-
PDF
- Split View
-
Views
-
Cite
Cite
Duncan McNab, Sadia N. Khan, Linda D. Sharples, Judy Y. Ryan, Carol Freeman, Noreen Caine, Sue Tait, Ian Hardy, Peter M. Schofield, An open label, single-centre, randomized trial of spinal cord stimulation vs. percutaneous myocardial laser revascularization in patients with refractory angina pectoris: the SPiRiT trial, European Heart Journal, Volume 27, Issue 9, May 2006, Pages 1048–1053, https://doi.org/10.1093/eurheartj/ehi827
Close - Share Icon Share
Abstract
Aims Refractory angina pectoris leads to significant morbidity. Treatment options include percutaneous myocardial laser revascularization (PMR) and spinal cord stimulation (SCS). This study was designed to compare these two treatments.
Methods and results Subjects with Canadian Cardiovascular Society (CCS) class 3/4 angina and reversible perfusion defects were randomized to SCS (34) or PMR (34). The primary outcome was to compare exercise treadmill time on a modified Bruce protocol over 12 months. Thirty subjects in both groups completed 12-month follow-up. The mean total exercise time was 6.38±3.45 min in the SCS group and 7.41±3.68 min in the PMR group at baseline and 7.08±0.67 min in the SCS group and 7.12±0.71 min in the PMR group at 12 months (95% confidence limits for the difference between the groups −1.02 to +2.2 min, P=0.466). There were no differences in angina-free exercise capacity, CCS class, and quality of life between treatments. SCS patients had more adverse events in the first 12 months, mainly angina or SCS system related (P=0.001).
Conclusion There was little evidence of a difference in effectiveness between SCS and PMR in patients with refractory angina.
Introduction
There is an increasing group of patients with ‘refractory angina pectoris’ who are unsuitable for conventional revascularization by coronary artery bypass surgery or percutaneous intervention.1 This patient group often have significant disability, with limiting symptoms, multiple medications, and frequent hospital admissions contributing to this. Many techniques have been used to try to improve quality of life including lifestyle and exercise modification, enhanced external counterpulsation, laser revascularization, spinal cord stimulation (SCS), and gene therapy-based techniques. There is little evidence directly comparing these multiple therapeutic modalities, and currently, treatment is allocated on the basis of clinician bias and local availability. Ideally, the data forming this evidence base should come from randomized trials. Of the therapies mentioned above, percutaneous myocardial laser revascularization (PMR) is the only technique supported by data from a placebo controlled trial2 (in which the ‘sham’ method cannot be distinguished) and from other randomized controlled trials,3,4 whereas SCS is supported by a number of observational studies and one randomized controlled trial.5 Patient selection criteria for SCS and PMR are very similar and at the time this study started there were few data to inform clinicians and patients on relative efficacy and safety. The European Society of Cardiology Joint Study group published its recommendations in 2002.1 However, since this time further data, particularly the randomized trial of PMR, have become available.2
The technique of transmyocardial laser revascularization uses laser ablation to create transmural channels in ischaemic myocardium, usually via a left anterior thoracotomy. Some trials have shown symptomatic benefit but with significant peri-operative mortality and morbidity.6,7 In this context, PMR, in which channels are created from the endocardium partially through the myocardium, is an attractive alternative as the peri-procedural complication rate is significantly lower.3,4 At the time this study started, PMR was the only procedure offered to this group of patients in our institution and was considered to be the best available treatment. SCS has been used for many years in the treatment of chronic pain and obtained CE (European product compliance) mark for the treatment of refractory angina pectoris in 1995. Observational studies have found a reduction in angina frequency and an improvement in quality of life while neither preventing nor concealing the symptoms of myocardial infarction (MI).8–10
The primary objective of this study was to compare the effect of SCS vs. PMR on treadmill exercise time on a modified Bruce protocol over a period of 12 months. Secondary measures of effectiveness were angina as measured by the Canadian Cardiovascular Society (CCS) angina scale, morbidity/mortality, and health-related quality of life.
Methods
This open label, single-centre, parallel group randomized controlled trial was carried out in a tertiary referral centre for patients with cardiovascular disease. The recruitment period was from December 2000 to December 2003 and is summarized in Figure 1. Twelve-month follow-up was completed in January 2005 and is reported here. Further follow-up is ongoing. Approval was obtained from the local research Ethics Committee prior to study commencement. During the study assessment period, the medical history was reviewed, written informed consent obtained, and the subject underwent baseline testing which included an echocardiogram, myocardial perfusion scan, and a modified Bruce treadmill test. A screening angiogram was also carried out if this had not been done within the last 9 months or if the clinical situation had changed within this period. The main inclusion criteria were limiting angina despite maximally tolerated anti-anginal medication, angiographically documented coronary disease unsuitable for conventional revascularization (this judgement was made by a consultant interventional cardiologist in conjunction with the referring consultant cardiologist/cardiothoracic surgeon), and reversible ischaemia on 99 msestamibi-technetium scanning. Exclusion criteria included myocardial wall thickness <8 mm in the areas to be treated by PMR, implanted pacemakers or defibrillators or comorbidity that was considered by the assessing clinician to be of greater significance than angina pectoris.
