-
PDF
- Split View
-
Views
-
Cite
Cite
H.M. Kristiansen, G. Vollan, T. Hovstad, H. Keilegavlen, S. Faerestrand, The impact of left ventricular lead position on left ventricular reverse remodelling and improvement in mechanical dyssynchrony in cardiac resynchronization therapy, European Heart Journal - Cardiovascular Imaging, Volume 13, Issue 12, December 2012, Pages 991–1000, https://doi.org/10.1093/ehjci/jes114
Close - Share Icon Share
Abstract
To investigate the influence of left ventricular (LV) lead position on LV dyssynchrony in cardiac resynchronization therapy (CRT).
The LV lead was prospectively targeted to the latest activated LV segment (concordant) evaluated by two-dimensional speckle tracking radial strain (ST-RS) echocardiography in 103 CRT recipients (67 ± 12 years). Mechanical dyssynchrony was assessed by anteroseptal-to-posterior (AS-P) delay and interventricular mechanical delay (IVMD). Concordant LV leads were obtained in 72 (70%) patients. Superior LV reverse remodelling (LV-RR; ≥15% LV end-systolic volume reduction at 6-month follow-up) was observed in the concordant LV leads compared with the discordant LV leads [51 (76%) vs. 13 (45%); P = 0.003]. Mechanical resynchronization responders (≥50% AS-P delay reduction at 6-month follow-up) obtained in the concordant LV leads [44 (66%)] was greater than in the discordant LV leads [10 (34%); P = 0.005]. The discordant LV leads located adjacent to the concordant LV leads (+1 segment; n = 22) and 2 segments apart (+2 segments; n = 9) were evaluated in a subgroup analysis. Mechanical resynchronization responders 6 months after CRT were as follows: in +1 segment [n = 10 (48%)] and in +2 segments (n = 0; P = 0.001). The concordant LV lead was the only independent predictor of LV-RR at 6-month follow-up (odds ratio, 4.177; P = 0.004). Independent predictors of mechanical resynchronization responders were AS-P delay (odds ratio, 1.007; P = 0.032), IVMD (odds ratio, 1.024; P = 0.038), and concordant LV lead (odds ratio, 4.691; P = 0.004).
Concordant LV leads in CRT provided more responders according to both LV reverse remodelling and mechanical resynchronization.
Trial registration: http://clinicaltrials.gov. Unique identifier: NCT01035489.
Introduction
Cardiac resynchronization therapy (CRT) can reduce mortality and induce left ventricular (LV) volume reduction (LV reverse remodelling) in symptomatic heart failure (HF) patients with a depressed LV function and wide QRS duration.1 The extent of LV dyssynchrony and the segmental LV activation delay in patients eligible for CRT can be assessed by using two-dimensional (2D) speckle tracking radial strain (ST-RS) echocardiography.2–4 Furthermore, CRT may reduce the magnitude of LV dyssynchrony through biventricular pacing,2 and the improved synchronicity in CRT recipients has been related to improved clinical long-term outcome.5
LV lead placement in CRT at the LV segment with latest mechanical contraction (concordant LV lead), evaluated by ST-RS echocardiography, can provide superior improvement in LV reverse remodelling and prolongation of survival.6,7 However, it is not fully understood if the extent of mechanical resynchronization is related to LV lead position in CRT. We hypothesized that concordant LV leads in CRT could provide better synchronicity in patients undergoing CRT. Accordingly, the objective of this study was to investigate the LV dyssynchrony reduction effected by LV leads when targeted to the latest contracting LV segment in prospective settings. Similar effects of randomized right ventricular (RV) lead position located in the RV apex (RV-A) vs. RV high posterior septum (RV-HS) on LV dyssynchrony in CRT have previously been published.8 The current substudy reports the impact of LV lead position on LV reverse remodelling and mechanical resynchronization in CRT.
Methods
Patient population and study protocol
One hundred and four consecutive HF patients scheduled for CRT were prospectively included in this study. Inclusion criteria were HF patients in New York Heart Association (NYHA) functional class III or IV on optimal pharmacological treatment, LV ejection fraction (LVEF) ≤35% and QRS duration ≥120 ms or paced QRS duration ≥200 ms. Native QRS morphologies included left bundle branch block (LBBB) and non-specific intraventricular conduction delay. Right bundle branch block was not included. The patients underwent echocardiographic examination, clinical assessment according to the NYHA functional class and distance walked in 6 min prior to CRT implantation, and they were re-evaluated 6 months after CRT. New RV lead implants were prospectively randomized to RV-A or RV-HS. The LV lead was prospectively targeted to the latest contracting segment identified in preoperative ST-RS echocardiography. The regional ethics committee approved this study, and the patients were included after informed consent.
Cardiac resynchronization therapy device implantation
A CRT device was implanted under local anaesthesia. Guided by fluoroscopy, the coronary sinus was cannulated using a guide catheter. Next, by using a balloon catheter (Attain 6215, Medtronic, Minneapolis, MN, USA), a coronary venogram recording of the coronary vein tributaries was obtained. Prior to the CRT procedure, the results from the preoperative ST-RS echocardiographic examination were discussed with the implanting physician. Thus, the operator was aware of the latest mechanical contracting LV segment in order to guide the LV lead concordantly. Fluoroscopy in the left anterior oblique 30° (LAO) view was considered to correspond to the mid-ventricular ST-RS echocardiography in a parasternal short axis. A venogram in an LAO fluoroscopic view was divided into five equal segments: anterior, anterolateral, lateral, posterolateral, and posterior.9 Subsequently, the LV lead was targeted to the latest activated LV segment identified by the preoperative ST-RS echocardiography. Further, the LV lead was positioned in a basal segment close to the mid-ventricular segment or in a mid-ventricular segment, and not positioned apically, demonstrated in the right anterior oblique fluoroscopic view.9 The RV lead was positioned in RV-A or in RV-HS,10,11 in which new implants of RV leads were prospectively randomized to receive RV-A or RV-HS in a 1:1 ratio. The right atrial (RA) lead was placed in the RA appendage. The pacing leads were connected to a CRT device, similar in all patients (Medtronic, Minneapolis, MN, USA).
Left ventricular lead concordance
The LV lead position was several months after the CRT device implantation confirmed in a double-blind set-up. Two experienced CRT implanters independently evaluated the LV lead position by using the digitally stored CRT implant procedure fluoroscopic images. In this set up, neither the judging physician nor the control person was aware of the latest contracting LV segment obtained in the preoperative ST-RS echocardiography or the success of this targeted LV lead position. Based on these analyses, the concordant LV lead was accredited if the LV lead was located in the targeted LV segment, or the adjacent one. Subsequently, LV leads located 2 segments or more from the targeted LV segments were assigned discordant LV leads. A subgroup classification on discordant LV leads was derived. The discordant LV leads located adjacent to the concordant LV leads were assigned +1 segment, whereas the discordant LV leads positioned 2 segments apart from the concordant LV leads were named +2 segments.
