-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Spies, Roland Strasheim, Ines Timmermanns, Rainer Schraeder, Patent foramen ovale closure in patients with cryptogenic thrombo-embolic events using the Cardia PFO occluder, European Heart Journal, Volume 27, Issue 3, February 2006, Pages 365–371, https://doi.org/10.1093/eurheartj/ehi617
Close - Share Icon Share
Abstract
Aims We report our experience with three generations of the Cardia patent foramen ovale (PFO) occluder in patients with cryptogenic thrombo-embolic events (TE).
Methods and results Between 1998 and 2004, interventional PFO closure was attempted in 403 patients. Prior to PFO closure, 605 TE occurred, translating into an annual incidence of 3.1%. PFO closure was successful in all patients. Peri-procedural complications occurred in eight patients (2.0%). At 6 months follow-up, residual shunt was present in 10.8% of patients. Transient thrombi developed on 10 devices (predominantly generation II) and asymptomatic wire fractures were detected in 14 cases (generation I and II). The annual incidence of recurrent TE was 2.0% (n=13). Atrial septal aneurysm and prior device-related thrombus formation were identified as predictors of recurrent TE.
Conclusion Owing to technical improvements and modified adjunctive pharmacotherapy, the rate of thrombus formation has declined and wire fractures are virtually absent in generation III devices. The overall rate of recurrent TE is reduced by transcatheter PFO closure with the Cardia PFO occluder, and seems comparable to recurrence rates reported for other devices used for this purpose.
See page 258 for the editorial comment on this article (doi:10.1093/eurheartj/ehi671)
Introduction
Shortly after the first report of a higher prevalence of patent foramen ovale (PFO) in patients with cryptogenic stroke, suggesting an association between the presence of PFO and cryptogenic thrombo-embolic events (TE), Bridges et al. reported a series of 36 patients who underwent transcatheter PFO closure following cerebral ischaemia due to presumed paradoxical embolism.1,2 Despite the ongoing controversy about the value of transcatheter intervention vs. medical therapy, transcatheter PFO closure is performed in annually increasing numbers and has virtually replaced the hypothetical alternative of open surgical closure.3 Despite the growing popularity of interventional PFO closure, only a limited number of larger series with more than 100 patients has been published.4–13
Following the introduction of the Cardia PFO occluder (Cardia Inc., Burnsville, MN, USA) in 1998, it has been systematically modified without changing the general concept of the device, which consists of a double umbrella design with Ivalon sails mounted on Nitinol wires. Aside from two large series of the Cardia PFO occluder published by Braun and colleagues, only a few studies are available where a minority of patients underwent PFO closure with the Cardia device.6,10,12–15 We report our experience with the first three generations of the Cardia PFO occluder (current model is generation IV) in patients with at least one TE of cryptogenic stroke, transient ischaemic attack (TIA), or peripheral thrombo-embolism.
Methods
Patient population
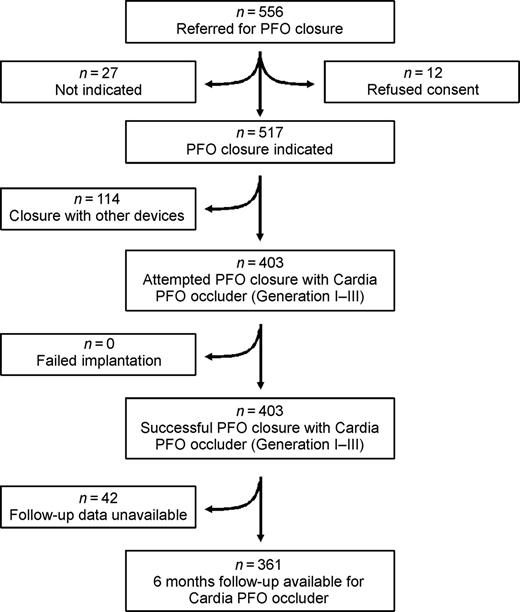
Between April 1998 and April 2004, 556 consecutive patients were referred to our tertiary referral centre in Frankfurt, Germany for possible transcatheter PFO closure. Five hundred and seventeen of those patients were deemed acceptable candidates for PFO closure after exclusion of patients who refused to give consent for the procedure and according to the criteria listed below (Figure 1). A total of 114 patients underwent PFO closure with other devices [fourth generation Cardia PFO occluder (n=63), Amplatzer PFO occluder (n=43), Cardioseal/Starflex occluder (n=8)]. In the remaining 403 patients, PFO closure was attempted with the first three generations of the Cardia PFO occluder (generation I–III), which are included in the present analysis.
