-
PDF
- Split View
-
Views
-
Cite
Cite
Jürgen Pache, Alban Dibra, Julinda Mehilli, Josef Dirschinger, Albert Schömig, Adnan Kastrati, Drug-eluting stents compared with thin-strut bare stents for the reduction of restenosis: a prospective, randomized trial, European Heart Journal, Volume 26, Issue 13, July 2005, Pages 1262–1268, https://doi.org/10.1093/eurheartj/ehi098
Close - Share Icon Share
Abstract
Aim Drug-eluting stents have considerably reduced restenosis. Their relative merits have been assessed on the basis of comparisons made with control bare stents with thick struts. However, increased strut thickness negatively affects restenosis. No direct comparisons between drug-eluting stents and bare stents with thin struts have been performed. The aim of this study was to evaluate the relative efficacy of sirolimus-eluting stents (Cypher™) as compared with that of bare stents with thin struts (BeStent™ 2).
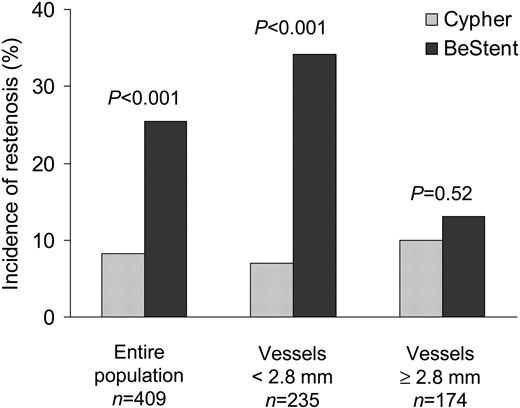
Methods and results A total of 500 patients with coronary artery disease were randomly assigned to receive a Cypher stent or BeStent. The primary endpoint was angiographic restenosis defined as a stenosis diameter ≥50% at 6-month angiographic follow-up. The secondary endpoint was the need for target vessel revascularization (TVR) during the year following the procedure. Follow-up angiography was performed in 81.8% of the patients. Patients treated with Cypher stents had a lower angiographic restenosis rate [8.3 vs. 25.5%, relative risk, 0.33 (95% confidence interval, 0.19–0.56), P<0.001] and a lower incidence of TVR [7.2 vs. 18.8%, relative risk, 0.38 (0.22–0.66), P<0.001]. For smaller vessels (<2.8 mm), the angiographic restenosis rates were 7.0% with the Cypher stent and 34.2% with the BeStent (P<0.001). For larger vessels (≥2.8 mm), angiographic restenosis rates were 10.0% with the Cypher stent and 13.1% with the BeStent (P=0.52).
Conclusion The drug-eluting stent, Cypher, is associated with a significantly lower risk of restenosis compared with the bare thin-strut BeStent. The advantage of the Cypher stent is vastly reduced in large vessels.
Introduction
Restenosis constitutes the major drawback of percutaneous coronary interventions. Despite leading to better results compared with plain balloon angioplasty, the long-term success of coronary stenting has also been limited by a significant rate of restenosis, particularly in some high-risk lesions.1–4 The use of the recently developed drug-eluting stents, coated with substances that actively inhibit muscle cell proliferation and possess anti-inflammatory properties, has led to a spectacular reduction in the incidence of coronary restenosis in studies in which they have been compared with bare metal control stents with thick struts. Currently, these new devices are considered the most effective tool to prevent restenosis.5–7 Thus, in the Sirolimus-Coated BX Velocity Balloon-Expandable Stent in the Treatment of Patients with de novo Coronary Artery Lesions (SIRIUS) trial, patients treated with the drug-eluting stent had a 76% reduction in the restenosis rate compared with those treated with the control BX Velocity stent.6 However, while the efficacy of these devices for the reduction of restenosis in the studied populations is well established, there is no evidence that they can influence mortality or myocardial infarction (MI) rate. Consequently, their cost-effectiveness largely depends on the reduction of restenosis achieved with this new technology. Nowadays, there is ongoing debate about whether drug-eluting stents are cost-effective, and the current price of these devices is highly prohibitive for widespread use.8–10
The large amount of accumulated data shows that stent characteristics, such as stent design, composition, and strut thickness may have a significant impact on the outcome of patients undergoing coronary stenting.11–16 In particular, strut thickness of the stents has been found to play an important role in the development of in-stent restenosis with thin-strut stents reported to cause less neointima proliferation.15–18 It is also thought that thinner struts create more favourable conditions for re-endothelialization.19 Data from the Intracoronary Stenting and Angiographic Results: Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO-2) trial, which compared the thick-strut BX Velocity stent with a thin-strut stent, demonstrated a 43% reduction in the incidence of angiographic restenosis among patients assigned to the thin-strut stent.16 In addition, results from randomized trials comparing stenting with balloon angioplasty for lesions in small coronary vessels have been more favourable for stenting when the thin-strut BeStent has been used.20–24
Because all previous comparisons of drug-eluting stents have been made with control stents with thick struts, their effectiveness might have been overestimated. No prior studies have compared the relative efficacy of antiproliferative drug-eluting stents with bare metal stents with thin struts. Therefore, we undertook a randomized trial to compare the value of a sirolimus-eluting stent (Cypher) with a bare metal stent with thin struts (BeStent™ 2), for the prevention of restenosis.
