-
PDF
- Split View
-
Views
-
Cite
Cite
Diana Rinkevich, Sanjiv Kaul, Xin-Qun Wang, Khim Leng Tong, Todd Belcik, Saul Kalvaitis, Wolfgang Lepper, John M. Dent, Kevin Wei, Regional left ventricular perfusion and function in patients presenting to the emergency department with chest pain and no ST-segment elevation, European Heart Journal, Volume 26, Issue 16, August 2005, Pages 1606–1611, https://doi.org/10.1093/eurheartj/ehi335
Close - Share Icon Share
Abstract
Aims We hypothesized that the assessment of left ventricular regional function (RF) and myocardial perfusion (MP) will provide incremental value over routine evaluation in patients who present to the emergency department (ED) with chest pain (CP) and no ST-segment elevation.
Methods and results In addition to routine clinical evaluation, patients with suspected cardiac CP and no ST-segment elevation were evaluated in the ED for RF and MP using contrast echocardiography (CE). Cardiac-related death, acute myocardial infarction, unstable angina pectoris, congestive heart failure (CHF), and revascularization were considered as events within 48 h (early). Of the 1017 patients studied, 166 (16.3%) had early events. Adding RF increased the prognostic information of clinical and EKG variables significantly (Bonferroni corrected P<0.0001) for predicting these events. When MP was added, significant additional prognostic information was obtained (Bonferroni corrected P=0.0002). All patients were followed for a median of 7.7 months (25th–75th percentiles: 2.7–12.5) Of these, 292 (28.7%) had events. Adding RF increased the prognostic information of clinical and EKG variables for determining the risk of events significantly (Bonferroni corrected P<0.0001), which was further increased by adding MP (Bonferroni corrected P<0.0001).
Conclusion Early assessment of RF on CE adds significant diagnostic and prognostic value to routine evaluation in patients presenting to the ED with suspected cardiac CP and no ST-segment elevation. MP provides additional significant value. CE could be a valuable tool in the early triage and management of CP patients presenting to the ED.
See page 1573 for the editorial comment on this article (doi:10.1093/eurheartj/ehi381)
Introduction
In the USA, approximately 5 million people visit the emergency department (ED) each year with chest pain (CP). The vast majority are admitted either to the hospital or to a CP unit in the ED, but acute myocardial ischaemia or infarction (AMI) is confirmed in only 10–30%.1–3 Many have symptoms that are ultimately attributed to non-ischaemic causes.4,5 Of those discharged from the ED, an estimated 2–7% subsequently have acute coronary events.6,7 The initial EKG is diagnostic in only 30–40% of patients with ongoing AMI8 and cardiac enzymes do not become positive for several hours after coronary occlusion.9,10 Meanwhile, valuable time is lost before definitive therapy can be offered.
Assessment of either perfusion or function [generally single photon emission tomography (SPECT)11–13 for the former and echocardiography12–15 for the latter] has been shown to be of considerable value in risk-stratifying patients early after ED admission. Both abnormal left ventricular (LV) myocardial perfusion (MP) and regional function (RF) have been shown to identify high-risk patients, whereas the event rate in those with normal MP or RF has been shown to be low. In this study, we hypothesized that the assessment of both RF and MP will provide incremental value over routine clinical and EKG evaluation in patients presenting to the ED with CP and without ST elevation. To test our hypothesis, we used myocardial contrast echocardiography (MCE), which can provide a rapid bedside assessment of RF and MP.13,16
Methods
Study sample and protocol
This prospective study was approved by the Human Investigation Committee at the University of Virginia. Patients presenting to the ED with a complaint of CP not easily attributable to a non-cardiac cause (such as chest wall pain or pulmonary pathology), and who did not have ST-segment elevation on the EKG, were approached for enrolment. The inclusion criteria were age >30 years and CP lasting for at least 30 min and occurring within 12 h of ED admission. All patients provided written informed consent. After the history was obtained and physical examination was performed by a cardiologist, EKG, blood samples, and MCE were obtained sequentially. EKG and blood samples were repeated every 6 h as needed. RF or MP results were not shared with the ED physician, who admitted or discharged the patient based on routine criteria (clinical, EKG, and cardiac enzymes).