All patients were required to fulfil the following criteria: (i) (a) a history of unequivocal ischaemic stroke, documented clinically by a neurologist and radiographically by either cranial computed tomography or magnetic resonance imaging, or (b) TIA, defined as a transient neurological deficit or vision loss with full recovery within 24 h confirmed clinically by a neurologist, or (c) peripheral thrombo-embolism verified clinically by an internist or a cardiologist, and radiographically by either computed tomography or angiography; and (ii) the exclusion of other identifiable cause for this TE, with: (a) ultrasound of cerebral arteries and aorta, (b) 24-hour Holter-EKG, (c) 24-hour blood-pressure measurements; and (iii) finally, presence of PFO with or without atrial septal aneurysm (ASA) with spontaneous or inducible right-to-left shunt during contrast transoesophageal echocardiography or transthoracally, if clearly visible. A small shunt volume was defined as 3–20 bubbles and a large shunt volume as >20 bubbles passing the PFO into the left atrium. An ASA was defined as an inter-atrial septum of abnormal mobility with protrusion of the septum into the left or right atrium of at least 10 mm beyond baseline. Informed consent was obtained from all patients or their relatives prior to the procedure. The protocol was approved by the Institutional Committee on human research.
The device
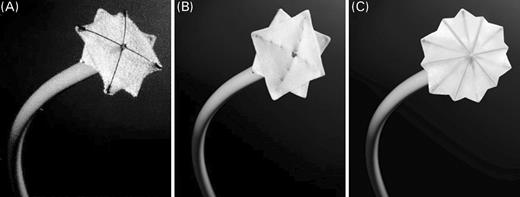
The Cardia PFO occluder consists of two square discs made of Ivalon (polyvinyl alcohol). Each umbrella has a diameter of 18, 22, 26, 30, or 35 mm and is expanded by four or six Nitinol arms (Figure 2).
Implantation procedure
Treatment with acetylsalicylic acid (100 mg) and clopidogrel (loading dose 300 mg) was initiated the day prior to the procedure. At the day of implantation, three doses of intravenous cefazolin (1.5 g) for prophylaxis of bacterial endocarditis were administered. After sheath placement in the right femoral vein, heparinization was performed with 100 units of unfractionated heparin per kilogram of body weight. Following premedication with metoclopramide (10 mg), atropine (0.5 mg) to decrease salivation, and midazolam (median dose 7.5 mg, range 2–25 mg) for conscious sedation, the transeosophageal echo probe was introduced.
A 5F multipurpose catheter was used for angiography of the fossa ovalis and to visualize the anatomy of the septum primum and secundum, as well as the length and orientation of the PFO-channel. Balloon sizing of the PFO was only performed until 2000, as estimation of the stretched diameter was not felt to be of any incremental value. After crossing the septum, the tip of the catheter was placed into the left superior pulmonary vein and the position was checked by injection of a small amount of contrast dye. The multipurpose catheter was then exchanged for a 9 to 11F trans-septal sheath by means of an extra stiff 0.035 in. exchange wire. Thereafter, angiography of the left atrium was performed to determine the size and shape of the atrium and the atrial septum. The Cardia PFO occluder was then delivered through the trans-septal sheath under fluoroscopic as well as transoesophageal echocardiographic guidance. After opening the left atrial disc, the device was pulled back together with the delivery system and sheath, against the septum. By keeping slight tension on the delivery system, the sheath was then pulled back until the right atrial part of the occluder opened. Before and after release, the position of the device was checked by right atrial contrast injection through the trans-septal sheath.
Post-interventional treatment and follow-up
Following an uncomplicated procedure, patients were discharged the following day. Before discharge, a complete blood count and C-reactive protein were measured. The position of the device was confirmed by chest X-ray and transthoracic echocardiography. For prophylaxis of TE after device implantation, patients were treated with clopidogrel (75 mg) for 4 weeks and with acetylsalicylic acid (100 mg) for 6 months. Standard bacterial endocarditis prophylaxis was recommended for 12 months. Patients were instructed to return to our institution after 6 months, at which time a chest X-ray for detection of device fractures, as well as transoesophageal contrast echocardiography for detection of residual shunting was performed. Residual shunting was graded following the definition used for evaluation of the PFO prior closure, as described earlier. Thereafter, patients were followed annually by telephone contact. They were further instructed to follow-up with their referring physicians who were asked to inform us of any change in clinical status. Recurrent TE (stroke, TIA, or peripheral embolism) was defined according to the prior stated inclusion criteria. Events were either recorded during patient follow-up, telephone contact, or reported through the referring physician.
Statistical analysis
Continuous data is presented as median with range. Incidence of ischaemic events before and after PFO closure was calculated as total number of events before and after defect closure, divided by cumulative patient-years pre- and post-intervention. Nominal variables were compared by two-sided Fisher's exact test. Survival free from recurrent TE was calculated with the Kaplan–Meier method, using the Wilcoxon–Gehan–Breslow test to identify potential predictors of recurrent TE. The follow-up time until the first recurrent TE in each individual was included in the analysis. For those patients without recurrent TE, available follow-up time was used for analysis purposes. Variables were chosen on the basis of potential confounders described in the literature, including the presence of any cardiovascular risk factors, defined as hypertension, diabetes mellitus, hyperlipidaemia, or smoking habit. Further, device-related parameters, which may confound the incidence of TE, were included in the analysis. The alpha-level was set at 0.05. Data were analysed with SAS (Version 8.2, SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 403 patients aged 17–80 years (median 49 years) were included in the present analysis. Patients had experienced 1–4 TE, with a median of one ischaemic episode per person prior to PFO closure. During 19 878 cumulative patient-years prior intervention, a total of 605 TE had occurred (Table 1), which translates into an annual incidence of TE of 3.1% (stroke: 1.9%; TIA: 1.0; peripheral thrombo-embolism: 0.2%). Most common comorbidities were hypertension (30%) and hyperlipidaemia (24%) (Table 1). An ASA was identified by echocardiography in 154 patients (38.2%).
