much of the likelihood of a first faint can be predicted by the age and sex of the person and, importantly, by whether there is a parental history of fainting. Identifying the gene(s) involved and the cause of the age-dependent onset might provide valuable clues to understand the cause of vasovagal syncope.
Acknowledgements
This study was supported by grant 73-1976 from the Canadian Institutes for Health Research, Ottawa, Canada.
Conflict of interest: none declared.