Echocardiographic imaging
A conventional transthoracic 2D echocardiographic examination was performed (Vivid 7, General Electric Vingmed, Milwaukee, WI, USA), with data acquisition using a 3.5-MHz transducer. Digitally stored cine loops were obtained for offline post-processing (EchoPac 108.1.5, GE Medical Systems, Horten, Norway). LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were obtained from apical four- and two-chamber views and LVEF was calculated using the Simpson rule.12 Three consecutive representative cardiac cycles were used, and the mean value was used for statistical calculations. Patients demonstrating ≥15% LVESV reduction at 6-month follow-up were considered as LV reverse remodelling responders.13 One single observer blinded to the LV lead position performed all the echocardiographic analysis.
Echocardiographic assessment of mechanical dyssynchrony
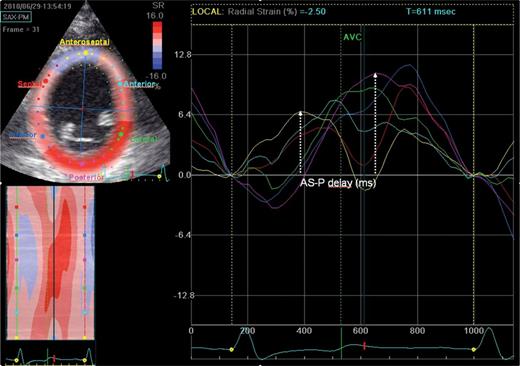
Intraventricular dyssynchrony was evaluated by speckle tracking echocardiography performed from a 2D greyscale mid-LV parasternal short-axis view during breath-hold, using frame rates ≥30 Hz (mean 50 ± 17, range 30–89), as demonstrated in Figure 1. Speckle tracking software automatically generated time–strain curves of the LV myocardium, and five representative cardiac cycles were averaged with tracking quality adequately in 94% of LV segments. The time delay from peak radial strain in anteroseptal-to-posterior LV segments (AS-P delay) by using ST-RS imaging was obtained, and AS-P delay ≥130 ms was classified as extensive intraventricular mechanical dyssynchrony.2,14 Further, the standard deviation (SD) of time delay from QRS onset to peak radial strain in six LV segments (SDt6) was calculated.2 Patients demonstrating ≥50% reduction in AS-P delay at 6-month follow-up were considered mechanical resynchronization responders.8 Prior to the CRT procedure and routinely the day before the CRT implantation, using ST-RS echocardiography, the peak radial strain of latest contracting anterior, lateral, and posterior segments were identified. If the latest contracting LV segments were separated by ≤10 ms from the five averaged strain curves, the segment between them was assigned the latest one. Subsequently, we identified five targeted LV lead positions: anterior, anterolateral, lateral, posterolateral, and posterior segments.8 If LV segments anterior, lateral, and posterior demonstrated low-amplitude strain, defined as the peak radial strain <10%, they were excluded if possible.15 This was used to avoid the LV lead position at segments demonstrating extensive LV scarring. In the event of low-amplitude strain in all these three LV segments, the latest contracting LV segment was assigned the targeted segment.
Preoperative two-dimensional ST-RS echocardiography in the mid-LV short axis. AS-P delay, anteroseptal (yellow) to posterior (magenta) time delay; AVC, aortic valve closure; LV, left ventricular; ST-RS, speckle tracking radial strain.
Interventricular mechanical delay (IVMD) was assessed by using pulse-wave Doppler of aortic and pulmonic flow velocities in the apical long-axis and parasternal short-axis views, respectively. The time delay from QRS onset to opening of aortic and pulmonic valves were obtained, and the IVMD was defined as the difference in time.16
Atrioventricular (AV) dyssynchrony was evaluated in an apical four-chamber view by using transmitral pulsed-wave Doppler of the LV diastolic filling. The LV filling time (LVFT) was divided by the duration of the cardiac cycle length (RR) and presented as percentages (LVFT/RR × 100%).16
To evaluate intraventricular-, interventricular-, and AV dyssynchrony, five representative cardiac cycles were analysed, and the average was used for statistical calculations. Intraobserver and interobserver variability has been described previously.8
Follow-up
Prior to hospital discharge and repeated at 3-month follow-up, AV optimization was performed using the iterative method before VV optimization from the maximal velocity time integrals obtained from pulsed-wave Doppler.17 At 6-month follow-up, the patients were evaluated with echocardiographic examination and clinically assessed with the NYHA classification and distance walked in 6 min.
Statistical analysis
All statistical analyses were conducted using SPSS software version 18.0 (SPSS, Inc., Chicago, IL, USA). Sample size calculations have previously been reported.8 Histograms and Q–Q plots were used to assess the normality of the continuous variables. Continuous variables are presented as the mean ± SD and were compared by using the paired or unpaired Student t-test as appropriate. A two-way analysis of variance for repeated measurements was used to evaluate subgroup interactions. Categorical data are listed as frequencies and percentages and were compared by Pearson χ2 or Fisher's exact test. To assess the interdependency of LV reverse remodelling and the extent of mechanical resynchronization, linear regression analyses were performed. Univariable and multivariable logistic regression analyses were used to assess nine preoperative variables to predict LV reverse remodelling responders and mechanical resynchronization responders. A P-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
One patient was excluded because of suboptimal echocardiographic imaging quality. Subsequently, the baseline characteristics of the 103 patients in the study population [67 ± 12 years; 84 men (82%)] are presented in Table 1. All HF patients were severe symptomatic [81 in the NYHA functional class III (79%)] with a wide QRS duration of 177 ± 30 ms. The patients were treated with either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker (98%), and a beta blocker (94%) at maximal tolerated dosages. The results of the echocardiographic examinations performed prior to CRT implantation are presented in Tables 2 and 3. The LV contractile function was severely depressed; the mean LVEF was 24 ± 4%. LV volumes were increased; the mean LVEDV was 226 ± 69 mL and the mean LVESV was 172 ± 55 mL. Intraventricular dyssynchrony was severe; the mean AS-P delay was 212 ± 96 ms.
Baseline characteristics of the patients in concordant and discordant left ventricular leads
| . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, years | 67 ± 12 | 67 ± 12 | 66 ± 12 | 0.89 |
| Gender, male (%) | 84 (82) | 58 (81) | 26 (84) | 0.69 |
| NYHA functional class III, n (%) | 81 (79) | 57 (79) | 24 (77) | 0.84 |
| Ischaemic cardiomyopathy, n (%) | 59 (57) | 37 (51) | 22 (71) | 0.07 |
| Sinus rhythm, n (%) | 80 (78) | 57 (79) | 23 (74) | 0.58 |
| Intrinsic rhythm, n (%) | 84 (82) | 60 (83) | 24 (77) | 0.48 |
| QRS intrinsic, ms | 169 ± 25 | 169 ± 25 | 168 ± 25 | 0.91 |
| QRS paced, ms | 215 ± 18 | 212 ± 15 | 220 ± 23 | 0.36 |
| LBBB, n (%) | 72 (70) | 52 (72) | 20 (65) | 0.43 |
| Medical therapy | ||||
| ACEI or ARB, n (%) | 101 (98) | 70 (97) | 31 (100) | 0.35 |
| Beta-blocker, n (%) | 97 (94) | 68 (94) | 29 (94) | 0.86 |
| Loop diuretics, n (%) | 78 (76) | 53 (74) | 25 (81) | 0.45 |
| Spironolactone, n (%) | 30 (29) | 19 (26) | 11 (35) | 0.35 |
| . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, years | 67 ± 12 | 67 ± 12 | 66 ± 12 | 0.89 |
| Gender, male (%) | 84 (82) | 58 (81) | 26 (84) | 0.69 |
| NYHA functional class III, n (%) | 81 (79) | 57 (79) | 24 (77) | 0.84 |
| Ischaemic cardiomyopathy, n (%) | 59 (57) | 37 (51) | 22 (71) | 0.07 |
| Sinus rhythm, n (%) | 80 (78) | 57 (79) | 23 (74) | 0.58 |
| Intrinsic rhythm, n (%) | 84 (82) | 60 (83) | 24 (77) | 0.48 |
| QRS intrinsic, ms | 169 ± 25 | 169 ± 25 | 168 ± 25 | 0.91 |
| QRS paced, ms | 215 ± 18 | 212 ± 15 | 220 ± 23 | 0.36 |
| LBBB, n (%) | 72 (70) | 52 (72) | 20 (65) | 0.43 |
| Medical therapy | ||||
| ACEI or ARB, n (%) | 101 (98) | 70 (97) | 31 (100) | 0.35 |
| Beta-blocker, n (%) | 97 (94) | 68 (94) | 29 (94) | 0.86 |
| Loop diuretics, n (%) | 78 (76) | 53 (74) | 25 (81) | 0.45 |
| Spironolactone, n (%) | 30 (29) | 19 (26) | 11 (35) | 0.35 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; Concordant, LV lead located at the latest or adjacent activated LV segment evaluated by ST-RS echocardiography; LBBB, left bundle branch block; NYHA, New York Heart Association.