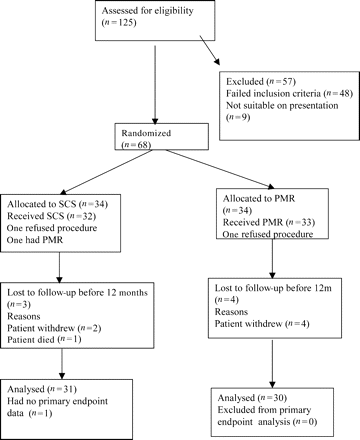
CONSORT diagram showing the flow of participants through the trial comparing SCS with PMR.
Procedures
Following screening subjects were randomized to either SCS or PMR using a computer-generated list that was held in the hospital R&D Unit and was not available to anyone directly involved in the conduct of the trial. Randomization was in blocks of size six and eight to ensure similar numbers in each group. The study was open label as blinding was not possible for either investigator or subject.
All procedures were carried out within 8 weeks of baseline. For PMR, biplane ventriculography was performed to provide landmarks for laser tip placement. A 9F Axcis guiding catheter was used to position the optical fibre attached to a Holmium:YAG laser. Each position was checked in two radiographic views to ensure placement of channels at least 1 cm apart and nine to 12 channels were created. All subjects underwent transthoracic echocardiography following the procedure to exclude a pericardial fluid collection.5 One procedural complication was reported, a femoral pseudo-aneurysm, which resolved within 24 h.
For SCS implantation, Medtronic fully implantable Itrel 3 systems were used for this study. The lead was advanced via the epidural space under radiographic screening to the high thoracic/low cervical spinal cord. When adequate paraesthesia was obtained, the pulse generator was implanted. Leads were positioned with the tip of the electrode at C6 (n=10), C7 (n=16), and T1 (n=6). There were no complications associated with implant of SCS device, but one subject reported a change in distribution of paraesthesia on the day following the implant procedure. For this subject, migration of the epidural lead was reported and a replacement lead was inserted 2 months after the initial procedure. Subjects were trained pre-and post-implant to try to achieve maximum benefit. The stimulation regime advised was a minimum of three 1 h sessions in each 24 h period. In addition, each patient was encouraged to use the device prior to carrying out activities known to cause angina symptoms and with each episode of angina for which sublingual nitrates would normally be used.
Follow-up protocol
Following treatment, subjects were seen at 3 and 12 months. At these visits, a clinical assessment of angina class was made, quality of life data collected, concomitant medications recorded, and a modified Bruce protocol exercise tolerance test was carried out. At 12 months, in addition, a myocardial perfusion scan was performed. For subjects with a spinal cord stimulator, the device was on for the purposes of the tests except for one subject at 3 months and two at 12 months in whom the device was switched off for technical reasons. Battery life and generator programming were checked at each visit. Adverse events were recorded as soon as they were reported by the subjects. Specifically, subjects were asked to report deterioration in angina status, all hospital admissions, and changes in the function of the SCS device.
Outcome measurements
The primary outcome was total exercise time on a modified Bruce protocol (ETT) at 12 months after SCS compared with PMR,11 and in this study, all tests were terminated by the subject. Both SCS and PMR aim to reduce the frequency and severity of angina, and exercise tolerance is a comparatively objective method of measuring angina-free functional capacity. Secondary outcomes were time to patient-reported angina during exercise test, angina class as measured by the CCS angina scale, morbidity and mortality, and the safety profiles of each therapy. In addition, health-related quality of life was assessed using questionnaires administered at follow-up clinic. The disease-specific Seattle Angina Questionnaire,12 the generic Short Form 36,13 and EuroQoL14 questionnaires were used.
Statistical methods
Sample size was estimated on the basis of the primary outcome measure, total ETT. On the basis of previous studies, we estimated that the minimum clinically significant difference in exercise time between SCS and PMR would be 1.5 min, with a standard deviation of 2 min.3,4,6,7,15 Assuming two-sided significance of 0.05, 80% power, and 15% dropout, 33 patients in each group were required.
Patients were analysed in the group to which they were allocated irrespective of actual treatment given. In an initial analysis, only patients who completed the study were included. The sensitivity of the results was assessed including all patients and using the last observation carried forward method. Under this analysis, differences were reduced to some extent but the conclusions were not altered and so these are not presented here. Adverse event rates were assumed Poisson and compared with a log-linear model. Total exercise time was normally distributed and was summarized as the mean and standard error. Analysis of variance was used to assess the difference in ETT between SCS and PMR patients and this was adjusted for baseline. Similar models were used to assess the difference in health-related quality of life scales. In addition, the change in ETT within each group was assessed using paired Student's t-tests. As not all patients experienced angina on the treadmill, the mean time to angina was estimated from the area under a Kaplan–Meier curve, with angina-free patients censored at the total ETT. The change in angina-free exercise time was calculated from the means and standard errors estimated in this way, with correlation between baseline and follow-up estimated from those patients who were not censored. The comparison between SCS and PMR groups in angina-free exercise time and the change in angina-free exercise time used Student's t-tests based on these Kaplan–Meier summaries. Bootstrapping was used to assess the sensitivity of the estimates to the normal distribution approximation. CCS class and change in CCS class was assessed using the Mantel–Haenszel test for ordered categorical variables. A decrease of two or more CCS classes was considered clinically significant and differences between the groups in the number who achieved this was assessed using Fisher's exact test.