Echocardiography
Echocardiography was performed using a Sonos 5500 system (Philips Ultrasound). Three millilitres of Optison (GE Healthcare) was diluted in 60 mL of saline and infused intravenously at a rate of 3 mL min−1 using a model AS40A pump (Baxter). The infusion rate was adjusted so as to obtain homogeneous LV cavity opacification with shadowing limited only to the left atrium in the apical views. Images were first acquired for RF analysis in the apical and parasternal views using a low mechanical index (<0.3). MP images were then obtained using high mechanical index (1.0) intermittent imaging (ultrasound transmission gated to end-systole) with transmit/receive frequency of 1.3/3.6 MHz (ultraharmonics). Ultrasound compression was set at 75%. If tissue signal was seen despite optimization of imaging settings, intermittent harmonic power Doppler imaging was performed to eliminate this signal. Typical settings for this approach included a pulse repetition frequency of 2 kHz, colour gain of 65%, medium line density, small packet size, and minimal persistence. All settings were optimized at the beginning of the study and then held constant. Image acquisition was completed in less than 10 min.
MP images were acquired in apical two-, three-, and four-chamber views at pulsing intervals of one, two, three, four, and five cardiac cycles. The transmit focus was initially set at the level of the mitral valve, but was readjusted to the apex if an apical defect was seen in order to discriminate between a true defect and an artefact. Off-axis images were acquired as needed. RF and MP data were stored separately on magneto-optical disc.
Image interpretation
RF and MP images were read by different experienced observers blinded to all information. Each observer had previously performed RF analysis on thousands of studies and MP analysis on at least 500 patients. RF and MP were scored as normal or abnormal using a 14-segment model, with six segments each in the basal and mid-papillary muscle levels and two segments in the apex. MP was scored as abnormal if maximal myocardial opacification was not seen within a segment by five cardiac cycles. A study was considered abnormal if one or more segments were abnormal for either RF or MP. If any segment within a vascular territory could not be assessed from any of the three views, the study was classified as not interpretable. Each visualized segment was also given an RF score, where 0 indicates normal; 1, hypokinesia; 2, akinesia; and 3, dyskinesia.
Follow-up and definition of events
Follow-up was obtained every 6 months for up to 2 years by questionnaire or telephone interview with the patient, patient's family, or the physician. All reported events were confirmed by review of the medical record or death certificate. ‘Hard’ cardiac events included non-fatal AMI and cardiac-related death. Sudden death occurring without another explanation was categorized as cardiac. ‘Soft’ events included unstable angina pectoris (UAP), CHF, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG). If a patient had more than one ‘soft’ or ‘hard’ event, only the first one was considered for analysis. If a ‘soft’ event preceded a ‘hard’ event, it was ignored.
AMI was defined by an abnormal troponin I level (≥0.6 ng mL−1).10 UAP was defined as CP with dynamic EKG changes and/or peak troponin I levels >0.08 but <0.6 ng mL−1.10 A diagnosis of CHF required confirmation by chest X-ray. The diagnosis of both UAP and CHF required hospital admission. Early events were defined as those occurring within 48 h of ED admission.
Statistical methods
The primary purpose of the statistical analysis was to assess whether each test performed (RF and/or MP) in a hierarchical order added incremental values to routine evaluation. Thus, the pre-specified demographic, clinical, and EKG variables were first analysed because these are routinely available in all patients presenting to the ED with suspected cardiac CP. Then, the added value of RF was assessed because it would be the first piece of information available on CE. After this, the added value of MP was assessed because it would be the next piece of information available on CE (a full model).
For early events, logistic regression models were used to discriminate between patients with and without events.17,18 The pre-specified explanatory variables included age, hypertension, diabetes, hypercholesterolaemia, smoking status, EKG (normal or abnormal defined with computer-assisted interpretation by a blinded observer), RF, and MP, as well as interaction between the latter two. Cox proportional hazard models were used to determine the risk of cardiac events for all 1017 patients.17 The Cox proportional hazard assumption was verified based on a χ2 goodness of fit test17. Adjusted survival probability was based on the Cox model.
A Wald χ2 test was used to assess the significance of each predictor, and the odds/hazard ratio was used to quantify its effect. The global χ2 statistic by likelihood ratio test was used to compare the overall performance of RF and of combined RF and MP vs. routine evaluation, and subsequently of MP vs. routine evaluation plus RF. Analyses were also adjusted for multiple comparisons in sequential testing between models and in predicted survival probability between different combinations of RF and MP using Bonferroni correction. The type I error rate was specified at a significance level of 0.05, and all tests were two-sided. All statistical analyses were performed using S-Plus version 2000 (Mathsoft, Inc.).