Baseline characteristics of the patients in concordant and discordant left ventricular leads
| . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, years | 67 ± 12 | 67 ± 12 | 66 ± 12 | 0.89 |
| Gender, male (%) | 84 (82) | 58 (81) | 26 (84) | 0.69 |
| NYHA functional class III, n (%) | 81 (79) | 57 (79) | 24 (77) | 0.84 |
| Ischaemic cardiomyopathy, n (%) | 59 (57) | 37 (51) | 22 (71) | 0.07 |
| Sinus rhythm, n (%) | 80 (78) | 57 (79) | 23 (74) | 0.58 |
| Intrinsic rhythm, n (%) | 84 (82) | 60 (83) | 24 (77) | 0.48 |
| QRS intrinsic, ms | 169 ± 25 | 169 ± 25 | 168 ± 25 | 0.91 |
| QRS paced, ms | 215 ± 18 | 212 ± 15 | 220 ± 23 | 0.36 |
| LBBB, n (%) | 72 (70) | 52 (72) | 20 (65) | 0.43 |
| Medical therapy | ||||
| ACEI or ARB, n (%) | 101 (98) | 70 (97) | 31 (100) | 0.35 |
| Beta-blocker, n (%) | 97 (94) | 68 (94) | 29 (94) | 0.86 |
| Loop diuretics, n (%) | 78 (76) | 53 (74) | 25 (81) | 0.45 |
| Spironolactone, n (%) | 30 (29) | 19 (26) | 11 (35) | 0.35 |
| . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, years | 67 ± 12 | 67 ± 12 | 66 ± 12 | 0.89 |
| Gender, male (%) | 84 (82) | 58 (81) | 26 (84) | 0.69 |
| NYHA functional class III, n (%) | 81 (79) | 57 (79) | 24 (77) | 0.84 |
| Ischaemic cardiomyopathy, n (%) | 59 (57) | 37 (51) | 22 (71) | 0.07 |
| Sinus rhythm, n (%) | 80 (78) | 57 (79) | 23 (74) | 0.58 |
| Intrinsic rhythm, n (%) | 84 (82) | 60 (83) | 24 (77) | 0.48 |
| QRS intrinsic, ms | 169 ± 25 | 169 ± 25 | 168 ± 25 | 0.91 |
| QRS paced, ms | 215 ± 18 | 212 ± 15 | 220 ± 23 | 0.36 |
| LBBB, n (%) | 72 (70) | 52 (72) | 20 (65) | 0.43 |
| Medical therapy | ||||
| ACEI or ARB, n (%) | 101 (98) | 70 (97) | 31 (100) | 0.35 |
| Beta-blocker, n (%) | 97 (94) | 68 (94) | 29 (94) | 0.86 |
| Loop diuretics, n (%) | 78 (76) | 53 (74) | 25 (81) | 0.45 |
| Spironolactone, n (%) | 30 (29) | 19 (26) | 11 (35) | 0.35 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; Concordant, LV lead located at the latest or adjacent activated LV segment evaluated by ST-RS echocardiography; LBBB, left bundle branch block; NYHA, New York Heart Association.
Echocardiographic measurements at baseline and at 6-month follow-up in patients with concordant and discordant left ventricular leads
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | ||||
| Baseline | 24 ± 4 | 24 ± 4 | 24 ± 5 | 0.56 |
| Follow-up | 32 ± 8* | 33 ± 8* | 29 ± 6* | 0.011 |
| LVEDV (mL) | ||||
| Baseline | 226 ± 69 | 222 ± 69 | 236 ± 70 | 0.34 |
| Follow-up | 193 ± 72* | 179 ± 69* | 224 ± 71† | 0.005 |
| LVESV (mL) | ||||
| Baseline | 172 ± 55 | 168 ± 53 | 181 ± 60 | 0.27 |
| Follow-up | 135 ± 60* | 123 ± 56* | 161 ± 60* | 0.003 |
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | ||||
| Baseline | 24 ± 4 | 24 ± 4 | 24 ± 5 | 0.56 |
| Follow-up | 32 ± 8* | 33 ± 8* | 29 ± 6* | 0.011 |
| LVEDV (mL) | ||||
| Baseline | 226 ± 69 | 222 ± 69 | 236 ± 70 | 0.34 |
| Follow-up | 193 ± 72* | 179 ± 69* | 224 ± 71† | 0.005 |
| LVESV (mL) | ||||
| Baseline | 172 ± 55 | 168 ± 53 | 181 ± 60 | 0.27 |
| Follow-up | 135 ± 60* | 123 ± 56* | 161 ± 60* | 0.003 |
Abbreviations as in Table 1; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume.
*P < 0.001 baseline vs. follow-up; †P < 0.05 baseline vs. follow-up.
Echocardiographic measurements at baseline and at 6-month follow-up in patients with concordant and discordant left ventricular leads
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | ||||
| Baseline | 24 ± 4 | 24 ± 4 | 24 ± 5 | 0.56 |
| Follow-up | 32 ± 8* | 33 ± 8* | 29 ± 6* | 0.011 |
| LVEDV (mL) | ||||
| Baseline | 226 ± 69 | 222 ± 69 | 236 ± 70 | 0.34 |
| Follow-up | 193 ± 72* | 179 ± 69* | 224 ± 71† | 0.005 |
| LVESV (mL) | ||||
| Baseline | 172 ± 55 | 168 ± 53 | 181 ± 60 | 0.27 |
| Follow-up | 135 ± 60* | 123 ± 56* | 161 ± 60* | 0.003 |
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| LVEF (%) | ||||
| Baseline | 24 ± 4 | 24 ± 4 | 24 ± 5 | 0.56 |
| Follow-up | 32 ± 8* | 33 ± 8* | 29 ± 6* | 0.011 |
| LVEDV (mL) | ||||
| Baseline | 226 ± 69 | 222 ± 69 | 236 ± 70 | 0.34 |
| Follow-up | 193 ± 72* | 179 ± 69* | 224 ± 71† | 0.005 |
| LVESV (mL) | ||||
| Baseline | 172 ± 55 | 168 ± 53 | 181 ± 60 | 0.27 |
| Follow-up | 135 ± 60* | 123 ± 56* | 161 ± 60* | 0.003 |
Abbreviations as in Table 1; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume.