Results
Recruitment and follow-up
Between December 2000 and December 2003, 125 patients with refractory angina were assessed, of whom 57 were not suitable for the study. The main reasons for exclusion being reversible ischaemia not identified on perfusion scans (n=20), myocardial wall thickness <8 mm in the area to be treated by PMR (n=13), or suitability for conventional revascularization (n=13). The remaining 68 patients were recruited and randomized (Figure 1). During the first year of follow-up, of 34 patients allocated to SCS, one refused the procedure and withdrew from the trial, one withdrew after device implantation, and one died so that 31 were available for follow-up at 12 months. Of these, one could not complete the exercise test at 12-month follow-up but did complete the quality of life assessment. One further SCS patient refused the procedure and subsequently had PMR. This patient is included in the SCS group under intention to treat. Of the patients allocated to PMR, one refused the procedure and withdrew from the trial and a further three patients withdrew from the study after their procedure but before the 12-month follow-up visit.
Baseline characteristics
Baseline measurements are summarized in Table 1. The patients in the two treatment groups had comparable mean age, sex distribution, medical history, and frequency of risk factors for CAD. All but three patients (one SCS, two PMR) had received at least one previous revascularization procedure, usually CABG. None of the differences were greater than that might be expected by chance. In addition, at baseline, CCS classification and exercise tolerance were not significantly different. All patients were being treated with maximally tolerated anti-anginal medication and all were receiving more than one anti-anginal medication at baseline with similar regimens being used in the two treatment groups and this did not change during follow-up.
Baseline characteristics
| . | SCS (n=34) . | PMR (n=34) . |
|---|---|---|
| Age at recruitment | 64.2 (7.3) | 62.9 (9.6) |
| Sex M:F | 29:5 | 31:3 |
| Previous revascularization | ||
| PTCA | 6 (18%) | 10 (29%) |
| Stents | 6 (18%) | 6 (18%) |
| CABG | 32 (94%) | 32 (94%) |
| Exercise tolerance test | ||
| Total exercise time, mean (SD) | 6.38 (3.45) | 7.41 (3.68) |
| Time to angina, mean (SEM)a | 4.68 (0.52) | 5.47 (0.68) |
| No angina during exercise | 7 (21%) | 7 (21%) |
| CCS class at baseline | ||
| 2 | 0 (0%) | 0 (0%) |
| 3 | 22 (65%) | 25 (74%) |
| 4 | 12 (35%) | 9 (26%) |
| Short Form 36 | ||
| Aggregate physical score, mean (SD) | 21.1 (10.8) | 19.8 (10.3) |
| Aggregate mental score, mean (SD) | 34.1 (13.1) | 32.2 (12.0) |
| Seattle Angina Questionnaire | ||
| Exertional capacity scale, mean (SD) | 62.9 (27.3) | 66.9 (27.2) |
| Anginal stability scale, mean (SD) | 40.4 (17.4) | 44.9 (16.0) |
| Anginal frequency scale, mean (SD) | 28.2 (20.5) | 24.4 (16.2) |
| Treatment satisfaction scale, mean (SD) | 80.5 (15.7) | 73.0 (17.5) |
| Disease perception scale, mean (SD) | 35.8 (22.1) | 36.3 (18.6) |
| EuroQoL | ||
| EQ5D, mean (SD) | 0.41 (0.33) | 0.48 (0.27) |
| . | SCS (n=34) . | PMR (n=34) . |
|---|---|---|
| Age at recruitment | 64.2 (7.3) | 62.9 (9.6) |
| Sex M:F | 29:5 | 31:3 |
| Previous revascularization | ||
| PTCA | 6 (18%) | 10 (29%) |
| Stents | 6 (18%) | 6 (18%) |
| CABG | 32 (94%) | 32 (94%) |
| Exercise tolerance test | ||
| Total exercise time, mean (SD) | 6.38 (3.45) | 7.41 (3.68) |
| Time to angina, mean (SEM)a | 4.68 (0.52) | 5.47 (0.68) |
| No angina during exercise | 7 (21%) | 7 (21%) |
| CCS class at baseline | ||
| 2 | 0 (0%) | 0 (0%) |
| 3 | 22 (65%) | 25 (74%) |
| 4 | 12 (35%) | 9 (26%) |
| Short Form 36 | ||
| Aggregate physical score, mean (SD) | 21.1 (10.8) | 19.8 (10.3) |
| Aggregate mental score, mean (SD) | 34.1 (13.1) | 32.2 (12.0) |
| Seattle Angina Questionnaire | ||
| Exertional capacity scale, mean (SD) | 62.9 (27.3) | 66.9 (27.2) |
| Anginal stability scale, mean (SD) | 40.4 (17.4) | 44.9 (16.0) |
| Anginal frequency scale, mean (SD) | 28.2 (20.5) | 24.4 (16.2) |
| Treatment satisfaction scale, mean (SD) | 80.5 (15.7) | 73.0 (17.5) |
| Disease perception scale, mean (SD) | 35.8 (22.1) | 36.3 (18.6) |
| EuroQoL | ||
| EQ5D, mean (SD) | 0.41 (0.33) | 0.48 (0.27) |
aCalculated from Kaplan–Meier time to angina curves because some patients stopped exercising before onset of angina.