Results
A power analysis indicated that 500 participants would provide at least 90% power for the analyses that were conducted in this study. Fortunately, we were able to recruit more than twice as many patients. Of the 1209 patients approached, 1183 agreed to be recruited and complete assessment was performed in 1182 (one did not get an EKG). Of these, 80 were lost to follow-up and 85 had RF or MP studies deemed to be of inadequate quality. Therefore, complete data were available in 1017. We recognized that the deletion of the patients with incomplete information could cause selection bias. However, we did not observe significant difference in baseline characteristics or in the incidence of events between patients with complete vs. incomplete information.
Early events within 48 h
AMI was seen in 9% of patients, most of whom had subsequent PCI. UAP was seen in ∼5% of patients, several of whom also had PCI. CHF was seen in a little over 1%, whereas cardiac-related death, emergent PCI, and CABG without a diagnosis of either AMI or UAP occurred in <1% each. The total early event rate was a little over 16%.
Table 1 lists the summary statistics of patients with early cardiac events. Of 20 such patients who had norma RF, only four (25%) had a hard event (AMI). Others had soft events: eight had UAP, seven had PTCA, and one had CABG. Of the 48 patients with early events who had normal MP, 21 (44%) had AMI; the others had soft events: 17 had UAP, three had CHF, six had PTCA, and one had CABG.
On the full multivariable logistic regression analysis, the significant predictors of early cardiac events were the EKG (P=0.0009), RF (P<0.0001), and MP (P=0.0001). There was no significant interaction between RF and MP, so the interaction term was not included in the full model. Patients with abnormal RF were 6.1 times as likely to have an early event compared with those with normal RF [95% confidence interval (CI): 3.5–10.6]. Those with abnormal MP were 2.4 times as likely to experience an event compared with those with normal MP (95% CI: 1.5–3.7). When both RF and MP were abnormal, the odds ratio for an early event was 14.3 (95% CI: 8.3–24.8, P<0.0001) compared with when both were normal.
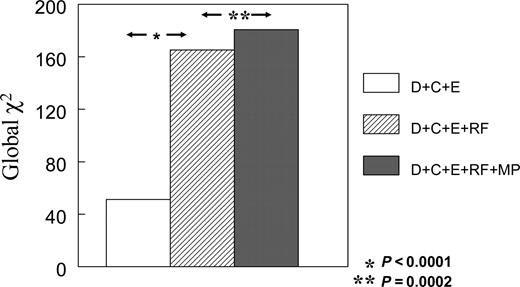
Figure 1 depicts the global χ2 values derived from multivariable logistic regression models for prediction of early events. The addition of RF to demographic, clinical, and EKG variables resulted in a highly significant increase in the global χ2 value for the prediction of early events (165.2 vs. 51.2, Bonferroni corrected P<0.0001). When MP was added to these variables the global χ2 value increased even further (180.7, Bonferroni corrected P=0.0002), indicating that while RF was a very powerful predictor of early events MP provided further incremental information.
Any events over a 2-year follow-up
Of the 1017 patients analysed, 292 (28.7%) had cardiac events over a median follow-up of 7.7 months (25th–75th percentiles: 2.7–12.5). Their characteristics are also depicted in Table 1. Again, the commonest event was AMI (13.1%) followed by UAP (6.7%), CHF (3.7%), PCI (2%), cardiac-related death (1.9%), and CABG (1.3%).
Of the 43 patients with events who had normal RF, only 10 had a hard event (AMI). Others had soft events: 13 had UAP, two had CHF, 12 had PTCA and six had CABG. Of the 100 patients with events who had normal MP, 35 had AMI and two experienced cardiac-related death. Others had soft events: 27 had UAP, 11 had CHF, 15 had PTCA, and six had CABG. Table 2 displays the number of events within the 2-year follow-up period, which were identified by combination of RF and MP, as well as EKG.
On the full multivariable Cox regression analysis, history of hypertension (P=0.028), EKG (P=0.0001), RF (P<0.0001), and MP (P<0.0001) were significant predictors of cardiac events. There was no significant interaction between RF and MP so the interaction was not included in the full model. Abnormal RF increased the risk of events by five-fold when compared with normal RF (95% CI: 3.4–7.2), whereas abnormal MP increased the risk by two-fold when compared with normal MP (95% CI: 1.5–2.7). When both RF and MP were abnormal, the risk increased to >10-fold (95% CI: 7.1–14.4, P<0.0001) compared with when both were normal.