Procedural success and immediate complications
The Cardia PFO occluder was implanted successfully in all patients. First generation devices were implanted in 14 cases (3.5%), second generation in 55 (13.7%), and third generation was implanted 334 times (82.8%). Median procedural and fluoroscopy time was 25 min (range 15–250 min) and 2.9 min (range 0.7–25.1 min), respectively. Peri-procedural complications occurred in eight patients (2.0%). In two cases the occluder embolized to the renal arteries, and following device removal via catheter the PFO was closed with a larger occluder. Transient ST-segment elevations occurred in four patients, presumably because of air embolism. Pericardial effusion was found in two patients within 1 month following the procedure. Neither patient required pericardiocentesis. Medical therapy resulted in resolution of the pericardial fluid collection and both patients had no recurrence during their 72 and 53 months follow-up period, respectively.
Recurrent thrombo-embolic events
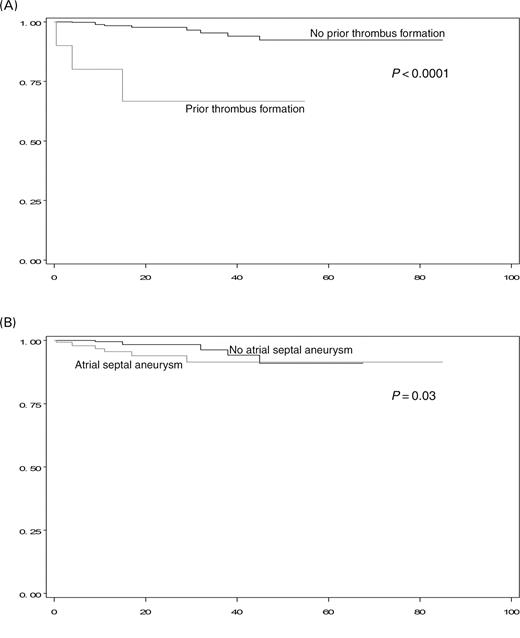
Prospective 6-month follow-up information including contrast transoesophageal echocardiography and chest X-ray is currently available for 361 patients (90%) (median follow-up time 12.6 months, range 0–85 months), totalling 645 cumulative patient-years. Recurrent strokes occurred in five patients and TIAs were noticed in eight cases, with one patient experiencing two events. This translates into an annual recurrence rate of 0.8% for strokes and 1.2% for TIAs, respectively (total incidence post-intervention 2.0%). The median time from PFO closure to recurrent TE was 15 months (range 0.5–47 months). Of those 12 patients experiencing a recurrent TE, seven had traditional cardiovascular risk factors, one had known thrombophilia, one new onset atrial fibrillation, seven an ASA. A stratified Kaplan–Meier analysis of potential predictors of recurrent TE is summarized in Table 2. An ASA at baseline was a predictor of recurrent events (annual incidence 2.6% [ASA present] vs. 1.6% [ASA absent]), although this finding was only statistically significant with the Wilcoxon–Gehan–Breslow test (P=0.03) and not with a log-rank analysis (P=0.2) (Figure 3). Prior device-related thrombus formation was identified as a predictor for recurrent TE [annual incidence 14.8% (prior thrombus formation) vs. 1.5% (no prior thrombus formation), P<0.0001]. The recurrent event rate was less in the subgroup of patients younger than 55 years of age [1.8% vs. 2.3% (≥55 years of age), P=0.6], but this finding was not statistically significant.
Residual shunt
A small residual shunt 6 months after implantation was present in 39 of 361 patients (10.8%). No large shunts were detected during follow-up, and all patients had a much smaller shunt volume at 6 months follow-up when compared with prior PFO closure. Residual shunting occurred in approximately equal frequencies among the three PFO occluder generations (Table 3). Among those found to have a residual shunt at 6 months, only one recurrent TE was observed, whereas among those without residual shunt, 12 events occurred.
Complications during follow-up
Three patients had pericardial effusions during follow-up. One case was considered due to peri-myocarditis supported by positive serologic viral markers and clinical course 10 months following PFO closure. The second effusion was found to be malignant, confirmed by positive cytology of pericardial fluid in a patient who was diagnosed with pancreatic cancer 28 months after his PFO closure. The third patient underwent surgical exploration 27 months after PFO closure, at which time a bleeding source at the coronary sinus with no evidence for cardiac perforation was found.