Baseline characteristics
| . | SCS (n=34) . | PMR (n=34) . |
|---|---|---|
| Age at recruitment | 64.2 (7.3) | 62.9 (9.6) |
| Sex M:F | 29:5 | 31:3 |
| Previous revascularization | ||
| PTCA | 6 (18%) | 10 (29%) |
| Stents | 6 (18%) | 6 (18%) |
| CABG | 32 (94%) | 32 (94%) |
| Exercise tolerance test | ||
| Total exercise time, mean (SD) | 6.38 (3.45) | 7.41 (3.68) |
| Time to angina, mean (SEM)a | 4.68 (0.52) | 5.47 (0.68) |
| No angina during exercise | 7 (21%) | 7 (21%) |
| CCS class at baseline | ||
| 2 | 0 (0%) | 0 (0%) |
| 3 | 22 (65%) | 25 (74%) |
| 4 | 12 (35%) | 9 (26%) |
| Short Form 36 | ||
| Aggregate physical score, mean (SD) | 21.1 (10.8) | 19.8 (10.3) |
| Aggregate mental score, mean (SD) | 34.1 (13.1) | 32.2 (12.0) |
| Seattle Angina Questionnaire | ||
| Exertional capacity scale, mean (SD) | 62.9 (27.3) | 66.9 (27.2) |
| Anginal stability scale, mean (SD) | 40.4 (17.4) | 44.9 (16.0) |
| Anginal frequency scale, mean (SD) | 28.2 (20.5) | 24.4 (16.2) |
| Treatment satisfaction scale, mean (SD) | 80.5 (15.7) | 73.0 (17.5) |
| Disease perception scale, mean (SD) | 35.8 (22.1) | 36.3 (18.6) |
| EuroQoL | ||
| EQ5D, mean (SD) | 0.41 (0.33) | 0.48 (0.27) |
| . | SCS (n=34) . | PMR (n=34) . |
|---|---|---|
| Age at recruitment | 64.2 (7.3) | 62.9 (9.6) |
| Sex M:F | 29:5 | 31:3 |
| Previous revascularization | ||
| PTCA | 6 (18%) | 10 (29%) |
| Stents | 6 (18%) | 6 (18%) |
| CABG | 32 (94%) | 32 (94%) |
| Exercise tolerance test | ||
| Total exercise time, mean (SD) | 6.38 (3.45) | 7.41 (3.68) |
| Time to angina, mean (SEM)a | 4.68 (0.52) | 5.47 (0.68) |
| No angina during exercise | 7 (21%) | 7 (21%) |
| CCS class at baseline | ||
| 2 | 0 (0%) | 0 (0%) |
| 3 | 22 (65%) | 25 (74%) |
| 4 | 12 (35%) | 9 (26%) |
| Short Form 36 | ||
| Aggregate physical score, mean (SD) | 21.1 (10.8) | 19.8 (10.3) |
| Aggregate mental score, mean (SD) | 34.1 (13.1) | 32.2 (12.0) |
| Seattle Angina Questionnaire | ||
| Exertional capacity scale, mean (SD) | 62.9 (27.3) | 66.9 (27.2) |
| Anginal stability scale, mean (SD) | 40.4 (17.4) | 44.9 (16.0) |
| Anginal frequency scale, mean (SD) | 28.2 (20.5) | 24.4 (16.2) |
| Treatment satisfaction scale, mean (SD) | 80.5 (15.7) | 73.0 (17.5) |
| Disease perception scale, mean (SD) | 35.8 (22.1) | 36.3 (18.6) |
| EuroQoL | ||
| EQ5D, mean (SD) | 0.41 (0.33) | 0.48 (0.27) |
aCalculated from Kaplan–Meier time to angina curves because some patients stopped exercising before onset of angina.
Adverse events
There have been six deaths to the end of January 2005, four in the SCS group and two in the PMR group. Causes of death (days post-procedure) were ischaemic heart disease (18), metastatic squamous cell carcinoma (442), presumed malignancy (630), and acute MI (660) in the SCS group and stomach carcinoma (430) and ischaemic heart disease/MI (490) in the PMR group. Eighty-three non-fatal adverse events were recorded in the first year (Table 2). The SCS group reported significantly more adverse events than the PMR group (P=0.001). Fifty-seven events occurred in 20 patients in the SCS group, with 26 events categorized as being related to the SCS procedure. The majority of these events (18) were an undesirable change in the level of stimulation, which could be resolved by reprogramming (13) or by repositioning or replacing the lead (5). Twenty-six adverse events were reported by 15 patients in the PMR group. Four events were related to the PMR procedure, one of which occurred in a patient randomized to SCS. A further 30 events in the SCS group and 23 events in the PMR group were categorized as unrelated to the procedure; most were related to the underlying disease and the difference between the groups was not significant (P=0.342). Of all the adverse events, 41 in the SCS group and 24 in the PMR group were classed as severe (P=0.039) in that they either required admission, prolonged stay in hospital, required surgery, were life threatening or ultimately resulted in death.