Methods
Patients
This prospective, randomized trial was performed in the Deutsches Herzzentrum and the Medizinische Klinik, Klinikum rechts der Isar, Munich, Germany. A total of 500 patients with symptomatic coronary artery disease and significant angiographic stenosis in native coronary vessels were enrolled in this study. Exclusion criteria were acute MI, lesion in left main coronary artery, in-stent restenosis, contraindications to the antiplatelet drugs (clopidogrel, aspirin), and lack of consent to participate in the study.
The study was conducted according to the principles of the Declaration of Helsinki and approved by the institutional ethics committee. All patients had given their informed consent for participation in the trial as well as for the 6-month follow-up coronary angiography.
Randomization, stent placement, and post-stenting treatment
Immediately after successful passage of the guidewire through the target lesion, the patients were randomly assigned to receive one of the two pre-mounted stent types: BeStent™ 2 (Medtronic, Inc., Minneapolis, MN, USA) with a strut thickness of 0.076 mm, and the Cypher stent (J&J Cordis, Miami Lakes, FL, USA) with a strut thickness of 0.140 mm. Randomization was performed with the use of sealed envelopes containing the computer-generated randomization sequence. A block sequence of 50 was used for sequence generation. The procedure was considered successful when stent placement was associated with a residual stenosis of <30% and thrombolysis in myocardial infarction (TIMI) flow grade ≥2. All patients received a loading dose of 600 mg clopidogrel at least 2–4 h prior to the procedure and intravenous aspirin plus heparin during the procedure. After the intervention, the patients received aspirin (100 mg bid), indefinitely and clopidogrel 2×75 mg for the first 3 days and 75 mg for at least 6 months. The use of additional antithrombotic drugs was at the discretion of the operators. Beta-blockers, statins, nitrates, and angiotensin-converting enzyme (ACE-) inhibitors were given if clinically indicated.
Angiographic evaluation
Angiograms recorded before and immediately after the procedure as well as at 6-month follow-up were assessed with the aid of the automated edge-detection system CMS (Medis Medical Imaging System, Nuenen, Netherlands). Lesions were classified according to the modified American College of Cardiology (ACC)/American Heart Association (AHA) grading system.25 All measurements were performed on cine-angiograms recorded after intra-coronary nitroglycerin administration. The same projections were used at all time points. The contrast-filled non-tapered catheter tip was used for calibration. Late lumen loss was the difference in the minimal lumen diameter between that immediately after the procedure and that at follow-up. Angiographic restenosis was defined as a diameter stenosis ≥50% at angiographic follow-up at 6 months measured at any point within the stented segment or in the 5-mm proximal or distal segments adjacent to the stent.
Clinical evaluation
Adverse events were monitored throughout the follow-up period: by telephone interview at 30 days, a clinical visit at 6 months, and an additional telephone interview at 1 year after the intervention. If patients reported cardiac symptoms during the telephone interview, at least a clinical and electrocardiographic follow-up visit was performed at the outpatient clinic or by the referring physician. All information available from hospital re-admission records, the referring physician, or the outpatient clinic was entered into a computer database. Death, MI, and target-vessel revascularization [TVR—percutaneous transluminal coronary angioplasty (PTCA), or bypass surgery] were considered as major adverse cardiac events. The diagnosis of MI was based on the presence of new pathological Q-waves or the rise in creatinine kinase or its MB isoenzyme>3 times the upper limit of normal. The criteria for TVR included the presence of angiographic restenosis accompanied by symptoms and/or a positive exercise test.
Endpoints of the study and sample size calculation
The primary endpoint of the study was the incidence of angiographic restenosis (see the definition in the preceding text). The secondary endpoint was the TVR at 1 year. The sample size of the trial was calculated on the basis of the following assumptions: a 10% restenosis rate with the Cypher stent and a 20% restenosis rate with the thin-strut stent. We intended to be able to show a similar difference for the two stents in this study, with an 80% power at a two-sided α-level of 0.05. To achieve this objective, 200 patients with follow-up angiography in each group were required. Assuming a follow-up coronary angiography rate of at least 75%, we included 500 patients to accommodate expected missing angiographic examinations at 6 months.
Statistical analysis
The main analyses were performed on an intention-to-treat basis. As most of the continuous data were not normally distributed, they are expressed as median (25th, 75th percentiles). Categorical data are presented as counts or proportions (percentages). The differences between groups were assessed using the non-parametric Wilcoxon rank-sum test for continuous data and χ2 test or Fisher's exact test for categorical data. All statistical tests were two-sided. Survival parameters were compared using the log-rank test. The relative risk (RR) and its 95% confidence interval (CI) were calculated for each study endpoint. Main analyses were also performed for subgroups of interest defined by the presence of diabetes, complex lesions, chronic occlusions, lesion length, and vessel size. A P-value <0.05 was considered statistically significant.
Results
Baseline characteristics and procedural results
As shown in Table 1, there were no differences between patients in the Cypher stent and the BeStent groups with respect to baseline clinical characteristics. Baseline angiographic characteristics are shown in Table 2. The number of patients with multi-vessel disease was high in both groups and statistically not different between them. Almost identical proportions of interventions were performed in all coronary vessels in the two groups. Patients treated with the Cypher stent more frequently had complex lesions (B2 or C lesions) than those treated with the BeStent (72 vs. 63%, P=0.03), as well as a higher percentage of chronic occlusions (6.8 vs. 2.8%, P=0.04). There were no significant differences between the two groups with respect to vessel size, lesion length, and diameter stenosis.