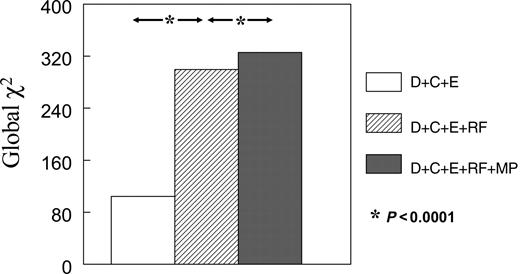
Figure 2 illustrates the global χ2 values from multivariable Cox regression models for determining the risk of late events. The addition of RF to demographic, clinical, and EKG variables resulted in a significant increase in the global χ2 value for determining risk of late cardiac events (299.4 vs. 104.3, Bonferroni corrected P<0.0001). When MP was added to these models, a further increase was noted (325.7, Bonferroni corrected P<0.0001). Thus, even though RF was a very powerful predictor of late events, MP provided significant additional information.
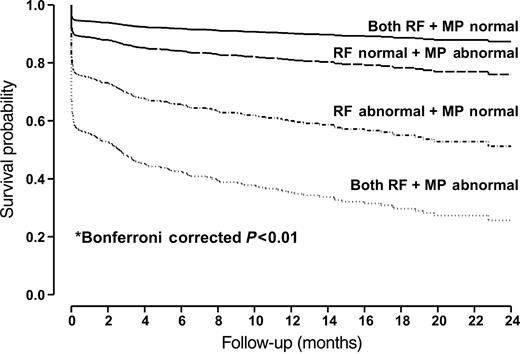
Figure 3 depicts the adjusted survival probability for patients with different combinations of RF and MP values. When both RF and MP were normal, the 1- and 2-year cardiac event rates were 9.8 and 12.6%, respectively. Most events were ‘soft’ in nature. These rates increased, respectively, to 18.9 and 21% when RF was normal but MP was abnormal (Bonferroni corrected P<0.0001), and to 40.1 and 48.8% when RF was abnormal but MP was normal (Bonferroni corrected P<0.0001). These rates increased even further (64.7 and 74.4%, respectively) when both RF and MP were abnormal (Bonferroni corrected P<0.0001). Thus, adding MP information further risk-stratified patients. Similar results were noted when cardiac events were divided into hard (death and AMI) or soft (UAP, CABG, CHF, and PCI). The overall results of the study were not different whether MP or RF was expressed in binary terms or as a proportion of segments that were abnormal. Similar conclusion can be made when the 18% patients with a prior Q-wave on the EKG and/or thinned out and scarred myocardium on echocardiography were excluded from the analysis.
Discussion
In this study, we have defined the incremental value of MP and RF over routine clinical and EKG evaluation in patients presenting to the ED with suspected cardiac CP and no ST-segment elevation on the initial EKG. Not only did the study include a large patient sample and employ a prospective design, but it also used a new clinical tool that can be employed rapidly at the bedside to provide direct online assessment of both RF and MP. This approach can assist a physician in determining disposition of patients immediately upon arrival to the ED, thus potentially increasing efficiency and decreasing cost.
Incremental value of RF and MP
The incremental value of RF over demographic, clinical, and EKG data in this study is even more impressive than in a previously published smaller study.14,15 Although MP alone also adds significant incremental information to routine clinical evaluation, the increase is not as marked as that provided by RF alone. This should not be surprising because resting LV function has been repeatedly demonstrated to be the most important predictor of future cardiac events.19,20
This information is contrary to findings of a previous smaller study in a similar patient cohort.12 In that study, we found MP to be superior to RF in predicting events. However, we used an old MCE technique (bolus injections of contrast) which has been found to be suboptimal and has been largely discarded. We also limited the follow-up to 48 h and did not include UAP as an event. Finally, the study cohort was one-fifth in size compared with the present study, the results of which are more relevant to contemporary echocardiography.
Interestingly, in the current study, MP added the most in patients who already had abnormal RF, which is a new finding. Those with normal MP but abnormal RF were at much lower risk than those in whom both MP and RF were abnormal. The latter probably indicates patients with very low myocardial blood flow, whereas the former represents those with near normal flow, and could include patients with myocardial stunning due to repetitive ischaemia or spontaneous reperfusion rather than those with total coronary occlusion. They could also represent patients with extensive collateral flow who have near normal MP, despite total coronary occlusion.21 Finally, patients with non-ischaemic cardiomyopathy (from hypertension, etc.) would also have normal MP despite LV dysfunction. All these patients would typically be at less risk than those in whom MP was persistently reduced, such as most patients with AMI and UAP.