Adverse Events reported in the first year
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| Disease related | |||
| Unstable anginaa | 18 | 12 | 0.284 |
| MIb | 4 | 1 | 0.244 |
| Worsening angina | 6 | 3 | 0.350 |
| All disease related | 28 | 16 | 0.077 |
| SCS related | |||
| Infection of SCS system | 0 | NA | |
| Undesirable change in stimulation | 18 | NA | |
| Pain at neurostimulator site | 3 | NA | |
| Neurostimulator generator migration | 2 | NA | |
| Lead migration | 1 | NA | |
| PMR related | |||
| Femoral pseudoaneurysm | 0 | 1 | |
| Groin haematoma | 1 | 2 | |
| Other miscellaneous | 2 | 7 | 0.132 |
| Total events | 57 | 26 | 0.001 |
| Total events excluding SCS/PMR related | 30 | 23 | 0.342 |
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| Disease related | |||
| Unstable anginaa | 18 | 12 | 0.284 |
| MIb | 4 | 1 | 0.244 |
| Worsening angina | 6 | 3 | 0.350 |
| All disease related | 28 | 16 | 0.077 |
| SCS related | |||
| Infection of SCS system | 0 | NA | |
| Undesirable change in stimulation | 18 | NA | |
| Pain at neurostimulator site | 3 | NA | |
| Neurostimulator generator migration | 2 | NA | |
| Lead migration | 1 | NA | |
| PMR related | |||
| Femoral pseudoaneurysm | 0 | 1 | |
| Groin haematoma | 1 | 2 | |
| Other miscellaneous | 2 | 7 | 0.132 |
| Total events | 57 | 26 | 0.001 |
| Total events excluding SCS/PMR related | 30 | 23 | 0.342 |
aUnstable angina: admission to hospital with severe/worsening chest pain not meeting the definition of MI.
bMI: admission to hospital with prolonged chest pain associated with biochemical/ECG evidence of infarction.
Adverse Events reported in the first year
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| Disease related | |||
| Unstable anginaa | 18 | 12 | 0.284 |
| MIb | 4 | 1 | 0.244 |
| Worsening angina | 6 | 3 | 0.350 |
| All disease related | 28 | 16 | 0.077 |
| SCS related | |||
| Infection of SCS system | 0 | NA | |
| Undesirable change in stimulation | 18 | NA | |
| Pain at neurostimulator site | 3 | NA | |
| Neurostimulator generator migration | 2 | NA | |
| Lead migration | 1 | NA | |
| PMR related | |||
| Femoral pseudoaneurysm | 0 | 1 | |
| Groin haematoma | 1 | 2 | |
| Other miscellaneous | 2 | 7 | 0.132 |
| Total events | 57 | 26 | 0.001 |
| Total events excluding SCS/PMR related | 30 | 23 | 0.342 |
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| Disease related | |||
| Unstable anginaa | 18 | 12 | 0.284 |
| MIb | 4 | 1 | 0.244 |
| Worsening angina | 6 | 3 | 0.350 |
| All disease related | 28 | 16 | 0.077 |
| SCS related | |||
| Infection of SCS system | 0 | NA | |
| Undesirable change in stimulation | 18 | NA | |
| Pain at neurostimulator site | 3 | NA | |
| Neurostimulator generator migration | 2 | NA | |
| Lead migration | 1 | NA | |
| PMR related | |||
| Femoral pseudoaneurysm | 0 | 1 | |
| Groin haematoma | 1 | 2 | |
| Other miscellaneous | 2 | 7 | 0.132 |
| Total events | 57 | 26 | 0.001 |
| Total events excluding SCS/PMR related | 30 | 23 | 0.342 |
aUnstable angina: admission to hospital with severe/worsening chest pain not meeting the definition of MI.
bMI: admission to hospital with prolonged chest pain associated with biochemical/ECG evidence of infarction.
Exercise tolerance tests
The results from the exercise tolerance tests are summarized in Table 3. The mean total exercise time at 3 months was almost identical in the two groups (mean difference 0.01 min, 95% CI −1.75–1.78, P=0.989). Adjusting for baseline, the difference between the groups was 0.61 min (95% CI −0.55–1.77, P=0.353). The mean total exercise time at 12 months remained very similar in the two groups (mean difference −0.04 min, 95% CI −1.94–1.86, P=0.970). Adjusting for baseline, the difference in total exercise time between groups was 0.59 min (95% CI −1.02–2.20, P=0.466).