Table 3 shows the procedural data. Stenting procedure was successful in 100% of the patients in the BeStent group and 99.6% of the patients in the Cypher stent group (P=0.32). There were 383 lesions treated in the Cypher stent group and 376 lesions in the BeStent group. Compared with the patients in the Cypher stent group, there was a trend toward a higher balloon-to-vessel ratio among patients in the BeStent group; also, a larger final minimal lumen diameter and a smaller final diameter stenosis were achieved in the latter group. More stents were implanted in the BeStent group.
Early 30-day outcome
There were two cases of angiographically documented stent thrombosis, one in each group. One was acute (within the first 24 h after the procedure) and one occurred after 15 days. While no deaths occurred in either of the groups, MI occurred in nine patients (3.6%) in the Cypher stent group and 11 patients (4.4%) in the BeStent group (P=0.65). One urgent revascularization was performed in each group: one repeat PTCA in the Cypher stent group and one coronary bypass procedure in the BeStent group. The composite of major cardiac events at 30 days was not significantly different with 3.6% (nine patients) in the Cypher stent group and 4.8% (12 patients) in the BeStent group (P=0.50).
Follow-up angiographic results
The number of eligible patients for follow-up coronary angiography at 6 months (patients with successful procedure who survived without the need for an urgent revascularization within the first 30 days) was identical for each stent group (248, 99.2%). Of these, 204 patients (82.3%) in the BeStent group, and 205 patients in the Cypher stent group (82.7%) (P=0.91) underwent the 6-month coronary angiography. Three patients in the BeStent group and five patients in the Cypher stent group died before the scheduled angiographic follow-up. The remaining patients without follow-up angiography declined to undergo it. Table 4 shows the results of the quantitative assessment of the angiograms for lesions of both groups of patients. The primary endpoint of the study, angiographic restenosis, was achieved in 8.3% of the patients in the Cypher stent group and 25.5% of the patients in the BeStent group [RR, 0.33 (95% CI, 0.19–0.56), P<0.001]. Similarly, all the other measures of restenosis significantly favoured the Cypher stent.
The incidence of restenosis was also lower among patients in the Cypher stent compared with those in the BeStent group in various subgroups. Thus, the rate of angiographic restenosis for complex lesions (ACC/AHA type B2 and C lesions) was 8.9% (13/146) in the Cypher stent group and 28.7% (37/129) in the BeStent group (P<0.001). The rate of angiographic restenosis for chronic total occlusions was 6.7% (1/15) in the Cypher stent group and 60% (3/5) in the BeStent group (P=0.03). Among diabetics, the restenosis rate was 6.7% in the Cypher stent group and 31.8% in the BeStent group (P<0.001). Among non-diabetics, the restenosis rates were 9.0% with the Cypher stent and 22.5% with the BeStent (P=0.002). For lesions ≥15 mm, restenosis rates were 14.9 and 35.7% (P=0.004), and for lesions <15 mm restenosis rates were 4.6 and 20.1% (P<0.001) in the Cypher stent and BeStent groups, respectively.
We also analysed restenosis according to vessel size using a cut-off of 2.8 mm in vessel size. Among patients in the subgroup with vessel size <2.8 mm, median actual vessel diameter was 2.4 mm (25th, 75th percentiles: 2.2, 2.6 mm) in the Cypher stent group and 2.5 mm (2.3, 2.7 mm) in the BeStent group (P=0.04). The other baseline clinical and lesion characteristics were not significantly different between the two stent groups. Angiographic restenosis rates were 7.0% (8/115) with the Cypher stent and 34.2% (41/120) with the BeStent (P<0.001). Among patients in the subgroup with vessel size ≥2.8 mm, median actual vessel diameter was 3.1 mm (3.0, 3.3 mm) in the Cypher stent group and 3.0 mm (2.9, 3.2 mm) in the BeStent group (P=0.002). Also in this subgroup, the other baseline clinical and lesion characteristics were not significantly different between the two stent groups. In this subgroup, angiographic restenosis rates were not significantly different between the Cypher stent and BeStent groups [10.0% (9/90) vs. 13.1% (11/84), respectively, P=0.52] (Figure 1). Restenosis did not occur in any of the nine patients in the Cypher stent group with vessel size ≥2.8 mm, in whom the use of a balloon larger than 3.5 mm was needed. Median late loss in the Cypher stent group was 0.16 mm (–0.04, 0.60 mm) in the subgroup with vessel size ≥2.8 mm and 0.14 mm (–0.05, 0.36 mm) in the subgroup with vessel size <2.8 mm (P=0.20).