*P < 0.001 baseline vs. follow-up; †P < 0.05 baseline vs. follow-up.
Echocardiographic dyssynchrony at baseline and at 6-month follow-up of patients in concordant and discordant left ventricular leads
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| AS-P delay (ms) | ||||
| Baseline | 212 ± 96 | 213 ± 100 | 209 ± 87 | 0.84 |
| Follow-up | 114 ± 97* | 106 ± 101* | 132 ± 85* | 0.23 |
| Change | −96 ± 122 | −102 ± 131 | −80 ± 98 | 0.42 |
| SDt6 (ms) | ||||
| Baseline | 100 ± 50 | 103 ± 50 | 93 ± 51 | 0.39 |
| Follow-up | 56 ± 46* | 55 ± 48* | 58 ± 42* | 0.78 |
| Change | −42 ± 53 | −45 ± 56 | −37 ± 47 | 0.53 |
| IVMD (ms) | ||||
| Baseline | 39 ± 24 | 40 ± 24 | 37 ± 23 | 0.55 |
| Follow-up | 15 ± 13* | 15 ± 13* | 16 ± 14* | 0.71 |
| Change | −25 ± 25 | −26 ± 26 | −22 ± 25 | 0.45 |
| LVFT/RR (%) | ||||
| Baseline | 45 ± 8 | 44 ± 7 | 48 ± 10 | 0.045 |
| Follow-up | 48 ± 6† | 48 ± 6† | 49 ± 5 | 0.32 |
| Change | 3 ± 10 | 3 ± 8 | 3 ± 14 | 0.26 |
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| AS-P delay (ms) | ||||
| Baseline | 212 ± 96 | 213 ± 100 | 209 ± 87 | 0.84 |
| Follow-up | 114 ± 97* | 106 ± 101* | 132 ± 85* | 0.23 |
| Change | −96 ± 122 | −102 ± 131 | −80 ± 98 | 0.42 |
| SDt6 (ms) | ||||
| Baseline | 100 ± 50 | 103 ± 50 | 93 ± 51 | 0.39 |
| Follow-up | 56 ± 46* | 55 ± 48* | 58 ± 42* | 0.78 |
| Change | −42 ± 53 | −45 ± 56 | −37 ± 47 | 0.53 |
| IVMD (ms) | ||||
| Baseline | 39 ± 24 | 40 ± 24 | 37 ± 23 | 0.55 |
| Follow-up | 15 ± 13* | 15 ± 13* | 16 ± 14* | 0.71 |
| Change | −25 ± 25 | −26 ± 26 | −22 ± 25 | 0.45 |
| LVFT/RR (%) | ||||
| Baseline | 45 ± 8 | 44 ± 7 | 48 ± 10 | 0.045 |
| Follow-up | 48 ± 6† | 48 ± 6† | 49 ± 5 | 0.32 |
| Change | 3 ± 10 | 3 ± 8 | 3 ± 14 | 0.26 |
Abbreviations as in Tables 1 and 2; AS-P delay, LV anteroseptal-to-posterior time difference in peak radial strain measured by ST-RS imaging; SDt6, standard deviation of time from QRS onset to peak radial strain measured by ST-RS echocardiography in 6 LV segments; IVMD, interventricular mechanical delay; LVFT/RR, LV filling time/cardiac cycle length × 100%.
Echocardiographic dyssynchrony at baseline and at 6-month follow-up of patients in concordant and discordant left ventricular leads
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| AS-P delay (ms) | ||||
| Baseline | 212 ± 96 | 213 ± 100 | 209 ± 87 | 0.84 |
| Follow-up | 114 ± 97* | 106 ± 101* | 132 ± 85* | 0.23 |
| Change | −96 ± 122 | −102 ± 131 | −80 ± 98 | 0.42 |
| SDt6 (ms) | ||||
| Baseline | 100 ± 50 | 103 ± 50 | 93 ± 51 | 0.39 |
| Follow-up | 56 ± 46* | 55 ± 48* | 58 ± 42* | 0.78 |
| Change | −42 ± 53 | −45 ± 56 | −37 ± 47 | 0.53 |
| IVMD (ms) | ||||
| Baseline | 39 ± 24 | 40 ± 24 | 37 ± 23 | 0.55 |
| Follow-up | 15 ± 13* | 15 ± 13* | 16 ± 14* | 0.71 |
| Change | −25 ± 25 | −26 ± 26 | −22 ± 25 | 0.45 |
| LVFT/RR (%) | ||||
| Baseline | 45 ± 8 | 44 ± 7 | 48 ± 10 | 0.045 |
| Follow-up | 48 ± 6† | 48 ± 6† | 49 ± 5 | 0.32 |
| Change | 3 ± 10 | 3 ± 8 | 3 ± 14 | 0.26 |
| Variable . | All (n = 103) . | Concordant (n = 72) . | Discordant (n = 31) . | P-value . |
|---|---|---|---|---|
| AS-P delay (ms) | ||||
| Baseline | 212 ± 96 | 213 ± 100 | 209 ± 87 | 0.84 |
| Follow-up | 114 ± 97* | 106 ± 101* | 132 ± 85* | 0.23 |
| Change | −96 ± 122 | −102 ± 131 | −80 ± 98 | 0.42 |
| SDt6 (ms) | ||||
| Baseline | 100 ± 50 | 103 ± 50 | 93 ± 51 | 0.39 |
| Follow-up | 56 ± 46* | 55 ± 48* | 58 ± 42* | 0.78 |
| Change | −42 ± 53 | −45 ± 56 | −37 ± 47 | 0.53 |
| IVMD (ms) | ||||
| Baseline | 39 ± 24 | 40 ± 24 | 37 ± 23 | 0.55 |
| Follow-up | 15 ± 13* | 15 ± 13* | 16 ± 14* | 0.71 |
| Change | −25 ± 25 | −26 ± 26 | −22 ± 25 | 0.45 |
| LVFT/RR (%) | ||||
| Baseline | 45 ± 8 | 44 ± 7 | 48 ± 10 | 0.045 |
| Follow-up | 48 ± 6† | 48 ± 6† | 49 ± 5 | 0.32 |
| Change | 3 ± 10 | 3 ± 8 | 3 ± 14 | 0.26 |
Abbreviations as in Tables 1 and 2; AS-P delay, LV anteroseptal-to-posterior time difference in peak radial strain measured by ST-RS imaging; SDt6, standard deviation of time from QRS onset to peak radial strain measured by ST-RS echocardiography in 6 LV segments; IVMD, interventricular mechanical delay; LVFT/RR, LV filling time/cardiac cycle length × 100%.