Critique of our methods
Contrast improves endocardial border detection, thus enabling a better evaluation of RF.22,23 With this approach, RF could be assessed in all vascular territories in all but 2% of our patients, an incidence much lower than previously reported when contrast was not used. It also reduces observer variability and enhances the confidence of the reader.23
For MP, we used intermittent high mechanical index ultraharmonic imaging because the signal-to-noise ratio is more robust than with the ‘real-time’ low mechanical index approaches.24 Using this method, we were able to obtain complete evaluation of MP in all but 4% of our patients. We did not quantify MCE data for two principal reasons. First, we wanted to use an approach that would be currently applicable to the ED. Quantification requires offline image analysis and is time consuming at present. Secondly, we have previously shown that in a sizeable minority of patients, quantification is not possible because of the quality of the original studies.24 Perhaps, these limitations could be minimized in the future and rapid quantification of MCE data could be used routinely in any clinical setting.
We missed some patients who came to the ED at night. However, because patients could be recruited for up to 12 h after CP occurrence, most were enrolled the following morning. The proportion of patients with hard events that were missed by echocardiography alone was smaller than the number reported in patients discharged from the ED after routine evaluation.6,7 None of these events was fatal.
Clinical implications
The evaluation of patients presenting to the ED with CP has not changed appreciably over the past four decades. It still relies on the EKG and blood tests. Although newer blood tests (troponin, myoglobin, etc.) have increased the sensitivity of AMI detection, these still take several hours to become positive. Our results show that the rapid assessment of RF and MP provides significant incremental information when compared with routine evaluation of patients presenting to the ED with CP.
On the basis of our results, we suggest the following paradigm in patients presenting to the ED with suspected cardiac CP who do not have ST-segment elevation on the EKG. Rather than wait for serum troponin levels, these patients should immediately undergo CE to assess RF. If RF is normal and there is a low pre-test likelihood of CAD, the patients could be discharged. If the pre-test likelihood for CAD is moderate to high, the patient should undergo stress imaging.
If RF is abnormal, the patient should undergo MP assessment. If MP is abnormal, the patient should undergo coronary angiography. If MP is normal, the patient should be monitored until the troponin results become available. If troponin is positive, the patient should undergo coronary angiography; if it is negative and the patient is stable, they should undergo stress imaging for detection of ischaemia (reversible MP or RF defects).
We believe that such an approach will not only help identify patients experiencing myocardial ischaemia or AMI early and offer them appropriate therapy, but will also identify low-risk patients who can be safely discharged, thus reducing cost. Obviously, such an algorithm needs to be applied prospectively in the ED to determine its value in patient management and curtailing cost.
Acknowledgements
Supported in part by grants from the National Institutes of Health, Bethesda, MD, USA (R01-RO1-HL66034) and the American Society of Echocardiography, Durham, NC, USA awarded to S.K. Microbubbles were provided by GE Healthcare (Princeton, NJ, USA) and the ultrasound equipment was provided by Philips Ultrasound (Andover, MA, USA). K.L.T. was the recipient of a Research Fellowship Award from the National Medical Research Council of Singapore. K.W. was the recipient of a Mentored Clinical Scientist Development Award (K08-HL03909) of the National Institutes of Health. T.B. was the recipient of the Sonographer Research Award of the American Society of Echocardiography.
Presented at the Young Investigator Award Competition at the Annual Scientific Meeting of the American Society of Echocardiography, San Diego, CA, USA in June 2004.
Figure 1 Incremental value of tests performed for prediction of early events. D, demographics; C, clinical; E, EKG. P-values as Bonferroni corrected.
Figure 2 Incremental value of tests performed for determining risk of all events. Abbreviations used are as in Figure 1. P-values are Bonferonni corrected.
Figure 3 Adjusted survival probabilities for patients with different combinations of RF and MP values. *Bonferroni corrected P<0.01 indicated there were significant differences in survival probabilities between any two groups. The survival probabilities have been adjusted for patients' age, hypertension, diabetes, hypercholesterolaemia, smoking status, and EKG.