One-year clinical outcome
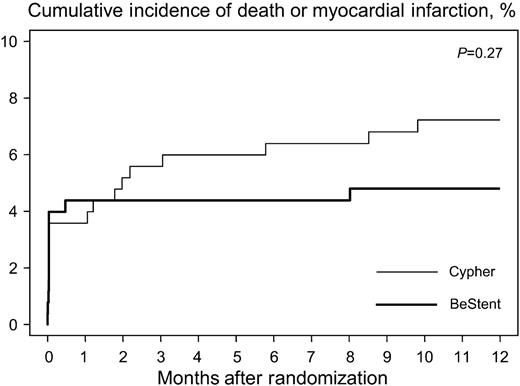
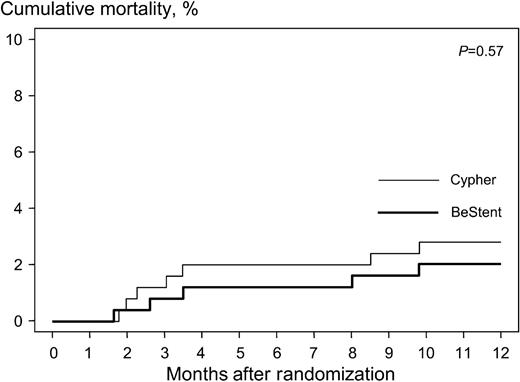
One-year clinical follow-up was complete in all but 13 patients (2.6%). The median follow-up interval in these 13 patients was 6.2 months (25th, 75th percentiles: 2.3, 6.8 months). In the period between 30 days and 1 year after the procedure, three cases of total stent occlusion were documented angiographically: two in the Cypher group and one in the BeStent group. In only one of these three patients (of the Cypher group), stent occlusion led to acute MI 173 days after the procedure; the 1-year clinical course of the remaining two patients was uneventful. Table 5 shows the events observed during the 1-year period in each group. In the Cypher stent group, 18 patients (7.2%) died or suffered an MI compared with 12 patients (4.8%) in the BeStent group (Figure 2). Seven patients (2.8%) in the Cypher group and five patients (2.0%) in the BeStent group died during this period. Of these cases, one patient in the Cypher stent group committed suicide. All other death cases were of cardiac origin. Figure 3 shows the time at which the deaths occurred in each group. TVR was performed in 18 patients (7.2%) in the Cypher stent group and in 47 patients (18.8%) in the BeStent group [RR, 0.38 (95% CI, 0.22–0.66), P<0.001].
Discussion
A large body of evidence demonstrates that antiproliferative drug-eluting stents are the most effective way to reduce in-stent restenosis. Their efficacy has been established in several trials where drug-eluting stents have been compared with bare control stents with thick struts. However, drug-eluting stents have not been shown to reduce mortality and MI rates, and their cost-effectiveness, mainly depending on the reduction of restenosis, is not yet definitely established. Previous work on the impact of bare metal stents with different strut thickness on restenosis shows that the occurrence of restenosis is lower when stents with thin struts are used. In a randomized study, for the first time, we compared the relative efficacy of a stent with antiproliferative drug-eluting coating, the Cypher stent, and a bare metal stent with thin struts, the BeStent. We found that, although the BeStent yielded excellent results for bare metal stents considering the clinical and angiographic characteristics of the patients enrolled, the use of the Cypher stent was clearly more beneficial; this benefit was due to a 67% reduction of the risk of restenosis, mainly in smaller vessels.
Vascular injury induced by stent struts after coronary stenting leads to a cascade of events that is responsible for increased neointima proliferation and the subsequent in-stent restenosis.26–28 Stenting strategies that have appropriately addressed the mechanisms responsible have been associated with a decreased rate of restenosis. In particular, drug-eluting stents with antiproliferative properties have had remarkable results in this regard. Most of the experience with an antiproliferative drug has been accumulated so far with the cytostatic and immunosuppressive compound, sirolimus. In the Randomized Double-Blind Study with the Sirolimus-Eluting BX Velocity Balloon-Expandable Stent in the Treatment of Patients with De Novo Native Coronary Lesions (RAVEL) trial, patients were randomly assigned to a sirolimus-eluting stent or bare metal control stent.5 At 6 months, the angiographic restenosis rate in the population of this study, which had restricted inclusion criteria, was 0% in the drug-eluting stent group compared with 26% in the bare metal stent group.5 After RAVEL, another trial with more liberal inclusion criteria, the SIRIUS trial, demonstrated the superiority of the sirolimus-eluting BX Velocity stent over the bare metal version of the same stent. But compared with RAVEL, in the SIRIUS trial the rate of angiographic restenosis at 8 months was higher in both stent groups: 8.9% in the Cypher stent group, and 36.3% in the bare metal stent group, which represented a 76% reduction of the risk of restenosis.6 We also observed a similar rate of angiographic restenosis in the Cypher stent group (8.3%) in our study, whose inclusion criteria, although slightly more liberal, were similar to SIRIUS. However, differently from the findings in the Cypher stent group, the incidence of angiographic restenosis in our bare metal stent group was almost 12% lower compared with the respective group in SIRIUS. It could be supposed that the difference in the restenosis rate between the bare metal stent groups in our study and in that of SIRIUS might derive from the differences in strut thickness between bare metal stents employed as controls in these studies: BeStent with thin struts in our study, and the BX Velocity stent with thick struts in the SIRIUS study.
Thinner struts have been shown to possess an endothelialization capacity which is lost with increased strut thickness.19 Also, in comparative studies with thick-strut stents, stents with thinner struts have been associated with a lower rate of restenosis and neointima proliferation, probably as a result of reduced vascular injury caused by the thinner struts.15,16,18 In particular, BeStent has been shown to be effective in reducing restenosis even in small vessels, a subset in which the superiority of stenting over plain balloon angioplasty has not always been demonstrated.20–22 In our study, patients treated with the BeStent had fewer complex lesions, fewer chronic occlusions, a bigger final minimal lumen diameter, and a smaller final diameter stenosis. These characteristics have been found to predict a lower incidence of restenosis.29,30 However, when compared with the Cypher stent in our study, BeStent, despite the advantages conferred by its thinner struts and more favourable angiographic and procedural characteristics, was associated with significantly higher angiographic and clinical restenosis rates. This finding applied to the whole population as well as to the majority of subgroups, thus, not only adding to the existing body of evidence on the efficacy of sirolimus-eluting stents on the reduction of restenosis, but also further extending the current knowledge on the superiority of these devices, even when compared with bare metal thin-strut stents.