Unadjusted comparisons between SCS and PMR of mean exercise tolerance test at 3 and 12 months
| . | SCS . | PMR . | Difference adjusted for baseline 95% CI . | P-value . |
|---|---|---|---|---|
| Exercise tolerance at 3 months | (n=32) | (n=33) | ||
| Total exercise time, mean (SEM) | 7.33 (0.62) | 7.32 (0.66) | 0.61 (-0.55 – 1.77) | 0.353 |
| Time to angina, mean (SEM)a | 7.31 (0.73) | 6.26 (0.65) | 1.84 (0.19 – 3.49) | 0.028 |
| No angina during exercise | 10 (31%) | 7 (21%) | 0.474 | |
| Exercise tolerance at 12 months | (n=30) | (n=30) | ||
| Total exercise time, mean (SEM) | 7.08 (0.67) | 7.12 (0.71) | 0.59 (−1.02–2.20) | 0.466 |
| Time to angina, meana (SEM) | 7.30 (0.90) | 6.86 (0.82) | 1.23 (−0.61–3.07) | 0.191 |
| No angina during exercise | 11 (37%) | 10 (33%) | 1.00 | |
| . | SCS . | PMR . | Difference adjusted for baseline 95% CI . | P-value . |
|---|---|---|---|---|
| Exercise tolerance at 3 months | (n=32) | (n=33) | ||
| Total exercise time, mean (SEM) | 7.33 (0.62) | 7.32 (0.66) | 0.61 (-0.55 – 1.77) | 0.353 |
| Time to angina, mean (SEM)a | 7.31 (0.73) | 6.26 (0.65) | 1.84 (0.19 – 3.49) | 0.028 |
| No angina during exercise | 10 (31%) | 7 (21%) | 0.474 | |
| Exercise tolerance at 12 months | (n=30) | (n=30) | ||
| Total exercise time, mean (SEM) | 7.08 (0.67) | 7.12 (0.71) | 0.59 (−1.02–2.20) | 0.466 |
| Time to angina, meana (SEM) | 7.30 (0.90) | 6.86 (0.82) | 1.23 (−0.61–3.07) | 0.191 |
| No angina during exercise | 11 (37%) | 10 (33%) | 1.00 | |
aCalculated from area under the Kaplan–Meier time to angina curves because some patients stopped exercising before onset of angina. Comparisons are based on change from baseline and within patient correlation estimated from uncensored patients from both groups combined (ρ=0.40 at 3 months and 0.28 at 12 months).
Unadjusted comparisons between SCS and PMR of mean exercise tolerance test at 3 and 12 months
| . | SCS . | PMR . | Difference adjusted for baseline 95% CI . | P-value . |
|---|---|---|---|---|
| Exercise tolerance at 3 months | (n=32) | (n=33) | ||
| Total exercise time, mean (SEM) | 7.33 (0.62) | 7.32 (0.66) | 0.61 (-0.55 – 1.77) | 0.353 |
| Time to angina, mean (SEM)a | 7.31 (0.73) | 6.26 (0.65) | 1.84 (0.19 – 3.49) | 0.028 |
| No angina during exercise | 10 (31%) | 7 (21%) | 0.474 | |
| Exercise tolerance at 12 months | (n=30) | (n=30) | ||
| Total exercise time, mean (SEM) | 7.08 (0.67) | 7.12 (0.71) | 0.59 (−1.02–2.20) | 0.466 |
| Time to angina, meana (SEM) | 7.30 (0.90) | 6.86 (0.82) | 1.23 (−0.61–3.07) | 0.191 |
| No angina during exercise | 11 (37%) | 10 (33%) | 1.00 | |
| . | SCS . | PMR . | Difference adjusted for baseline 95% CI . | P-value . |
|---|---|---|---|---|
| Exercise tolerance at 3 months | (n=32) | (n=33) | ||
| Total exercise time, mean (SEM) | 7.33 (0.62) | 7.32 (0.66) | 0.61 (-0.55 – 1.77) | 0.353 |
| Time to angina, mean (SEM)a | 7.31 (0.73) | 6.26 (0.65) | 1.84 (0.19 – 3.49) | 0.028 |
| No angina during exercise | 10 (31%) | 7 (21%) | 0.474 | |
| Exercise tolerance at 12 months | (n=30) | (n=30) | ||
| Total exercise time, mean (SEM) | 7.08 (0.67) | 7.12 (0.71) | 0.59 (−1.02–2.20) | 0.466 |
| Time to angina, meana (SEM) | 7.30 (0.90) | 6.86 (0.82) | 1.23 (−0.61–3.07) | 0.191 |
| No angina during exercise | 11 (37%) | 10 (33%) | 1.00 | |
aCalculated from area under the Kaplan–Meier time to angina curves because some patients stopped exercising before onset of angina. Comparisons are based on change from baseline and within patient correlation estimated from uncensored patients from both groups combined (ρ=0.40 at 3 months and 0.28 at 12 months).
At 3 months, mean time to onset of angina increased significantly from baseline in the SCS group (2.63±0.58 vs. 0.79±0.61 min in the PMR group) with a difference between the two groups at 3 months of 1.84 min (95% CI 0.19–3.49 min, P=0.028). The increase in angina-free exercise time over baseline was significant for both groups at 12 months (Table 3) but the difference between the two groups was not significant at 1.23 min (95% CI −0.61–3.07 min, P=0.191).
CCS class
At 3 months, SCS patients were in a significantly lower CCS class (P=0.049) and more in this group had a clinically significant decrease of two CCS classes (P=0.077) (Table 4). When viewed as a trend, the change in CCS score at 3 months was significantly greater for SCS patients (P=0.018). This trend continued to 12 months, with SCS patients having greater improvement in CCS class (P=0.042). In this analysis, we have excluded the patients who were not available for follow-up at 3 and 12 months. Treating deaths and dropouts as failures would reduce the success rate to 12/34 (35%) in the SCS group and 5/34 (15%) in the PMR group at 3 months (P=0.093) and to 11/34 (32%) and 6/34 (15%) at 12 months (P=0.263).