Transient thrombi developed on 10 devices of generation II and III, and were treated with coumadin (Table 3). None of those patients experienced any thrombo-embolic or bleeding complication when the thrombus was present, or during the period of oral anticoagulation. No intra-cardiac, non-device-related thrombi were seen during follow-up. Asymptomatic wire fractures were detected in 14 cases, occurring only in the earlier models (Table 3).
Discussion
The present data evaluating the Cardia PFO occluder represents the largest single-device series of transcatheter PFO closure in patients with cryptogenic TE published to date. This single centre experience further establishes the Cardia PFO occluder as a reliable and safe device in a wide variety of patients presenting with cryptogenic TE and coexisting PFO. Our data also provides additional evidence that PFO closure may reduce the risk of recurrent TE in patients with PFOs.
Comparison with other closure devices
Several devices are currently available for PFO closure, among which are the Cardia, the Cardioseal/Starflex, and the Amplatzer PFO occluder. Direct comparison of these devices is difficult, as most series consist of a variety of different occluders, each usually with a fairly small sample size. Further, some of these devices now are rarely used in clinical practice for transcatheter PFO closure. In an effort to overcome this limitation, Braun et al. compared the safety features and implantation characteristics of the abovementioned three devices in 307 patients, and comes to the conclusion that despite differences in design and concept of defect closure among those occluders, there is no obvious advantage of one device over the others.12 In contrast to this observation, is a recent report comparing the Amplatzer PFO occluder with the earlier generation Cardia device in 100 patients finding more complications and a higher incidence of residual shunts with the latter occluder.16 However, the reported complication rate of 16%, and incidence of residual shunt of 34% with the Cardia PFO occluder reported by Schwerzmann and colleagues seems unusually high, compared with a complication rate of 2.0% in our series which compares well with the incidence of major and minor complications of 1.5–7.9% reported in a recent review comparing large PFO closure studies with medical therapy.17
A residual shunt was found in approximately 10% of patients at 6 months follow-up, which appears favourable to the wide range of residual shunt reported at various follow-up times of 0%, in a series predominantly utilizing the Amplatzer devices to 49% with the Cardioseal occluder.18,19
Changes in the design of the Nitinol wires and improved retractile strength of the wires implemented in the II and III generation devices led to the absence of wire fractures. Although wire fractures are mostly asymptomatic, they potentially can result in catastrophic complications, such as cardiac perforation, making an eradication of wire fractures essential. This has been achieved in the case of the Amplatzer PFO occluder, but is a well-known complication of the Cardioseal device, with fracture rates of, i.e. 38%, in one series.20
Thrombus formation on the surface of inter-atrial closure devices has been estimated to occur in 3–27%.21,22 In particular, thrombi on the surface of the left atrial disc, as seen in a few cases in the present series, can result in potentially catastrophic TE. This complication has been rarely described with the Amplatzer PFO occluder, probably because the device causes a rather ‘controlled’ thrombosis within its meshwork.12,22 In umbrella shaped closure devices with metallic arms, such as the Cardia, Cardioseal, or Starflex occluder, most thrombi seem to adhere to the expanded Nitinol arms or the metallic centre post of the discs.22 Hence, placement of all Nitinol parts medial to the Ivalon sails, oriented towards the inter-atrial septum, a design change implemented in the III generation Cardia PFO occluder, may have led to the remarkable reduction in thrombus formation seen with the III generation device. Further, as the implementation of dual anti-platelet therapy with acetylsalicylic acid and clopidogrel for the first 4 weeks post-procedure, no device-related thrombus formation was seen with the Cardia PFO occluder at our institution. Additional considerations with regard to thrombus formation include the recent suggestion that prior anecdotal use of post-procedural prothrombotic (protamine) therapy may have some culpability in thrombus formation rates reported in certain early series. However, this interesting potential association has yet to be fully ascertained.23
Pericardial effusions were detected in five cases of which at most two may have been because of procedure or device-related cardiac perforation. This incidence of cardiac perforation is comparable to the Amplatzer device, where this potentially lethal complication occurs rarely, although the incidence of perforations may be under-reported.24 Overall, PFO closure in general, and specifically with the Cardia PFO occluder appears to be a safe procedure, a feature especially important for this kind of transcatheter therapy considering the ongoing controversy about the appropriateness of defect closure vs. medical therapy.25–27
Recurrence of TE events
The annual incidence of recurrent TIA and stroke following transcatheter PFO closure with the Cardia occluder was 2.0%; a value which falls right in-between the range of 0–4.9% found in prior series utilizing a variety of different occluder devices.17 Implantation of the Cardia PFO occluder appears to be associated with a relative risk reduction of 35% for the recurrence of any TE. However, a reduction in the incidence of TE events in this setting may not be simply because of PFO closure alone. Several confounding aspects need to be considered. First, all patients were receiving anti-platelet therapy temporarily following occluder placement, what represents an accepted treatment option for patients with PFO and cryptogenic TE in itself. Secondly, a detailed medication history prior PFO closure is not available for comparison with the post-procedural regimen. Thirdly, patients are older following transcatheter closure, what increases the possibility of worsening atherosclerosis or new onset atrial fibrillation as factors for recurrent TE. Nevertheless, in light of an annual incidence of 5.6% for recurrent stroke or TIA in the medical treatment arm of a recent non-randomized trial comparing PFO closure with medical therapy, transcatheter PFO closure may in fact have a significant treatment effect.13
Although a combined ASA with PFO in patients with cryptogenic stroke harbours a high risk of recurrence in medically treated patients, prior studies have shown that patients with an ASA undergoing transcatheter PFO closure do not appear to have an increased risk for recurrent TE compared with those having PFO alone.28,29 Our finding of an ASA being a possible predictor of TE following PFO closure is difficult to interpret, in particular as the identified difference reaches only statistical significance with the Wilcoxon–Gehan–Breslow test, which emphasizes earlier events after the intervention. On the other hand, evaluating this parameter with a log-rank test does not reveal statistically significant results, a finding underscored by the initially separated Kaplan–Meier curves, which merge completely with additional follow-up time (Figure 2). Stabilization of the atrial septum by the occluder has been a proposed mechanism explaining the absence in the association of ASA and PFOs with recurrent TE following the closure of the inter-atrial defect. Still, this mechanism may apply to our patient collective as well, and one might speculate that complete endothelialization occurring over the first several weeks is required for maximal stabilization of the septum, eventually explaining the statistical difference in ‘earlier’ recurrent events.