For vessels 2.8 mm in size or larger, there were no significant differences in the angiographic and clinical restenosis rates between the Cypher stent group and BeStent group. Such a finding has not been reported from other studies with the Cypher stent where thick strut stents have been used as controls.5,6 While the subgroup of patients with vessels ≥2.8 mm in diameter is too small to draw any conclusion, the results in this group may have important implications. Considering the currently high cost of FDA-approved antiproliferative drug-eluting stents, it may contribute to guide strategies aimed at the reduction of restenosis by helping in the selection of candidates undergoing coronary stenting who derive real benefit from the use of these devices. However, we should note that the evaluation of restenosis in large vessels was performed only in the settings of a subset analysis and this study was underpowered to address this issue.
Although not designed as endpoints of our study, we also monitored other clinical outcomes. At 30-day follow-up, no significant difference was observed between the study groups with respect to individual or combined major adverse cardiac events. At the end of the follow-up period, there was a trend for a higher percentage of patients that experienced the combined endpoint of death and MI in the Cypher group compared with the BeStent group. The insufficient power of the present study to assess this combined endpoint minimizes the relevance of this, otherwise worrying, finding. In contrast, the composite of major adverse cardiac events was significantly higher in the group of patients treated with BeStent compared with those treated with the Cypher stent. The entire difference between the two groups came from the difference in TVR, which constituted most of the adverse events observed at the end of the follow-up period.
Conclusions
There is a considerable benefit with use of the drug-eluting stent Cypher, even when compared with the bare thin-strut BeStent. The advantage of the Cypher stent is vastly reduced in large vessels.
Figure 1 Angiographic restenosis rates with Cypher stent and BeStent, in the entire population, and in the subgroups of patients with vessel size <2.8 mm and ≥2.8 mm.
Figure 2 Kaplan–Meier analysis of cumulative incidence of death or MI.
Figure 3 Kaplan–Meier analysis of cumulative mortality.
Baseline clinical characteristics
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Age, years | 67.4 (59.0, 75.4) | 66.7 (59.9, 74.7) | 0.66 |
| Women, n (%) | 54 (22) | 55 (22) | 0.91 |
| Diabetes, n (%) | 72 (29) | 82 (33) | 0.33 |
| Current smoker, n (%) | 49 (20) | 40 (16) | 0.29 |
| Arterial hypertension, n (%) | 137 (55) | 154 (62) | 0.12 |
| Hypercholesterolaemia, n (%) | 134 (54) | 128 (51) | 0.59 |
| Unstable angina, n (%) | 97 (39) | 112 (45) | 0.17 |
| Previous MI, n (%) | 81 (32) | 76 (30) | 0.63 |
| Previous bypass surgery, n (%) | 19 (8) | 18 (7) | 0.86 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Age, years | 67.4 (59.0, 75.4) | 66.7 (59.9, 74.7) | 0.66 |
| Women, n (%) | 54 (22) | 55 (22) | 0.91 |
| Diabetes, n (%) | 72 (29) | 82 (33) | 0.33 |
| Current smoker, n (%) | 49 (20) | 40 (16) | 0.29 |
| Arterial hypertension, n (%) | 137 (55) | 154 (62) | 0.12 |
| Hypercholesterolaemia, n (%) | 134 (54) | 128 (51) | 0.59 |
| Unstable angina, n (%) | 97 (39) | 112 (45) | 0.17 |
| Previous MI, n (%) | 81 (32) | 76 (30) | 0.63 |
| Previous bypass surgery, n (%) | 19 (8) | 18 (7) | 0.86 |
Data are number of patients (%) or median (25th, 75th percentiles).
Baseline clinical characteristics
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Age, years | 67.4 (59.0, 75.4) | 66.7 (59.9, 74.7) | 0.66 |
| Women, n (%) | 54 (22) | 55 (22) | 0.91 |
| Diabetes, n (%) | 72 (29) | 82 (33) | 0.33 |
| Current smoker, n (%) | 49 (20) | 40 (16) | 0.29 |
| Arterial hypertension, n (%) | 137 (55) | 154 (62) | 0.12 |
| Hypercholesterolaemia, n (%) | 134 (54) | 128 (51) | 0.59 |
| Unstable angina, n (%) | 97 (39) | 112 (45) | 0.17 |
| Previous MI, n (%) | 81 (32) | 76 (30) | 0.63 |
| Previous bypass surgery, n (%) | 19 (8) | 18 (7) | 0.86 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Age, years | 67.4 (59.0, 75.4) | 66.7 (59.9, 74.7) | 0.66 |
| Women, n (%) | 54 (22) | 55 (22) | 0.91 |
| Diabetes, n (%) | 72 (29) | 82 (33) | 0.33 |
| Current smoker, n (%) | 49 (20) | 40 (16) | 0.29 |
| Arterial hypertension, n (%) | 137 (55) | 154 (62) | 0.12 |
| Hypercholesterolaemia, n (%) | 134 (54) | 128 (51) | 0.59 |
| Unstable angina, n (%) | 97 (39) | 112 (45) | 0.17 |
| Previous MI, n (%) | 81 (32) | 76 (30) | 0.63 |
| Previous bypass surgery, n (%) | 19 (8) | 18 (7) | 0.86 |
Data are number of patients (%) or median (25th, 75th percentiles).