Concordant left ventricular lead positions
The location of the targeted LV segments were anterior [10 (10%)], anterolateral [8 (8%)], lateral [29 (28%)], posterolateral [33 (32%)], and posterior [23 (22%)]. In the preoperative analysis, the excluded LV segments due to low-amplitude ST-RS were: anterior (n = 7; 7%), lateral (n = 1; 1%), and posterior (n = 7; 7%). The LV leads were positioned in the anterior [2 (2%)], anterolateral [28 (27%)], lateral [50 (49%)], posterolateral [19 (18%)], and posterior [4 (4%)] segments. Subsequently, the concordant LV leads were achieved in 72 patients (70%) in the study population. The discordant LV lead position had to be accepted due to the lack of suitable coronary veins [21 (68%)], LV lead instability [4 (13%)], phrenic nerve stimulation [3 (10%)], and insufficient LV pacing capture [3 (10%)]. Longer CRT implantation duration was observed in the discordant LV leads than in the concordant LV leads (140 ± 57 vs. 117 ± 45 min; P = 0.03). The peak radial strain in the LV segment containing the LV lead was similar in the concordant LV vs. discordant LV leads (18.2 ± 7.9 vs. 21.3 ± 11.7%; P = 0.18). Nine patients (9%) demonstrated low-amplitude ST-RS at the LV lead location, equally distributed between the concordant and discordant LV leads [6 (8%) vs. 3(10%); P = 0.83]. After AV optimization, similar settings were used in the concordant and discordant LV leads (120 ± 34 vs. 118 ± 31 ms; P = 0.76). After VV optimization, comparable programmed VV delays were used between the concordant and discordant LV leads, predominantly with LV pre-exitation (24 ± 27 vs. 30 ± 34 ms; P = 0.39). During the 6-month follow-up period, 7 (7%) LV lead dislodgements were observed, similarly in the concordant and discordant LV leads [6(8%) vs. 1(3%); P = 0.67]. All LV leads were successfully repositioned at the same LV lead segment.
Clinical response to cardiac resynchronization therapy
During the 6-month observation period, one patient underwent heart transplantation, 5 patients died from HF (n = 2) or intercurrent disease (n = 3), and one patient was lost to follow-up. After 6 months of CRT, the patients improved in the NYHA functional class from 3.2 ± 0.4 to 2.3 ± 0.5 (P < 0.001), although similar in the concordant and discordant LV leads (3.2 ± 0.4–2.3 ± 0.5 vs. 3.2 ± 0.4–2.2 ± 0.4; P = 0.23). The distance walked in 6 min increased from 385 ± 107 to 411 ± 113 m (P = 0.003), comparable in the concordant and discordant LV leads (384 ± 114–410 ± 122 m vs. 386 ± 90–415 ± 89 m; P = 0.92).
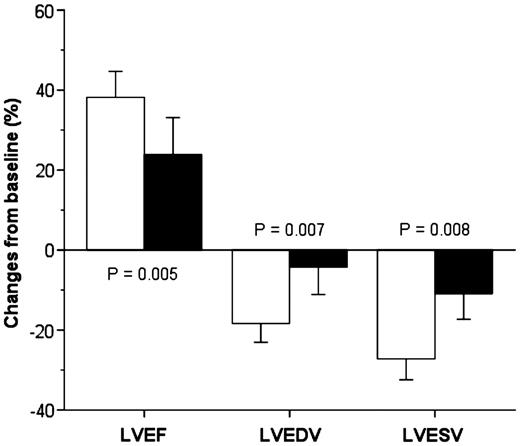
Left ventricular ejection fraction and reverse remodelling
The echocardiographic assessment of LV function at baseline and 6 months after CRT are presented in Table 2, and the relative improvements observed are presented in Figure 2. Both the concordant and discordant LV leads demonstrated improvement in LVEF and LV reverse remodelling at 6-month follow-up; however, the benefits of CRT were superior in concordant LV leads. Six months after the CRT implantation, the numbers of patients defined as LV reverse remodelling responders were 64 (67%), and according to the LV lead position were 51 (76%) in the concordant LV leads and 13 (45%) in the discordant LV leads (P = 0.003). The improvement in the NYHA functional class and increased distance walked in 6 min were not statistically different between LV reverse remodelling responders and non-responders (3.2 ± 0.4–2.3 ± 0.4 vs. 3.2 ± 0.4–2.4 ± 0.5; P = 0.067) and (382 ± 114–416 ± 118 m vs. 395 ± 85–403 ± 105 m; P = 0.072), respectively.
The echocardiographic response from baseline to 6 months after CRT in patients with concordant and discordant LV lead positions. Abbreviations as in Figure 1. LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; open bars = concordant LV leads located at the latest activated segment, or adjacent one, identified by ST-RS echocardiography; solid bars = discordant LV leads.
Mechanical resynchronization responders
LV dyssynchrony at baseline and 6 months after CRT are presented in Table 3. The numbers of patients classified as mechanical resynchronization responders at 6 month follow-up were 54 (56%), and according to the LV lead position 44 (66%) in the concordant LV leads and 10 (34%) in the discordant LV leads (P = 0.005). The improvement in the NYHA functional class and increased distance walked in 6 min were similar between mechanical resynchronization responders and non-responders (3.2 ± 0.4–2.3 ± 0.5 vs. 3.1 ± 0.4–2.3 ± 0.5; P = 0.55) and (387 ± 117–412 ± 123 vs. 387 ± 91–410 ± 99 m; P = 0.95), respectively. Concordant LV leads demonstrated more reduction in LV dyssynchrony than discordant LV leads, assessed by speckle tracking echocardiography as AS-P delay and SDt6, but the overall difference did not reach statistical significance. IVMD was reduced 6 months after CRT, similarly in the concordant and discordant LV lead positions. AV dyssynchrony expressed as LVFT/RR percentage was improved 6 months after CRT. However, baseline LVFT/RR ratio was lower in the concordant LV lead group, and this group constituted the improvement in the AV dyssynchrony. The effect of CRT in patients with extensive preoperative intraventricular dyssynchrony separated by AS-P delay of 130 ms, short- and long AS-P delay demonstrated comparable improvements in LVEF (10 ± 7 vs. 7 ± 6%; P = 0.12) and similar LVESV reduction (−39 ± 32 vs. −37 ± 40 mL; P = 0.83).