The finding of prior thrombus formation on the closure device as a predictor of recurrent TE is concerning, but not unexpected. As all TE occurred following resolution of the initially identified device-related thrombus, transoesophageal echocardiography may have missed residual thrombi during surveillance examinations, or thrombi have recurred after initial resolution of the clot leading to recurrent thrombo-embolism. As discussed earlier, changes and device design and anti-platelet therapy have eradicated device-related thrombus formation, thus minimizing this potential source of recurrent TE.
Comprehensibly, presence of residual shunt may be associated with an increased risk of recurrent TE. However, only one recurrent event was observed in patients with residual shunts at 6 months, with the remaining 12 recurrent TE occurring in patients with complete PFO closure. Since, having a residual shunt can hardly be expected to be protective, it seems reasonable to assume that the presence of residual shunt is not associated with the recurrence of TE in our study. Absence of this association adds to the varying findings in the literature with some authors reporting a correlation between residual shunt and treatment failure,15 whereas others do not.6,20
Despite an overall reduction in TE, we found a small increase in the incidence of TIAs following PFO closure. This finding most likely represents an overestimation of these, rather ‘soft’ events. By definition, there is no permanent objective evidence for a TIA, and patients or their primary physicians may have misinterpreted non-specific symptoms as TIAs reported to us. Nevertheless, we have elected to include all possible recurrent events, probably leading to an overestimation of recurrent TE, as only five patients had recurrent events with objective, permanent neurological sequelae.
Randomized controlled trials comparing medical therapy utilizing anticoagulants or anti-platelet medications with transcatheter PFO closure are needed; and although these trials are on their way, these data are not yet available. Clearly, our study does not answer the question about the superiority of transcatheter PFO closure vs. medical management in patients with cryptogenic TE; however, it further supports our understanding that actual PFO closure might reduce the risk of recurrent cryptogenic stroke or TIAs. This piece of evidence is in line with Khairy's recent review comparing interventional PFO closure with medical management which concludes that transcatheter closure of PFO may prevent a substantial proportion of cryptogenic strokes.17
Limitations
Aside from prior mentioned shortcomings, several limitations apply to our study. First, referral bias is a major limitation that applies to every case series reported by large volume referral centres. Secondly, although we have 645 patient-years of follow-up, 6-month data is only available for 90% of patients. Thirdly, the small number of recurrent events under powers our study to identify possible predictors of recurrent TE.
Conclusions
The Cardia PFO occluder is a safe and feasible device for transcatheter closure of PFOs. Owing to technical improvements and modified adjunctive pharmacotherapy, the rate of initial shortcomings, including thrombus formation and wire fractures, significantly improved. The overall clinical recurrence rate for cerebral ischaemic events is reduced by transcatheter PFO closure with the Cardia PFO occluder, and seems comparable to recurrence rates reported for other devices used for this purpose.
Note: Results addressing thrombus formation of 123 patients included in this series have been reported unauthorized elsewhere.22 Furthermore, partial data of our patients have been reported in other articles.6,9,12
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors gratefully thank Peter M. Meyer, PhD and Zhen Chen from the Section of Biostatistics at Rush University Medical Center for their assistance with the statistical analysis and critical review of the manuscript.
Conflict of interest: R. Schraeder has received travel reimbursement from Cardia, Inc. and was a proctor for the company. C. Spies, R. Strasheim, and I. Timmermanns have no potential conflict of interest.
Figure 1 Flow diagram of all patients referred for transcatheter PFO closure (for details see text).