Baseline angiographic characteristics
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multivessel disease, n (%) | 204 (82) | 199 (80) | 0.57 |
| Coronary vessel, n (%) | 0.95 | ||
| LAD | 107 (43) | 108 (43) | |
| LCx | 74 (29) | 76 (30) | |
| RCA | 69 (28) | 66 (27) | |
| ACC/AHA lesion type, n (%) | 0.09 | ||
| A | 11 (4) | 11 (4) | |
| B1 | 59 (24) | 82 (33) | |
| B2 | 141 (56) | 130 (52) | |
| C | 39 (16) | 27 (11) | |
| Chronic occlusions, n (%) | 17 (7) | 7 (3) | 0.04 |
| Restenotic lesions, n (%) | 4 (2) | 3 (1) | 0.70 |
| Lesion length, mm | 13.0 (8.9, 18.0) | 12.2 (8.4, 17.0) | 0.33 |
| Vessel size, mm | 2.7 (2.4, 3.1) | 2.7 (2.4, 3.0) | 0.77 |
| Vessel size ≥2.8 mm, n (%) | 104 (42) | 98 (39) | 0.58 |
| Diameter stenosis, n (%) | 59.9 (51.1, 69.0) | 62.6 (50.1, 73.7) | 0.33 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multivessel disease, n (%) | 204 (82) | 199 (80) | 0.57 |
| Coronary vessel, n (%) | 0.95 | ||
| LAD | 107 (43) | 108 (43) | |
| LCx | 74 (29) | 76 (30) | |
| RCA | 69 (28) | 66 (27) | |
| ACC/AHA lesion type, n (%) | 0.09 | ||
| A | 11 (4) | 11 (4) | |
| B1 | 59 (24) | 82 (33) | |
| B2 | 141 (56) | 130 (52) | |
| C | 39 (16) | 27 (11) | |
| Chronic occlusions, n (%) | 17 (7) | 7 (3) | 0.04 |
| Restenotic lesions, n (%) | 4 (2) | 3 (1) | 0.70 |
| Lesion length, mm | 13.0 (8.9, 18.0) | 12.2 (8.4, 17.0) | 0.33 |
| Vessel size, mm | 2.7 (2.4, 3.1) | 2.7 (2.4, 3.0) | 0.77 |
| Vessel size ≥2.8 mm, n (%) | 104 (42) | 98 (39) | 0.58 |
| Diameter stenosis, n (%) | 59.9 (51.1, 69.0) | 62.6 (50.1, 73.7) | 0.33 |
Data are number of patients (%) or median (25th, 75th percentiles). LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
Baseline angiographic characteristics
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multivessel disease, n (%) | 204 (82) | 199 (80) | 0.57 |
| Coronary vessel, n (%) | 0.95 | ||
| LAD | 107 (43) | 108 (43) | |
| LCx | 74 (29) | 76 (30) | |
| RCA | 69 (28) | 66 (27) | |
| ACC/AHA lesion type, n (%) | 0.09 | ||
| A | 11 (4) | 11 (4) | |
| B1 | 59 (24) | 82 (33) | |
| B2 | 141 (56) | 130 (52) | |
| C | 39 (16) | 27 (11) | |
| Chronic occlusions, n (%) | 17 (7) | 7 (3) | 0.04 |
| Restenotic lesions, n (%) | 4 (2) | 3 (1) | 0.70 |
| Lesion length, mm | 13.0 (8.9, 18.0) | 12.2 (8.4, 17.0) | 0.33 |
| Vessel size, mm | 2.7 (2.4, 3.1) | 2.7 (2.4, 3.0) | 0.77 |
| Vessel size ≥2.8 mm, n (%) | 104 (42) | 98 (39) | 0.58 |
| Diameter stenosis, n (%) | 59.9 (51.1, 69.0) | 62.6 (50.1, 73.7) | 0.33 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multivessel disease, n (%) | 204 (82) | 199 (80) | 0.57 |
| Coronary vessel, n (%) | 0.95 | ||
| LAD | 107 (43) | 108 (43) | |
| LCx | 74 (29) | 76 (30) | |
| RCA | 69 (28) | 66 (27) | |
| ACC/AHA lesion type, n (%) | 0.09 | ||
| A | 11 (4) | 11 (4) | |
| B1 | 59 (24) | 82 (33) | |
| B2 | 141 (56) | 130 (52) | |
| C | 39 (16) | 27 (11) | |
| Chronic occlusions, n (%) | 17 (7) | 7 (3) | 0.04 |
| Restenotic lesions, n (%) | 4 (2) | 3 (1) | 0.70 |
| Lesion length, mm | 13.0 (8.9, 18.0) | 12.2 (8.4, 17.0) | 0.33 |
| Vessel size, mm | 2.7 (2.4, 3.1) | 2.7 (2.4, 3.0) | 0.77 |
| Vessel size ≥2.8 mm, n (%) | 104 (42) | 98 (39) | 0.58 |
| Diameter stenosis, n (%) | 59.9 (51.1, 69.0) | 62.6 (50.1, 73.7) | 0.33 |
Data are number of patients (%) or median (25th, 75th percentiles). LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
Procedural data
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multilesion intervention, n (%) | 128 (51) | 118 (47) | 0.37 |
| Maximal balloon pressure, atm | 12.7 (12, 15) | 13 (12, 14) | 0.41 |
| Maximal balloon diameter, mm | 3.1 (2.8, 3.4) | 3.1 (2.9, 3.4) | 0.68 |
| Number of implanted stents | 1.1±0.4 | 1.3±0.5 | 0.002 |
| Length of stented segment, mm | 18 (18, 33) | 18 (15, 27) | 0.03 |
| Final minimal lumen diameter, mm | 2.7 (2.4, 2.9) | 2.7 (2.5, 3.0) | 0.03 |
| Final diameter stenosis, % | 6.2 (2.4, 10.4) | 3.8 (–2.1, 8.3) | <0.001 |
| Procedural success, n (%) | 249 (100) | 250 (100) | 0.32 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multilesion intervention, n (%) | 128 (51) | 118 (47) | 0.37 |
| Maximal balloon pressure, atm | 12.7 (12, 15) | 13 (12, 14) | 0.41 |
| Maximal balloon diameter, mm | 3.1 (2.8, 3.4) | 3.1 (2.9, 3.4) | 0.68 |
| Number of implanted stents | 1.1±0.4 | 1.3±0.5 | 0.002 |
| Length of stented segment, mm | 18 (18, 33) | 18 (15, 27) | 0.03 |
| Final minimal lumen diameter, mm | 2.7 (2.4, 2.9) | 2.7 (2.5, 3.0) | 0.03 |
| Final diameter stenosis, % | 6.2 (2.4, 10.4) | 3.8 (–2.1, 8.3) | <0.001 |
| Procedural success, n (%) | 249 (100) | 250 (100) | 0.32 |
Data are number of patients (%) or median (25th, 75th percentiles) except for number of stents presented as mean±standard deviation.