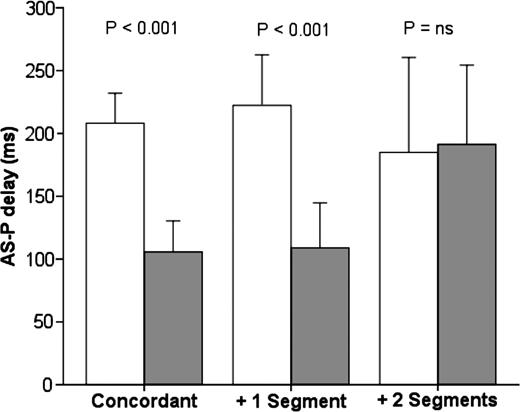
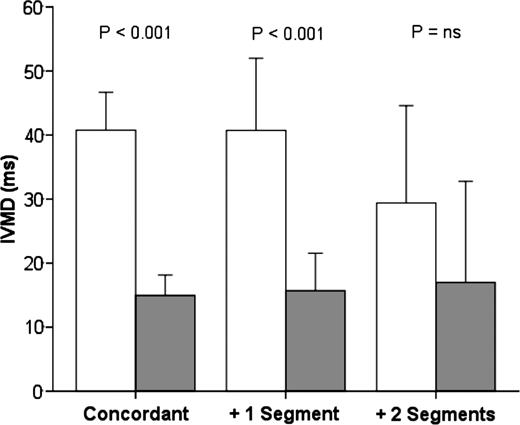
A subgroup analysis of the LV dyssynchrony reduction observed from baseline to 6-month follow-up in subsequent LV lead locations was performed. AS-P delay at baseline and 6 months after CRT according to LV lead positions are presented in Figure 3. Discordant LV leads located +1 segment [n = 22 (21%)] demonstrated similar AS-P delay reduction as concordant LV leads. However, discordant LV leads positioned +2 segments [n = 9 (9%)] did not demonstrate a decrease in AS-P delay. A subgroup interaction was identified in the AS-P delay reduction between the three LV lead groups (P = 0.04). The numbers of mechanical resynchronization responders were as follows: in the concordant LV leads [n = 44 (66%)], in +1 segment [n = 10 (48%)] and in +2 segments (n = 0; P = 0.001). IVMD at baseline and 6 months after CRT according to the LV lead position are presented in Figure 4. Similarly, the LV leads located in +2 segments did not demonstrate a decrease in IVMD. No subgroup interactions were identified in IVMD reduction between the three LV lead groups (P = 0.37).
Echocardiographic intraventricular dyssynchrony at baseline and 6-month follow-up evaluated by ST-RS echocardiography. Abbreviations as in Figures 1 and 2. Open bars = baseline; solid bars = 6-month follow-up; +1 segment = LV lead position in LV segment adjacent to concordant LV lead; +2 segments = LV lead location 2 segments apart from concordant LV lead.
Echocardiographic interventricular dyssynchrony at baseline and 6-month follow-up evaluated by pulse-wave Doppler. Abbreviations as in Figure 3; IVMD, interventricular mechanical delay.
The reduction in mechanical dyssynchrony observed at 6-month follow-up correlated to baseline dyssynchrony for AS-P delay (r = −0.63; P < 0.001), IVMD (r = −0.86; P < 0.001), and LVFT/RR (r = −0.76; P < 0.001).
Left ventricular reverse remodelling and mechanical dyssynchrony
Only a weak correlation was found in the relative AS-P delay reduction from baseline to 6-month follow-up and the relative improvements in LVEF (r = −0.23; P = 0.02), LVEDV (r = 0.24; P = 0.02), and LVESV (r = 0.27; P = 0.009), respectively. Similarly, the IVMD reduction correlated weakly with the relative improvements in LVEF (r = −0.30; P = 0.003), LVEDV (r = 0.23; P = 0.03), and LVESV (r = 0.27; P = 0.009), respectively. More mechanical resynchronization responders were observed among LV reverse remodelling responders than non-responders [n = 42 (66%) vs. n = 12 (38%); P = 0.009]. Further, the AS-P delay reduction was more extensive in LV reverse remodelling responders to CRT compared with non-responders (210 ± 102–93 ± 84 vs. 208 ± 80–154 ± 108 ms; P = 0.016). Similarly, the improvement in IVMD was greater in LV reverse remodelling responders than non-responders (44 ± 24–15 ± 13 vs. 32 ± 22–17 ± 15 ms; P = 0.013).
Right ventricular lead position
Comparable LV reverse remodelling responders were observed between the RV-A lead position (n = 60; 58%) and the RV-HS lead position (n = 43; 42%) at 6-month follow-up [RV-A vs. RV-HS; n = 38 (68%) vs. n = 26 (65%); P = 0.77)]. Mechanical resynchronization responders were similar in RV-A and RV-HS [n = 30 (54%) vs. n = 24 (60%); P = 0.53)].
Regression analyses
Univariable and multivariable logistic regression analyses were performed to assess nine variables to predict ≥15% reduction in LVESV and ≥50% AS-P delay reduction 6 months after CRT. The variables tested were gender, ischaemic cardiomyopathy, QRS duration, LBBB, radial strain ≥10% at the LV lead position, AS-P delay, IVMD, LVFT/RR and concordant LV lead position (Table 4). In the univariable analysis, the radial strain ≥10% at the LV lead position, IVMD, and concordant LV lead were positively associated with LV volume reduction. However, in the multivariable analysis, only concordant LV lead could independently predict LV reverse remodelling at 6-month follow-up. In the univariable analysis, LBBB, AS-P delay, IVMD, and concordant LV lead was associated with AS-P delay reduction. In the multivariable analysis, AS-P delay, IVMD, and concordant LV lead could independently predict mechanical resynchronization responders.
Univariable and multivariable logistic regression analyses to identify predictors to left ventricular reverse remodelling and mechanical resynchronization
| . | LV reverse remodeling . | Mechanical resynchronization . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Univariable | ||||
| Male | 0.726 (0.234–2.253) | 0.580 | 1.364 (0.488–3.809) | 0.554 |
| Ischaemic cardiomyopathy | 0.724 (0.304–1.726) | 0.466 | 1.011 (0.447–2.284) | 0.979 |
| QRS (per 1 ms) | 1.012 (0.997–1.028) | 0.117 | 1.004 (0.990–1.018) | 0.558 |
| LBBB | 0.852 (0.324–2.243) | 0.745 | 4.312 (1.638–11.354) | 0.003 |
| Radial strain ≥10% | 4.692 (1.090–20.204) | 0.038 | 0.615 (0.145–2.621) | 0.511 |
| AS-P delay (per 1 ms) | 1.000 (0.996–1.005) | 0.916 | 1.008 (1.003–1.013) | 0.002 |
| IVMD (per 1 ms) | 1.021 (1.002–1.041) | 0.033 | 1.024 (1.005–1.043) | 0.012 |