Figure 2 Generations of the Cardia PFO occluder. (A) Generation I devices were constructed with 2 mm centre posts, 2 mm thick foam Ivalon sails, and titanium protective end caps. (B) In generation II, the frame was strengthened using ‘stranded’ wires, and the left atrial disc was mounted on the outside of the frame. Further, the Ivalon sail was thinned. (C) Generation III devices were constructed with two additional arms per umbrella resulting in hexagonal shaped umbrellas. See online supplementary material for a colour version of this figure.
Figure 3 Kaplan–Meier curves of freedom from recurrent TE. Stratified results based on the presence of prior device-related thrombus formation (A) and ASA at baseline (B). X-axis represents duration of follow-up in months, y-axis is percent freedom from recurrent TE. See online supplementary material for a colour version of this figure.
Baseline characteristics
| . | Number (n=403) . | %a . |
|---|---|---|
| Female | 178 | 44 |
| Male | 225 | 56 |
| Atrial septal aneurysms | 154 | 38.2 |
| Type of event (n=605)b | ||
| Stroke | 373 | 61.7 |
| TIA | 195 | 32.2 |
| Peripheral thrombo-embolism | 37 | 5.7 |
| Cardiovascular risk factors | 196 | 49 |
| Hypertension | 121 | 30 |
| Hyperlipidaemia | 97 | 24.1 |
| Smoking | 47 | 11.7 |
| Diabetes mellitus | 25 | 6.2 |
| Coronary artery disease | 17 | 4.2 |
| Thrombophilia | 35 | 8.7 |
| . | Number (n=403) . | %a . |
|---|---|---|
| Female | 178 | 44 |
| Male | 225 | 56 |
| Atrial septal aneurysms | 154 | 38.2 |
| Type of event (n=605)b | ||
| Stroke | 373 | 61.7 |
| TIA | 195 | 32.2 |
| Peripheral thrombo-embolism | 37 | 5.7 |
| Cardiovascular risk factors | 196 | 49 |
| Hypertension | 121 | 30 |
| Hyperlipidaemia | 97 | 24.1 |
| Smoking | 47 | 11.7 |
| Diabetes mellitus | 25 | 6.2 |
| Coronary artery disease | 17 | 4.2 |
| Thrombophilia | 35 | 8.7 |
aPercent calculated as variable frequency divided by total number of patients (n=403); except for type of events, where the number of events (stroke, TIA, or peripheral thrombo-embolism) is divided by the total number of thrombo-embolic events (n=605).
bTotal number of events (n=605) occurring in 403 patients.
Baseline characteristics
| . | Number (n=403) . | %a . |
|---|---|---|
| Female | 178 | 44 |
| Male | 225 | 56 |
| Atrial septal aneurysms | 154 | 38.2 |
| Type of event (n=605)b | ||
| Stroke | 373 | 61.7 |
| TIA | 195 | 32.2 |
| Peripheral thrombo-embolism | 37 | 5.7 |
| Cardiovascular risk factors | 196 | 49 |
| Hypertension | 121 | 30 |
| Hyperlipidaemia | 97 | 24.1 |
| Smoking | 47 | 11.7 |
| Diabetes mellitus | 25 | 6.2 |
| Coronary artery disease | 17 | 4.2 |
| Thrombophilia | 35 | 8.7 |
| . | Number (n=403) . | %a . |
|---|---|---|
| Female | 178 | 44 |
| Male | 225 | 56 |
| Atrial septal aneurysms | 154 | 38.2 |
| Type of event (n=605)b | ||
| Stroke | 373 | 61.7 |
| TIA | 195 | 32.2 |
| Peripheral thrombo-embolism | 37 | 5.7 |
| Cardiovascular risk factors | 196 | 49 |
| Hypertension | 121 | 30 |
| Hyperlipidaemia | 97 | 24.1 |
| Smoking | 47 | 11.7 |
| Diabetes mellitus | 25 | 6.2 |
| Coronary artery disease | 17 | 4.2 |
| Thrombophilia | 35 | 8.7 |
aPercent calculated as variable frequency divided by total number of patients (n=403); except for type of events, where the number of events (stroke, TIA, or peripheral thrombo-embolism) is divided by the total number of thrombo-embolic events (n=605).
bTotal number of events (n=605) occurring in 403 patients.