Procedural data
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multilesion intervention, n (%) | 128 (51) | 118 (47) | 0.37 |
| Maximal balloon pressure, atm | 12.7 (12, 15) | 13 (12, 14) | 0.41 |
| Maximal balloon diameter, mm | 3.1 (2.8, 3.4) | 3.1 (2.9, 3.4) | 0.68 |
| Number of implanted stents | 1.1±0.4 | 1.3±0.5 | 0.002 |
| Length of stented segment, mm | 18 (18, 33) | 18 (15, 27) | 0.03 |
| Final minimal lumen diameter, mm | 2.7 (2.4, 2.9) | 2.7 (2.5, 3.0) | 0.03 |
| Final diameter stenosis, % | 6.2 (2.4, 10.4) | 3.8 (–2.1, 8.3) | <0.001 |
| Procedural success, n (%) | 249 (100) | 250 (100) | 0.32 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Multilesion intervention, n (%) | 128 (51) | 118 (47) | 0.37 |
| Maximal balloon pressure, atm | 12.7 (12, 15) | 13 (12, 14) | 0.41 |
| Maximal balloon diameter, mm | 3.1 (2.8, 3.4) | 3.1 (2.9, 3.4) | 0.68 |
| Number of implanted stents | 1.1±0.4 | 1.3±0.5 | 0.002 |
| Length of stented segment, mm | 18 (18, 33) | 18 (15, 27) | 0.03 |
| Final minimal lumen diameter, mm | 2.7 (2.4, 2.9) | 2.7 (2.5, 3.0) | 0.03 |
| Final diameter stenosis, % | 6.2 (2.4, 10.4) | 3.8 (–2.1, 8.3) | <0.001 |
| Procedural success, n (%) | 249 (100) | 250 (100) | 0.32 |
Data are number of patients (%) or median (25th, 75th percentiles) except for number of stents presented as mean±standard deviation.
Angiographic data at follow-up
| . | Cypher group (n=205) . | BeStent group (n=204) . | P . |
|---|---|---|---|
| Minimal lumen diameter, mm | 2.46 (2.12, 2.82) | 1.8 (1.26, 2.26) | <0.001 |
| Diameter stenosis, % | 12.0 (5.6, 20.9) | 30.7 (18.9, 50.8) | <0.001 |
| Late lumen loss, mm | 0.14 (–0.5, 0.43) | 0.94 (0.53, 1.30) | <0.001 |
| Angiographic restenosis, n (%) | 17 (8.3) | 52 (25.5) | <0.001 |
| . | Cypher group (n=205) . | BeStent group (n=204) . | P . |
|---|---|---|---|
| Minimal lumen diameter, mm | 2.46 (2.12, 2.82) | 1.8 (1.26, 2.26) | <0.001 |
| Diameter stenosis, % | 12.0 (5.6, 20.9) | 30.7 (18.9, 50.8) | <0.001 |
| Late lumen loss, mm | 0.14 (–0.5, 0.43) | 0.94 (0.53, 1.30) | <0.001 |
| Angiographic restenosis, n (%) | 17 (8.3) | 52 (25.5) | <0.001 |
Data are number of patients (%) or median (25th, 75th percentiles).
Angiographic data at follow-up
| . | Cypher group (n=205) . | BeStent group (n=204) . | P . |
|---|---|---|---|
| Minimal lumen diameter, mm | 2.46 (2.12, 2.82) | 1.8 (1.26, 2.26) | <0.001 |
| Diameter stenosis, % | 12.0 (5.6, 20.9) | 30.7 (18.9, 50.8) | <0.001 |
| Late lumen loss, mm | 0.14 (–0.5, 0.43) | 0.94 (0.53, 1.30) | <0.001 |
| Angiographic restenosis, n (%) | 17 (8.3) | 52 (25.5) | <0.001 |
| . | Cypher group (n=205) . | BeStent group (n=204) . | P . |
|---|---|---|---|
| Minimal lumen diameter, mm | 2.46 (2.12, 2.82) | 1.8 (1.26, 2.26) | <0.001 |
| Diameter stenosis, % | 12.0 (5.6, 20.9) | 30.7 (18.9, 50.8) | <0.001 |
| Late lumen loss, mm | 0.14 (–0.5, 0.43) | 0.94 (0.53, 1.30) | <0.001 |
| Angiographic restenosis, n (%) | 17 (8.3) | 52 (25.5) | <0.001 |
Data are number of patients (%) or median (25th, 75th percentiles).