Comparisons of CCS class between SCS and PMR at 3 and 12 months
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| CCS class at 3 months | (n=32) | (n=33) | |
| 1 | 9 (28%) | 4 (12%) | 0.049 |
| 2 | 12 (38%) | 11 (33%) | |
| 3 | 8 (25%) | 11 (33%) | |
| 4 | 3 (9%) | 7 (21%) | |
| Change in CCS ≥2 classes | |||
| No | 20 (63%) | 28 (85%) | 0.077 |
| Yes | 12 (37%) | 5 (15%) | |
| CCS class at 12 months | (n=30) | (n=30) | |
| 1 | 6 (20%) | 3 (10%) | 0.093 |
| 2 | 15 (50%) | 11 (37%) | |
| 3 | 4 (13%) | 8 (27%) | |
| 4 | 5 (17%) | 8 (27%) | |
| Change in CCS≥2 classes | |||
| No | 19 (63%) | 24 (80%) | 0.166 |
| Yes | 11 (37%) | 6 (20%) | |
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| CCS class at 3 months | (n=32) | (n=33) | |
| 1 | 9 (28%) | 4 (12%) | 0.049 |
| 2 | 12 (38%) | 11 (33%) | |
| 3 | 8 (25%) | 11 (33%) | |
| 4 | 3 (9%) | 7 (21%) | |
| Change in CCS ≥2 classes | |||
| No | 20 (63%) | 28 (85%) | 0.077 |
| Yes | 12 (37%) | 5 (15%) | |
| CCS class at 12 months | (n=30) | (n=30) | |
| 1 | 6 (20%) | 3 (10%) | 0.093 |
| 2 | 15 (50%) | 11 (37%) | |
| 3 | 4 (13%) | 8 (27%) | |
| 4 | 5 (17%) | 8 (27%) | |
| Change in CCS≥2 classes | |||
| No | 19 (63%) | 24 (80%) | 0.166 |
| Yes | 11 (37%) | 6 (20%) | |
aOn the basis of comparisons which were not adjusted for baseline.
Comparisons of CCS class between SCS and PMR at 3 and 12 months
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| CCS class at 3 months | (n=32) | (n=33) | |
| 1 | 9 (28%) | 4 (12%) | 0.049 |
| 2 | 12 (38%) | 11 (33%) | |
| 3 | 8 (25%) | 11 (33%) | |
| 4 | 3 (9%) | 7 (21%) | |
| Change in CCS ≥2 classes | |||
| No | 20 (63%) | 28 (85%) | 0.077 |
| Yes | 12 (37%) | 5 (15%) | |
| CCS class at 12 months | (n=30) | (n=30) | |
| 1 | 6 (20%) | 3 (10%) | 0.093 |
| 2 | 15 (50%) | 11 (37%) | |
| 3 | 4 (13%) | 8 (27%) | |
| 4 | 5 (17%) | 8 (27%) | |
| Change in CCS≥2 classes | |||
| No | 19 (63%) | 24 (80%) | 0.166 |
| Yes | 11 (37%) | 6 (20%) | |
| . | SCS . | PMR . | P-value . |
|---|---|---|---|
| CCS class at 3 months | (n=32) | (n=33) | |
| 1 | 9 (28%) | 4 (12%) | 0.049 |
| 2 | 12 (38%) | 11 (33%) | |
| 3 | 8 (25%) | 11 (33%) | |
| 4 | 3 (9%) | 7 (21%) | |
| Change in CCS ≥2 classes | |||
| No | 20 (63%) | 28 (85%) | 0.077 |
| Yes | 12 (37%) | 5 (15%) | |
| CCS class at 12 months | (n=30) | (n=30) | |
| 1 | 6 (20%) | 3 (10%) | 0.093 |
| 2 | 15 (50%) | 11 (37%) | |
| 3 | 4 (13%) | 8 (27%) | |
| 4 | 5 (17%) | 8 (27%) | |
| Change in CCS≥2 classes | |||
| No | 19 (63%) | 24 (80%) | 0.166 |
| Yes | 11 (37%) | 6 (20%) | |
aOn the basis of comparisons which were not adjusted for baseline.
Health-related quality of life
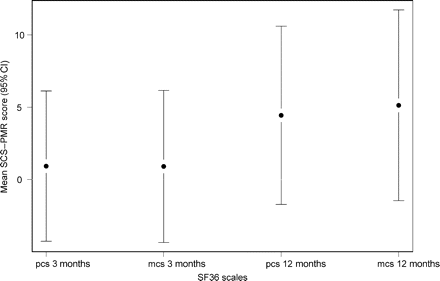
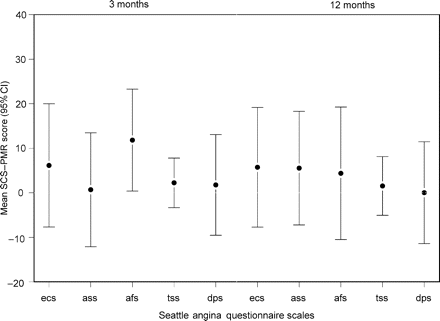
Figures 2 and 3 show the mean difference in health-related quality of life measures at 3 and 12 months after the allocated procedure adjusting for baseline. In general, both groups reported improvements at 3 and 12 months.