Summary statistics
| Variable . | All patients within 2 years (n=1017) . | No events within 2 years (n=725) . | Events within 48 h (n=166) . | Events within 2 years (n=292) . |
|---|---|---|---|---|
| Agea | 60 (49, 60, 70) | 59 (48, 58, 69) | 62 (51, 61, 72) | 63 (52, 63, 73) |
| Gender (Male) | 542 (53%) | 349 (48%) | 112 (67%) | 193 (66%) |
| Hypertension | 660 (65%) | 448 (62%) | 117 (70%) | 212 (73%) |
| Diabetes | 292 (29%) | 176 (24%) | 59 (36%) | 116 (40%) |
| Hypercholesterolaemia | 534 (53%) | 359 (50%) | 97 (58%) | 175 (60%) |
| Smoker | 280 (28%) | 190 (26%) | 47 (28%) | 90 (31%) |
| Abnormal EKG | 375 (37%) | 325 (45%) | 142 (86%) | 242 (83%) |
| Abnormal RF | 478 (47%) | 229 (32%) | 146 (88%) | 249 (85%) |
| Abnormal MP | 347 (34%) | 151 (21%) | 118 (71%) | 196 (67%) |
| Both RF and MP abnormal | 309 (30%) | 116 (16%) | 116 (70%) | 193 (66%) |
| Abnormal RF and normal MP | 169 (17%) | 113 (16%) | 30 (18%) | 56 (19%) |
| Normal RF and abnormal MP | 38 (4%) | 35 (5%) | 2 (1%) | 3 (1%) |
| Variable . | All patients within 2 years (n=1017) . | No events within 2 years (n=725) . | Events within 48 h (n=166) . | Events within 2 years (n=292) . |
|---|---|---|---|---|
| Agea | 60 (49, 60, 70) | 59 (48, 58, 69) | 62 (51, 61, 72) | 63 (52, 63, 73) |
| Gender (Male) | 542 (53%) | 349 (48%) | 112 (67%) | 193 (66%) |
| Hypertension | 660 (65%) | 448 (62%) | 117 (70%) | 212 (73%) |
| Diabetes | 292 (29%) | 176 (24%) | 59 (36%) | 116 (40%) |
| Hypercholesterolaemia | 534 (53%) | 359 (50%) | 97 (58%) | 175 (60%) |
| Smoker | 280 (28%) | 190 (26%) | 47 (28%) | 90 (31%) |
| Abnormal EKG | 375 (37%) | 325 (45%) | 142 (86%) | 242 (83%) |
| Abnormal RF | 478 (47%) | 229 (32%) | 146 (88%) | 249 (85%) |
| Abnormal MP | 347 (34%) | 151 (21%) | 118 (71%) | 196 (67%) |
| Both RF and MP abnormal | 309 (30%) | 116 (16%) | 116 (70%) | 193 (66%) |
| Abnormal RF and normal MP | 169 (17%) | 113 (16%) | 30 (18%) | 56 (19%) |
| Normal RF and abnormal MP | 38 (4%) | 35 (5%) | 2 (1%) | 3 (1%) |
aExpressed as mean [25th percentile, 50th percentile (Median), 75th percentile].
Summary statistics
| Variable . | All patients within 2 years (n=1017) . | No events within 2 years (n=725) . | Events within 48 h (n=166) . | Events within 2 years (n=292) . |
|---|---|---|---|---|
| Agea | 60 (49, 60, 70) | 59 (48, 58, 69) | 62 (51, 61, 72) | 63 (52, 63, 73) |
| Gender (Male) | 542 (53%) | 349 (48%) | 112 (67%) | 193 (66%) |
| Hypertension | 660 (65%) | 448 (62%) | 117 (70%) | 212 (73%) |
| Diabetes | 292 (29%) | 176 (24%) | 59 (36%) | 116 (40%) |
| Hypercholesterolaemia | 534 (53%) | 359 (50%) | 97 (58%) | 175 (60%) |
| Smoker | 280 (28%) | 190 (26%) | 47 (28%) | 90 (31%) |
| Abnormal EKG | 375 (37%) | 325 (45%) | 142 (86%) | 242 (83%) |
| Abnormal RF | 478 (47%) | 229 (32%) | 146 (88%) | 249 (85%) |
| Abnormal MP | 347 (34%) | 151 (21%) | 118 (71%) | 196 (67%) |
| Both RF and MP abnormal | 309 (30%) | 116 (16%) | 116 (70%) | 193 (66%) |
| Abnormal RF and normal MP | 169 (17%) | 113 (16%) | 30 (18%) | 56 (19%) |
| Normal RF and abnormal MP | 38 (4%) | 35 (5%) | 2 (1%) | 3 (1%) |
| Variable . | All patients within 2 years (n=1017) . | No events within 2 years (n=725) . | Events within 48 h (n=166) . | Events within 2 years (n=292) . |
|---|---|---|---|---|
| Agea | 60 (49, 60, 70) | 59 (48, 58, 69) | 62 (51, 61, 72) | 63 (52, 63, 73) |
| Gender (Male) | 542 (53%) | 349 (48%) | 112 (67%) | 193 (66%) |
| Hypertension | 660 (65%) | 448 (62%) | 117 (70%) | 212 (73%) |
| Diabetes | 292 (29%) | 176 (24%) | 59 (36%) | 116 (40%) |
| Hypercholesterolaemia | 534 (53%) | 359 (50%) | 97 (58%) | 175 (60%) |
| Smoker | 280 (28%) | 190 (26%) | 47 (28%) | 90 (31%) |
| Abnormal EKG | 375 (37%) | 325 (45%) | 142 (86%) | 242 (83%) |
| Abnormal RF | 478 (47%) | 229 (32%) | 146 (88%) | 249 (85%) |
| Abnormal MP | 347 (34%) | 151 (21%) | 118 (71%) | 196 (67%) |
| Both RF and MP abnormal | 309 (30%) | 116 (16%) | 116 (70%) | 193 (66%) |
| Abnormal RF and normal MP | 169 (17%) | 113 (16%) | 30 (18%) | 56 (19%) |
| Normal RF and abnormal MP | 38 (4%) | 35 (5%) | 2 (1%) | 3 (1%) |
aExpressed as mean [25th percentile, 50th percentile (Median), 75th percentile].