Stratified Kaplan–Meier analysis
| . | Events (annual incidence)a . | P-valueb . |
|---|---|---|
| Age | ||
| <55 years | 7 (1.8) | 0.6 |
| ≥55 years | 6 (2.3) | |
| Cardiovascular risk factors | ||
| Absent | 6 (2.1) | 0.3 |
| Present | 7 (2.0) | |
| Thrombophilia | ||
| Absent | 12 (2.0) | 0.99 |
| present | 1 (1.7) | |
| Atrial septal aneurysm | ||
| Absent | 6 (1.6) | 0.03 |
| Present | 7 (2.6) | |
| Device generation used | ||
| Generation I | 1 (1.5) | 0.5 |
| Generation II | 5 (2.4) | |
| Generation III | 7 (1.9) | |
| Thrombus formation | ||
| Absent | 9 (1.5) | <0.0001 |
| Present | 4 (14.8) | |
| Wire fractures | ||
| Absent | 10 (2.5) | 0.7 |
| Present | 1 (1.6) |
| . | Events (annual incidence)a . | P-valueb . |
|---|---|---|
| Age | ||
| <55 years | 7 (1.8) | 0.6 |
| ≥55 years | 6 (2.3) | |
| Cardiovascular risk factors | ||
| Absent | 6 (2.1) | 0.3 |
| Present | 7 (2.0) | |
| Thrombophilia | ||
| Absent | 12 (2.0) | 0.99 |
| present | 1 (1.7) | |
| Atrial septal aneurysm | ||
| Absent | 6 (1.6) | 0.03 |
| Present | 7 (2.6) | |
| Device generation used | ||
| Generation I | 1 (1.5) | 0.5 |
| Generation II | 5 (2.4) | |
| Generation III | 7 (1.9) | |
| Thrombus formation | ||
| Absent | 9 (1.5) | <0.0001 |
| Present | 4 (14.8) | |
| Wire fractures | ||
| Absent | 10 (2.5) | 0.7 |
| Present | 1 (1.6) |
aAbsolute number of events (total number is 13), and annual incidence calculated as number of events divided by patient follow-up years in each subgroup.
bWilcoxon–Gehan–Breslow test; the endpoint used was first recurrent thrombo-embolic event in each patient (total number is 12).
Stratified Kaplan–Meier analysis
| . | Events (annual incidence)a . | P-valueb . |
|---|---|---|
| Age | ||
| <55 years | 7 (1.8) | 0.6 |
| ≥55 years | 6 (2.3) | |
| Cardiovascular risk factors | ||
| Absent | 6 (2.1) | 0.3 |
| Present | 7 (2.0) | |
| Thrombophilia | ||
| Absent | 12 (2.0) | 0.99 |
| present | 1 (1.7) | |
| Atrial septal aneurysm | ||
| Absent | 6 (1.6) | 0.03 |
| Present | 7 (2.6) | |
| Device generation used | ||
| Generation I | 1 (1.5) | 0.5 |
| Generation II | 5 (2.4) | |
| Generation III | 7 (1.9) | |
| Thrombus formation | ||
| Absent | 9 (1.5) | <0.0001 |
| Present | 4 (14.8) | |
| Wire fractures | ||
| Absent | 10 (2.5) | 0.7 |
| Present | 1 (1.6) |
| . | Events (annual incidence)a . | P-valueb . |
|---|---|---|
| Age | ||
| <55 years | 7 (1.8) | 0.6 |
| ≥55 years | 6 (2.3) | |
| Cardiovascular risk factors | ||
| Absent | 6 (2.1) | 0.3 |
| Present | 7 (2.0) | |
| Thrombophilia | ||
| Absent | 12 (2.0) | 0.99 |
| present | 1 (1.7) | |
| Atrial septal aneurysm | ||
| Absent | 6 (1.6) | 0.03 |
| Present | 7 (2.6) | |
| Device generation used | ||
| Generation I | 1 (1.5) | 0.5 |
| Generation II | 5 (2.4) | |
| Generation III | 7 (1.9) | |
| Thrombus formation | ||
| Absent | 9 (1.5) | <0.0001 |
| Present | 4 (14.8) | |
| Wire fractures | ||
| Absent | 10 (2.5) | 0.7 |
| Present | 1 (1.6) |
aAbsolute number of events (total number is 13), and annual incidence calculated as number of events divided by patient follow-up years in each subgroup.
bWilcoxon–Gehan–Breslow test; the endpoint used was first recurrent thrombo-embolic event in each patient (total number is 12).
Comparison of device generations
| . | Generation I (n=14) . | Generation II (n=55) . | Generation III (n=292) . | All (n=361) . | P-value . |
|---|---|---|---|---|---|
| Wire fractures | 8 (57) | 6 (11) | 0 (0) | 14 (4) | <0.0001 |
| Thrombus formation | 0 (0) | 7 (13) | 3 (1) | 10 (3) | 0.0003 |
| Residual shunt | 1 (7) | 7 (13) | 31 (11) | 39 (11) | 0.88 |
| . | Generation I (n=14) . | Generation II (n=55) . | Generation III (n=292) . | All (n=361) . | P-value . |
|---|---|---|---|---|---|
| Wire fractures | 8 (57) | 6 (11) | 0 (0) | 14 (4) | <0.0001 |
| Thrombus formation | 0 (0) | 7 (13) | 3 (1) | 10 (3) | 0.0003 |
| Residual shunt | 1 (7) | 7 (13) | 31 (11) | 39 (11) | 0.88 |
Data are expressed as number and percentage. All P-values by Fisher's exact test (Freeman–Halton test).