| LVFT/RR (per 1%) | 0.010 (0.000–5.710) | 0.155 | 0.011 (0.000–4.558) | 0.143 |
| Concordant LV lead | 3.923 (1.560–9.869) | 0.004 | 3.635 (1.453–9.092) | 0.006 |
| Multivariable | ||||
| LBBB | — | 3.042 (0.982–9.427) | 0.054 | |
| Radial strain ≥10% | 4.554 (0.968–21.417) | 0.055 | — | |
| AS-P delay (per 1 ms) | — | 1.007 (1.001–1.013) | 0.032 | |
| IVMD (per 1 ms) | 1.020 (0.999–1.042) | 0.062 | 1.024 (1.001–1.047) | 0.038 |
| Concordant LV lead | 4.177 (1.579–11.049) | 0.004 | 4.691 (1.629–13.511) | 0.004 |
| . | LV reverse remodeling . | Mechanical resynchronization . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Univariable | ||||
| Male | 0.726 (0.234–2.253) | 0.580 | 1.364 (0.488–3.809) | 0.554 |
| Ischaemic cardiomyopathy | 0.724 (0.304–1.726) | 0.466 | 1.011 (0.447–2.284) | 0.979 |
| QRS (per 1 ms) | 1.012 (0.997–1.028) | 0.117 | 1.004 (0.990–1.018) | 0.558 |
| LBBB | 0.852 (0.324–2.243) | 0.745 | 4.312 (1.638–11.354) | 0.003 |
| Radial strain ≥10% | 4.692 (1.090–20.204) | 0.038 | 0.615 (0.145–2.621) | 0.511 |
| AS-P delay (per 1 ms) | 1.000 (0.996–1.005) | 0.916 | 1.008 (1.003–1.013) | 0.002 |
| IVMD (per 1 ms) | 1.021 (1.002–1.041) | 0.033 | 1.024 (1.005–1.043) | 0.012 |
| LVFT/RR (per 1%) | 0.010 (0.000–5.710) | 0.155 | 0.011 (0.000–4.558) | 0.143 |
| Concordant LV lead | 3.923 (1.560–9.869) | 0.004 | 3.635 (1.453–9.092) | 0.006 |
| Multivariable | ||||
| LBBB | — | 3.042 (0.982–9.427) | 0.054 | |
| Radial strain ≥10% | 4.554 (0.968–21.417) | 0.055 | — | |
| AS-P delay (per 1 ms) | — | 1.007 (1.001–1.013) | 0.032 | |
| IVMD (per 1 ms) | 1.020 (0.999–1.042) | 0.062 | 1.024 (1.001–1.047) | 0.038 |
| Concordant LV lead | 4.177 (1.579–11.049) | 0.004 | 4.691 (1.629–13.511) | 0.004 |
Univariable and multivariable logistic regression analyses to identify predictors to left ventricular reverse remodelling and mechanical resynchronization
| . | LV reverse remodeling . | Mechanical resynchronization . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Univariable | ||||
| Male | 0.726 (0.234–2.253) | 0.580 | 1.364 (0.488–3.809) | 0.554 |
| Ischaemic cardiomyopathy | 0.724 (0.304–1.726) | 0.466 | 1.011 (0.447–2.284) | 0.979 |
| QRS (per 1 ms) | 1.012 (0.997–1.028) | 0.117 | 1.004 (0.990–1.018) | 0.558 |
| LBBB | 0.852 (0.324–2.243) | 0.745 | 4.312 (1.638–11.354) | 0.003 |
| Radial strain ≥10% | 4.692 (1.090–20.204) | 0.038 | 0.615 (0.145–2.621) | 0.511 |
| AS-P delay (per 1 ms) | 1.000 (0.996–1.005) | 0.916 | 1.008 (1.003–1.013) | 0.002 |
| IVMD (per 1 ms) | 1.021 (1.002–1.041) | 0.033 | 1.024 (1.005–1.043) | 0.012 |
| LVFT/RR (per 1%) | 0.010 (0.000–5.710) | 0.155 | 0.011 (0.000–4.558) | 0.143 |
| Concordant LV lead | 3.923 (1.560–9.869) | 0.004 | 3.635 (1.453–9.092) | 0.006 |
| Multivariable | ||||
| LBBB | — | 3.042 (0.982–9.427) | 0.054 | |
| Radial strain ≥10% | 4.554 (0.968–21.417) | 0.055 | — | |
| AS-P delay (per 1 ms) | — | 1.007 (1.001–1.013) | 0.032 | |
| IVMD (per 1 ms) | 1.020 (0.999–1.042) | 0.062 | 1.024 (1.001–1.047) | 0.038 |
| Concordant LV lead | 4.177 (1.579–11.049) | 0.004 | 4.691 (1.629–13.511) | 0.004 |
| . | LV reverse remodeling . | Mechanical resynchronization . | ||
|---|---|---|---|---|
| . | OR (95% CI) . | P-value . | OR (95% CI) . | P-value . |
| Univariable | ||||
| Male | 0.726 (0.234–2.253) | 0.580 | 1.364 (0.488–3.809) | 0.554 |
| Ischaemic cardiomyopathy | 0.724 (0.304–1.726) | 0.466 | 1.011 (0.447–2.284) | 0.979 |
| QRS (per 1 ms) | 1.012 (0.997–1.028) | 0.117 | 1.004 (0.990–1.018) | 0.558 |
| LBBB | 0.852 (0.324–2.243) | 0.745 | 4.312 (1.638–11.354) | 0.003 |
| Radial strain ≥10% | 4.692 (1.090–20.204) | 0.038 | 0.615 (0.145–2.621) | 0.511 |
| AS-P delay (per 1 ms) | 1.000 (0.996–1.005) | 0.916 | 1.008 (1.003–1.013) | 0.002 |
| IVMD (per 1 ms) | 1.021 (1.002–1.041) | 0.033 | 1.024 (1.005–1.043) | 0.012 |
| LVFT/RR (per 1%) | 0.010 (0.000–5.710) | 0.155 | 0.011 (0.000–4.558) | 0.143 |
| Concordant LV lead | 3.923 (1.560–9.869) | 0.004 | 3.635 (1.453–9.092) | 0.006 |
| Multivariable | ||||
| LBBB | — | 3.042 (0.982–9.427) | 0.054 | |
| Radial strain ≥10% | 4.554 (0.968–21.417) | 0.055 | — | |
| AS-P delay (per 1 ms) | — | 1.007 (1.001–1.013) | 0.032 | |
| IVMD (per 1 ms) | 1.020 (0.999–1.042) | 0.062 | 1.024 (1.001–1.047) | 0.038 |
| Concordant LV lead | 4.177 (1.579–11.049) | 0.004 | 4.691 (1.629–13.511) | 0.004 |
Discussion
The main findings in this study can be summarized as follows: (i) prospectively targeted concordant LV leads demonstrated superior improvement in the LV contractile function and LV reverse remodelling compared with discordant LV leads in CRT, but no difference in clinical status was observed; (ii) more mechanical resynchronization responders in concordant than discordant LV leads. Subgroup analysis demonstrated no improvement in the LV leads positioned +2 segments apart from concordant LV leads; (iii) the extent of mechanical resynchronization correlated only weakly with the improvement in the LV contractile function and LV reverse remodelling; (iv) there was a moderate-to-strong correlation between mechanical dyssynchrony observed at baseline and the reduction at 6-month follow-up.