Mean difference between SCS and PMR in SF36 physical component score (pcs) and mental component score (mcs) at 3 and 12 months after procedure, adjusted for baseline scores. Values above zero favour SCS.
Mean difference between SCS and PMR in Seattle angina questionnaire scales at 3 and 12 months after procedure, adjusted for baseline scores. Values above zero favour SCS.
Discussion
This study compared two emerging treatment modalities and found no significant difference between the groups in the primary outcome of total exercise time, there were some significant differences in secondary outcomes.
PMR patients reported improved angina-free exercise capacity, CCS class, and health-related quality of life. These findings are similar to those previously reported using this laser system.2–4 We believe that both patient and laser system selection are important factors determining response to PMR treatment and can explain some of the differences between trials of laser revascularization.16,17 The laser used in this study was the Cardiogenesis system which differs from the Biosense DMR17 system in that angiographic and perfusion data are used to identify areas to be treated as opposed to electromechanical mapping and in that the DMR laser is a ‘contact’ system such that the depth of the channels created is significantly less. The patient group in our study suffered CCS class 3/4 angina as did the cohort reported by Stone et al.,16 but the latter group was potentially revascularizable by CABG and were less likely to have diffuse coronary artery disease. We were unable to demonstrate an increase in exercise time over 12 months as has previously been reported;3,4 however, this study was not powered to detect within group changes in exercise times.
The findings in the SCS group were similar. Adequate parasthesia coverage was obtained for all subjects at the time of implantation and there were few serious procedure-related complications showing that this procedure can be carried out safely. At study commencement, SCS was a new procedure for this institution and so was associated with a learning curve for the research team, particularly with regard to device insertion and programming. There was evidence to suggest that the SCS group had more advanced disease at baseline and this may account for some of the differences observed in the frequency of unstable coronary syndromes.
Previous studies suggest that refractory angina is indeed a chronic condition with little change over time. Control groups offer the best insight into the natural history of the condition. In our previous study,4 no one in the group managed medically reported a two-class improvement in CCS score over 12 months when compared with 36% of the group treated with PMR. The data from the randomized double-blind study of PMR2 and from longer follow-up (mean 43 months) in a study of TMR18 suggest that this improvement in angina scores cannot be explained solely by the placebo effect. Against this background natural history, improvement in angina scores and time to angina may be more important to the individual patient.
In this study, the impact of the placebo effect and of each of the two therapies on a patient's perceptions/expectations cannot be measured. PMR is a ‘one-off’ treatment, whereas commitment to SCS is long-term. This may have a significant impact on the reporting of the symptoms of a chronic condition and a differential placebo effect in that SCS may increase expectations of therapy or improve satisfaction with therapy because of greater patient control and multidisciplinary team involvement. These factors, in addition to a relatively small number of subjects, are limitations of this study. Significant differences observed in this study were in secondary outcomes for which this study was not powered. The cost-effectiveness of these treatments for refractory angina also needs to be considered, particularly against a clinical background where the major aim of treatment is the alleviation of disabling symptoms. There is retrospective data showing that the costs of SCS treatment may be offset by subsequent admission to hospital from small, uncontrolled studies19–21 or from a prospective study in another patient group.22 We intend to conduct a cost-effectiveness analysis at the 2-year time point including data from hospital admissions. On the basis of our current results, we would suggest that both SCS and PMR can be used as treatment options for refractory angina pectoris and that such treatment should be offered in centres with a specialist interest in this field, including multidisciplinary team involvement. In this institution, the development of the SCS service has enabled us to offer treatment to an increasing number of patients, who would not be suitable for PMR, such as those with thin myocardium and poor left ventricular function. Pre-existing comorbid conditions, which preclude the use of one or other treatment option, and patient preference should guide clinicians when choosing between these two modalities.
Acknowledgements
The authors would like to acknowledge the patients who participated in this study and the Departments of Cardiology, Cardiac Technology, Radiology, and Research and Development, Papworth Hospital for their support.
Contributions
Contributions to the study were as follows; DM; PMR treatment, SCS treatment, patient management and support, trial management, writing of the report (D.M.). Patient management, data analysis, writing of the report (S.N.K.). Study design, data analysis, writing of the report (L.D.S.). Data management, patient support (J.Y.R.). Trial management, data management, writing of the report (C.F.). Study design, trial management, writing of the report (N.C.). Study design, trial management, writing of the report (S.T.). Study design, SCS treatment, patient management, writing of the report (I.H.). Study design, PMR treatment, writing of the report, principal investigator, and acts as guarantor for this study (P.M.S.).
Conflict of interest: This study was sponsored by Medtronic SA, who were responsible for funding of the trial related investigations such as perfusion scans and treadmill tests, research staff for data collection, and travelling expenses for the subjects. The sponsor had no role in study design, data collection and interpretation, or in the decision to submit the report for publication.