Number of events during 2 years follow-up based on key variables
| Type of event . | Echocardiography . | EKG . | Total events . | ||||
|---|---|---|---|---|---|---|---|
| . | RF Nor+MP Nor . | RF Nor+MP Abn . | RF Abn+MP Nor . | RF Abn+MP Abn . | Nor . | Abn . | . |
| AMI | 9 | 1 | 26 | 98 | 21 | 113 | 134 |
| Cardiac death | 0 | 0 | 2 | 17 | 2 | 17 | 19 |
| CABG | 6 | 0 | 0 | 7 | 6 | 7 | 13 |
| CHF | 1 | 1 | 10 | 26 | 3 | 35 | 38 |
| PTCA | 11 | 1 | 4 | 4 | 7 | 13 | 20 |
| USAP | 13 | 0 | 14 | 41 | 11 | 57 | 68 |
| Total | 40 | 3 | 56 | 193 | 50 | 242 | 292 |
| Type of event . | Echocardiography . | EKG . | Total events . | ||||
|---|---|---|---|---|---|---|---|
| . | RF Nor+MP Nor . | RF Nor+MP Abn . | RF Abn+MP Nor . | RF Abn+MP Abn . | Nor . | Abn . | . |
| AMI | 9 | 1 | 26 | 98 | 21 | 113 | 134 |
| Cardiac death | 0 | 0 | 2 | 17 | 2 | 17 | 19 |
| CABG | 6 | 0 | 0 | 7 | 6 | 7 | 13 |
| CHF | 1 | 1 | 10 | 26 | 3 | 35 | 38 |
| PTCA | 11 | 1 | 4 | 4 | 7 | 13 | 20 |
| USAP | 13 | 0 | 14 | 41 | 11 | 57 | 68 |
| Total | 40 | 3 | 56 | 193 | 50 | 242 | 292 |
Nor, normal; Abn, abnormal.
Number of events during 2 years follow-up based on key variables
| Type of event . | Echocardiography . | EKG . | Total events . | ||||
|---|---|---|---|---|---|---|---|
| . | RF Nor+MP Nor . | RF Nor+MP Abn . | RF Abn+MP Nor . | RF Abn+MP Abn . | Nor . | Abn . | . |
| AMI | 9 | 1 | 26 | 98 | 21 | 113 | 134 |
| Cardiac death | 0 | 0 | 2 | 17 | 2 | 17 | 19 |
| CABG | 6 | 0 | 0 | 7 | 6 | 7 | 13 |
| CHF | 1 | 1 | 10 | 26 | 3 | 35 | 38 |
| PTCA | 11 | 1 | 4 | 4 | 7 | 13 | 20 |
| USAP | 13 | 0 | 14 | 41 | 11 | 57 | 68 |
| Total | 40 | 3 | 56 | 193 | 50 | 242 | 292 |
| Type of event . | Echocardiography . | EKG . | Total events . | ||||
|---|---|---|---|---|---|---|---|
| . | RF Nor+MP Nor . | RF Nor+MP Abn . | RF Abn+MP Nor . | RF Abn+MP Abn . | Nor . | Abn . | . |
| AMI | 9 | 1 | 26 | 98 | 21 | 113 | 134 |
| Cardiac death | 0 | 0 | 2 | 17 | 2 | 17 | 19 |
| CABG | 6 | 0 | 0 | 7 | 6 | 7 | 13 |
| CHF | 1 | 1 | 10 | 26 | 3 | 35 | 38 |
| PTCA | 11 | 1 | 4 | 4 | 7 | 13 | 20 |
| USAP | 13 | 0 | 14 | 41 | 11 | 57 | 68 |
| Total | 40 | 3 | 56 | 193 | 50 | 242 | 292 |
Nor, normal; Abn, abnormal.