The influence of the LV lead position on LV reverse remodelling and clinical outcome in CRT has recently been demonstrated.18 Singh et al.9 investigated 799 patients undergoing CRT and found an increased risk for death (hazard ratio, 2.91; P = 0.004) in patients with an apical vs. a non-apical LV lead position. In contrast, the empirical anterior, lateral, and posterior segments for an LV lead placement segment did not influence on long-term mortality.9 Moreover, combined posterolateral LV lead position concomitant with mechanical dyssynchrony has demonstrated improved outcome.19 Ypenburg et al.6 assessed 257 CRT recipients retrospectively with ST-RS echocardiography, and demonstrated more extensive reverse remodelling and superior hospitalization-free survival after long-term CRT (hazard ratio, 0.22; P = 0.004) in patients with concordant LV leads compared with discordant LV leads. In a recent double-blind prospective randomized trial, Khan et al.20 demonstrated greater LV reverse remodelling responders in the targeted LV lead to the latest contracting LV segment guided by ST-RS echocardiography as compared with the empirical LV lead position (70 vs. 55%; P = 0.031). In addition, combined all-cause mortality and HF-related hospitalizations were lower in the targeted LV leads (log-rank test, P = 0.031). Furthermore, extensive scarring has been associated with decreased effect of CRT.15,21 The empirical LV lead position can result in 15–21% low-amplitude strain at the LV lead location, assessed by ST-RS echocardiography.15,20 In the present study, LV leads were prospectively targeted to the latest activated LV segment without extensive LV scarring. Thus, areas with low-amplitude strain were to a certain degree avoided, but still present in 9%. Few patients with scarring at the implanted LV lead position might explain why LV scarring only demonstrated decreased LV volume reduction in univariable but not in multivariable analysis. Corrected for covariates, only concordant LV lead of the tested variables was independently predictive of LV reverse remodelling. Consequently, increased LV volume reduction provided by concordant LV leads in CRT is confirmed by the current study in prospective settings. Contrary, clinical response was not greater in concordant compared with discordant LV leads. However, there is not necessarily a strong relationship between improvement in HF symptoms and LV reverse remodelling.22 On the other hand, LV reverse remodelling is considered to be one of the best predictors to long-term prognosis in CRT recipients.13
The relation between mechanical resynchronization assessed by speckle tracking echocardiography and LV reverse remodelling provided by CRT has previously been reported. Delgado et al.2 demonstrated more extensive mechanical resynchronization in LV responders as compared with non-responders. Similarly, a more sophisticated approach by using a strain delay index has shown the decreased LV dyssynchrony in responders to CRT.23 The interdependency of absolute LVESV decrease and AS-P delay (r = 0.73; P < 0.001) has recently been demonstrated in 64 patients undergoing CRT.24 Furthermore, mechanical resynchronization in CRT recipients, evaluated by speckle tracking echocardiography, has been related to better outcome.5 The present study supports the results from the aforementioned studies. However, in contrast to previous findings we could not demonstrate a strong correlation between the relative LV reverse remodelling and mechanical resynchronization in the current study. In fact, a moderate-to-strong correlation was observed between baseline mechanical dyssynchrony and the mechanical resynchronization effected by CRT. Thus, it seems reasonable to evaluate the improvement in LV dyssynchrony relative to the extent of dyssynchrony observed prior to CRT. Consequently, AS-P reduction ≥50% could be used as a readily feasible marker of improved LV synchronicity in CRT.
To our knowledge, this is the first comprehensive study to demonstrate the effect of LV lead position on the degree of mechanical resynchronization in CRT recipients, assessed by speckle tracking echocardiography. Previous studies to assess mechanical dyssynchrony have used empirical LV lead location, without prospectively targeted LV lead positions according to latest contracting LV segment identified in echocardiographic examination prior to CRT implantation. As reported, more mechanical resynchronization responders were observed in concordant than in discordant LV leads. However, the absolute differences in mechanical resynchronization between the LV lead positions did not reach statistical significance. The sample size in the present study might be underpowered to distinguish the absolute mechanical resynchronization between the two LV lead positions. An important observation from the subgroup analysis was that LV leads located +2 segments apart from concordant LV leads showed no reduction in the extent of LV dyssynchrony. However, the LV leads 2 segments apart from concordant LV leads constituted 9% of the study population, and larger prospective studies are needed to confirm these findings. Interventricular dyssynchrony at baseline and 6 months after CRT were similar in the concordant and discordant LV leads. Contrary, the AV dyssynchrony was different respectively to the LV lead concordance at baseline, and the results of the LVFT/RR ratio in the current study are more difficult to interpret. From the identified subgroup interactions, the LV lead location seems to be more important in the improvement of intraventricular dyssynchrony rather than interventricular dyssynchrony in CRT.
Previous studies have evaluated baseline mechanical dyssynchrony to predict LV reverse remodelling in CRT. In these, long AS-P delay and IVMD have been associated with greater effects of CRT.7,25 In the multivariate analysis, only the concordant LV lead predicted LV reverse remodelling, but not preoperative mechanical dyssynchrony. In contrast, baseline AS-P delay and IVMD were independently predictive of mechanical resynchronization in the current study. Baseline QRS duration and AS-P delay were longer in our study population than in the aforementioned studies and might have influenced on the results. Importantly, the LV lead was prospectively targeted concordantly in the current study, whereas the aforementioned studies to our knowledge include empirical LV lead positions. Moreover, it could not be demonstrated that QRS duration, LBBB morphology and ischaemic cardiomyopathy were predictive of LV reverse remodelling in the current study. However, conflicting evidence on the predictive value of QRS duration1,26 and LBBB27,28 has been reported. Furthermore, the sample size or the observation period in our study might be insufficient to identify these preoperative characteristics as predictive of CRT response.
It is not established whether AV and VV optimization should be performed routinely. This is at least recommended in patients not responding to CRT.17 However, individually tailored AV and VV intervals can provide improved response to CRT.29 Nevertheless, similar AV and VV intervals were programmed in the concordant and discordant LV leads in the current study. Thus, AV and VV optimization is less likely to have influenced on mechanical resynchronization according to the LV lead position.
Clinical implications
The present study further demonstrates that it is feasible to target the LV lead according to baseline ST-RS echocardiography in CRT, and confirm the superior LV reverse remodelling effected by concordant LV leads.20 Subsequently, we advocate the use of preoperative imaging to assess mechanical activation pattern in order to guide the LV lead position in CRT. However, the major limitation to achieve concordant LV lead position was the absence of suitable coronary vein tributaries at the targeted LV segment. Thus, transvenous LV lead implantation technique may theoretically be suboptimal in ∼30% of CRT patients. To further assess the potential additive effect on targeted LV lead position according to LV contraction pattern, more randomized trials are needed.
Recent studies have reported concordant LV leads in 47–68%, assessed by ST-RS echocardiography.6,7,15,20 Moreover, the imaging techniques to evaluate the LV lead position have been different. Preoperative imaging of available coronary vein tributaries prior to CRT implantation could be performed to assess the targeted LV segment.30,31 In the absence of suitable coronary veins at the targeted LV segment, limited left lateral thoracotomy could be considered to facilitate concordant LV lead placement.32 Furthermore, stenotic coronary veins located at the latest activated LV segment may be dilated by vein venoplasty during the CRT implant procedure.33 Larger prospective studies are needed to further assess the feasibility and the potential additive effect of combining different imaging modalities to guide LV lead placement in CRT.
Study limitations
The present single-centre observational study may be limited by the relative small sample size and underpowered to identify statistical significant differences in overall mechanical resynchronization between the concordant and discordant LV lead positions in CRT. Echocardiography-guided LV lead implantation was not compared with empirical LV lead position in randomized settings. There were few patients in the subgroup analysis and the subgroup interactions identified should be interpreted with some caution. In the present study, frame rates ≥30 were used, which are comparable to recent publications using ST-RS echocardiography in CRT.2,4,6,7,15 Higher frame rates could have been used for higher imaging resolution. In contrast, the prospective trial design strengthens the results presented in this study.
Conclusions
Targeted LV leads to the latest contracting LV segment demonstrated superior improvement in LV contractile function and LV reverse remodelling as compared with discordant LV leads after 6 months of CRT. More mechanical resynchronization responders were observed in concordant than discordant LV leads. Subgroup analysis demonstrated that LV leads located distantly to concordant LV leads did not reduce LV dyssynchrony at 6-month follow-up. Mechanical resynchronization was related to the extent of baseline LV dyssynchrony, and greater mechanical synchronicity was obtained in LV reverse remodelling responders.
Conflict of interest: none declared.
Funding
This study was supported by Helse Vest, the Norwegian Research Council.