Major adverse cardiac events at 1-year follow-up
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Death, n (%) | 7 (2.8) | 5 (2.0) | 0.57 |
| Death or MI, n (%) | 18 (7.2) | 12 (4.8) | 0.27 |
| TVR | 18 (7.2) | 47 (18.8) | <0.001 |
| Repeat PTCA | 17 (6.8) | 43 (17.2) | <0.001 |
| CABG | 1 (0.4) | 5 (2.0) | 0.10 |
| Death or MI or TVR, n (%) | 34 (13.6) | 56 (22.4) | 0.01 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Death, n (%) | 7 (2.8) | 5 (2.0) | 0.57 |
| Death or MI, n (%) | 18 (7.2) | 12 (4.8) | 0.27 |
| TVR | 18 (7.2) | 47 (18.8) | <0.001 |
| Repeat PTCA | 17 (6.8) | 43 (17.2) | <0.001 |
| CABG | 1 (0.4) | 5 (2.0) | 0.10 |
| Death or MI or TVR, n (%) | 34 (13.6) | 56 (22.4) | 0.01 |
CABG, aortocoronary bypass surgery.
Major adverse cardiac events at 1-year follow-up
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Death, n (%) | 7 (2.8) | 5 (2.0) | 0.57 |
| Death or MI, n (%) | 18 (7.2) | 12 (4.8) | 0.27 |
| TVR | 18 (7.2) | 47 (18.8) | <0.001 |
| Repeat PTCA | 17 (6.8) | 43 (17.2) | <0.001 |
| CABG | 1 (0.4) | 5 (2.0) | 0.10 |
| Death or MI or TVR, n (%) | 34 (13.6) | 56 (22.4) | 0.01 |
| . | Cypher group (n=250) . | BeStent group (n=250) . | P . |
|---|---|---|---|
| Death, n (%) | 7 (2.8) | 5 (2.0) | 0.57 |
| Death or MI, n (%) | 18 (7.2) | 12 (4.8) | 0.27 |
| TVR | 18 (7.2) | 47 (18.8) | <0.001 |
| Repeat PTCA | 17 (6.8) | 43 (17.2) | <0.001 |
| CABG | 1 (0.4) | 5 (2.0) | 0.10 |
| Death or MI or TVR, n (%) | 34 (13.6) | 56 (22.4) | 0.01 |
CABG, aortocoronary bypass surgery.
References
Serruys PW, de Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease.
Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease.
Elezi S, Kastrati A, Neumann FJ, Hadamitzky M, Dirschinger J, Schömig A. Vessel size and long-term outcome after coronary stent placement.
Kastrati A, Schomig A, Elezi S et al. Predictive factors of restenosis after coronary stent placement.
Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization.
Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery.
Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease.
O'Neill WW, Leon MB. Drug-eluting stents: costs versus clinical benefit.
Cohen DJ, Bakhai A, Shi C et al. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: results from the Sirolimus-Eluting Balloon Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SIRIUS) trial.
Greenberg D, Bakhai A, Cohen DJ. Can we afford to eliminate restenosis? Can we afford not to?
Lansky AJ, Roubin GS, O'Shaughnessy CD et al. Randomized comparison of GR-II stent and Palmaz-Schatz stent for elective treatment of coronary stenoses.
Kastrati A, Dirschinger J, Boekstegers P et al. Influence of stent design on 1-year outcome after coronary stent placement: a randomized comparison of five stent types in 1,147 unselected patients.
Kastrati A, Schömig A, Dirschinger J et al. Increased risk of restenosis after placement of gold-coated stents: results of a randomized trial comparing gold-coated with uncoated steel stents in patients with coronary artery disease.
vom Dahl JJ, Haager PK, Grube E et al. Effects of gold coating of coronary stents on neointimal proliferation following stent implantation.
Kastrati A, Mehilli J, Dirschinger J et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial.
Pache J, Kastrati A, Mehilli J et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial.
Briguori C, Sarais C, Pagnotta P et al. In-stent restenosis in small coronary arteries: impact of strut thickness.
Rittersma SZ, de Winter RJ, Koch KT et al. Impact of strut thickness on late luminal loss after coronary artery stent placement.
Simon C, Palmaz JC, Sprague EA. Influence of topography on endothelialization of stents: clues for new designs.
Koning R, Eltchaninoff H, Commeau P et al. Stent placement compared with balloon angioplasty for small coronary arteries: in-hospital and 6-month clinical and angiographic results.
Kastrati A, Schömig A, Dirschinger J et al. A randomized trial comparing stenting with balloon angioplasty in small vessels in patients with symptomatic coronary artery disease. ISAR-SMART Study Investigators. Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries.
Moer R, Myreng Y, Molstad P et al. Stenting in small coronary arteries (SISCA) trial. A randomized comparison between balloon angioplasty and the heparin-coated BeStent.
BeStent™ 2 with Discrete Technology™ Over-the-Wire and Rapid Exchange Coronary Stent Delivery Systems—P000022. http://www.fda.gov/cdrh/pdf/p000022.html. (
Doucet S, Schalij MJ, Vrolix MC et al. Stent placement to prevent restenosis after angioplasty in small coronary arteries.
Ellis SG, Vandormael MG, Cowley MJ et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group.
Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans.
Moreno PR, Bernardi VH, Lopez-Cuellar J et al. Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina.
Farb A, Sangiorgi G, Carter AJ et al. Pathology of acute and chronic coronary stenting in humans.
Lemos PA, Hoye A, Goedhart D et al. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study.