References
Strussman BJ. National Hospital Ambulatory Medicine Care Survey: 1995 Emergency Department Summary. Advance data from vital and health statistics of the Center for Disease Control Prevention/National Center for Health Statistics.
Gilber WB, Lewis LM, Erb RE, Markens PK, Kaplan BC, Vaughn RH, Biagini AV, Blanton JD, Campbell WB. Early detection of acute myocardial infarction in patients presenting with chest pain and nondiagnostic ECGs: serial CK-MB sampling in the emergency department.
Gilber WB, Young GP, Hedges JR, Lewis LM, Smith MS, Carleton SC, Aghababian RV, Jordon RO, Allison EJ, Otten EJ. Acute myocardial infarction in chest pain patients with non-diagnostic ECGs: serial CK-MB sampling in the emergency department.
Lee TH, Cook EF, Weisberg M, Wilson C, Goldman L. Acute chest pain in the emergency room: identification and examination of low-risk patients.
Selker HP, Griffith JL, D'agostino RB. A tool for judging coronary care unit admission appropriateness, valid for both real-time and retrospective use: a time-insensitive predictive instrument (TIPI) for acute cardiac ischemia: a multicenter study.
Lee TH, Rouan GW, Weinsberg MC, Brand DA, Acampora A, Stasiulewicz C, Walshon J, Terranova G, Gottleib L, Goldstein-Wayne B. Clinical characteristics and natural history of patients with acute myocardial infarction sent home from the emergency room.
Rude RE, Poole WK, Muller JE, Turi Z, Rutherford J, Parker C, Roberts R, Raabe DS, Gold HK, Stone PH; for the MILIS study group. Electrocardiographic and clinical criteria for recognition of acute myocardial infarction based on analysis of 3,697 patients.
Short D. The earliest electrocardiographic evidence of myocardial infarction.
Goldmann BU, Hamm CW. Risk stratification in acute coronary syndrome.
The Joint European Society of Cardiology/American College of Cardiology Committee: myocardial infarction redefined: a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction.
Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Handler J, Heller GV, Hendel RC, Pope JH, Ruthazer R, Speigler EJ, Woolard RH, Selker HP. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial.
Kaul S, Senior R, Firschke C, Wang X, Lindner JR, Villanueva FS, Kontos MC, Firoozan S, Taylor A, Nixon JV, Watson DD, Harrell FE. Incremental value of cardiac imaging in patients presenting to the emergency department with chest pain and without ST-segment elevation: A multicenter study.
Kontos MC, Kurdziel K, McQueen R, Arrowood JA, Jesse RL, Ornato JP, Paulsen WH, Tatum JL, Nixon JV. Comparison of 2-dimensional echocardiography and myocardial perfusion imaging for diagnosing myocardial infarction in emergency department patients.
Sabia P, Afrookteh A, Touchstone DA, Keller MW, Esquivel L, Kaul S. Value of regional wall motion abnormality in the emergency room diagnosis of acute myocardial infarction: a prospective study using two-dimensional echocardiography.
Sabia P, Abbott RD, Afrookteh A, Keller MW, Touchstone DA, Kaul S. The importance of two-dimensional echocardiographic assessment of left ventricular systolic function in patients presenting to the emergency room with cardiac-related symptoms.
Kaul S, Senior R, Dittrich H, Raval U, Khattar R, Lahiri A. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography.
White HD, Norris RM, Brown MA, Takayama M, Meslowski A, Bass NM, Ormiston JA, Whitlock T: Left ventricular end-systolic volume as the major determinant of survival after recovery from acute myocardial infarction.
Senior R, Lahiri A, Kaul S. Effect of revascularization on left ventricular remodeling in patients with severe chronic ischemic left ventricular dysfunction: influence on mortality.
Coggins MP, Le DE, Wei K, Goodman NC, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow.
Lindner JR, Dent JM, Moos S, Jayaweera AR, Kaul S. Enhancement of left ventricular cavity opacification by harmonic imaging after venous injection of Albunex.
Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging.
Dawson D, Rinkevich D, Belcik T, Jayaweera AR, Rafter P, Kaul S, Wei K. Measurement of myocardial blood flow velocity reserve with myocardial contrast echocardio-graphy in patients with suspected coronary artery disease: comparison with Technetium 99msestamibi single photon emission tomography