Comparison of device generations
| . | Generation I (n=14) . | Generation II (n=55) . | Generation III (n=292) . | All (n=361) . | P-value . |
|---|---|---|---|---|---|
| Wire fractures | 8 (57) | 6 (11) | 0 (0) | 14 (4) | <0.0001 |
| Thrombus formation | 0 (0) | 7 (13) | 3 (1) | 10 (3) | 0.0003 |
| Residual shunt | 1 (7) | 7 (13) | 31 (11) | 39 (11) | 0.88 |
| . | Generation I (n=14) . | Generation II (n=55) . | Generation III (n=292) . | All (n=361) . | P-value . |
|---|---|---|---|---|---|
| Wire fractures | 8 (57) | 6 (11) | 0 (0) | 14 (4) | <0.0001 |
| Thrombus formation | 0 (0) | 7 (13) | 3 (1) | 10 (3) | 0.0003 |
| Residual shunt | 1 (7) | 7 (13) | 31 (11) | 39 (11) | 0.88 |
Data are expressed as number and percentage. All P-values by Fisher's exact test (Freeman–Halton test).
References
Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke.
Bridges ND, Hellenbrand W, Latson W, Filiano J, Newburger JW, Lock JE. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism.
Sievert H, Horvath K, Zadan E, Krumsdorf U, Fach A, Merle H, Scherer D, Schraeder R, Spies H, Nowak B, Lissmann-Jensen H. Patent foramen ovale closure in patients with transient ischemia attack/stroke.
Wahl A, Meier B, Haxel B, Nedeltchev K, Arnold M, Eicher E, Sturzenegger M, Seiler C, Mattle HP, Windecker S. Prognosis after percutaneous closure of patent foramen ovale for paradoxical embolism.
Braun MU, Fassbender D, Schoen SP, Haass M, Schraeder R, Scholtz W, Strasser RH. Transcatheter closure of patent foramen ovale in patients with cerebral ischemia.
Beitzke A, Schuchlenz H, Beitzke M, Gamillscheg A, Stein HI, Zartner P. Interventioneller Verschluss von Foramen ovale und Vorhofseptumdefekten nach paradox embolischen Ereignissen.
Martin F, Sanchez PL, Doherty E, Colon-Hernandez PJ, Delgado G, Inglessis I, Scott N, Hung J, King ME, Buonanno F, Demirjian Z, de Moor M, Palacios IF. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism.
Schraeder R. Indication and techniques of transcatheter closure of patent foramen ovale.
Onorato E, Melzi G, Casilli F, Pedon L, Rigatelli G, Carrozza A, Maiolino P, Zanchetta M, Morandi E, Angeli S, Anzola GP. Patent foramen ovale with paradoxical embolism: mid-term results of transcatheter closure in 256 patients.
Khositseth A, Cabalka AK, Sweeny JP, Fortuin FD, Reeder GS, Connolly HM, Hagler DJ. Transcatheter Amplatzer closure of atrial septal defects and patent foramen ovale in patients with presumed paradoxical embolism.
Braun M, Gliech V, Boscheri A, Schoen S, Gahn G, Reichmann H, Haass M, Schraeder R, Strasser RH. Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism.
Windecker S, Wahl A, Nedeltchev K, Arnold M, Schwerzmann M, Seiler C, Mattle HP, Meier B. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke.
Bruch L, Parsi A, Grad MO, Rux S, Burmeister T, Krebs H, Kleber FX. Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism.
Windecker S, Wahl A, Chatterjee T, Garachemani A, Eberli FR, Seiler C, Meier B. Percutaneous closure of patent foramen ovale in patients with paradoxical embolism: long-term risk of recurrent thromboembolic events.
Schwerzmann M, Windecker S, Wahl A, Mehta H, Nedeltchev K, Mattle H, Seiler C, Meier B. Percutaneous closure of patent foramen ovale: impact of device design on safety and efficacy.
Khairy P, O'Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli.
Bruch L, Parsi A, Grad MO, Rux S, Burmeister T, Krebs H, Kleber FX. Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism—single-center experience.
Martin F, Sanchez PL, Doherty E, Colon-Hernandez PJ, Delgado G, Inglessis I, Scott N, Hung J, King MEE, Buonanno F, Demirjian Z, de Moor M, Palacios IF. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism.
Hung J, Landzberg MJ, Jenkins KJ, King ME, Lock JE, Palacios IF, Lang P. Closure of patent foramen ovale for paradoxical emboli: intermediate-term risk of recurrent neurological events following transcatheter device placement.
Anzai H, Child J, Natterson B, Krivokapich J, Fishbein MC, Chan VK, Tobis JM. Incidence of thrombus formation on the CardioSEAL and the Amplatzer closure devices.
Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1000 consecutive patients.
Jux C, Bertram H. Thrombus formation on intracardiac devices: a complex issue.
Divekar A, Gaamangwe T, Shaikh N, Raabe M, Ducas J. Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder.
Adams HP. Patent foramen ovale: Paradoxical embolism and paradoxical data.
Halperin JL, Fuster V. Patent foramen ovale and stroke—another paradoxical twist.
Blackshear JL. Closure of patent foramen ovale in cryptogenic stroke. Ready of not, here come the trials.
Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both.
Wahl A, Krumsdorf U, Meier B, Sievert H, Ostermayer S, Billinger K, Schwerzmann M, Becker U, Seiler C, Arnold M, Mattle HP, Windecker S. Transcatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patients.