-
PDF
- Split View
-
Views
-
Cite
Cite
Antonino Casile, Eran Dayan, Vittorio Caggiano, Talma Hendler, Tamar Flash, Martin A. Giese, Neuronal Encoding of Human Kinematic Invariants during Action Observation, Cerebral Cortex, Volume 20, Issue 7, July 2010, Pages 1647–1655, https://doi.org/10.1093/cercor/bhp229
Close - Share Icon Share
Abstract
Human movements, besides entailing the presence of a body shape, comply with characteristic kinematic laws of motion. Psychophysical studies show that low-level motion perception is biased toward stimuli complying with these laws. However, the neuronal structures that are sensitive to the kinematic laws of observed bodily movements are still largely unknown. We investigated this issue by dissociating, by means of computer-generated characters, form and motion information during the observation of human movements. In a functional imaging experiment, we compared the levels of blood oxygen level–dependent activity elicited by human actions complying with or violating the kinematic laws of human movements. Actions complying with normal kinematic laws of motion differentially activated the left dorsal premotor and dorsolateral prefrontal cortex as well as the medial frontal cortex. These findings suggest that the kinematic laws of human movements specifically modulate the responses of neuronal circuits also involved in action recognition and that are predominantly located in the left frontal lobe.
Introduction
The ability to perceive and understand the actions of others is among the most fundamental capabilities of the human brain, lying at the basis of social cognition. The high behavioral relevance of visual stimuli depicting the movements of conspecifics is underscored by several psychophysical studies showing specificities in their processing compared with other types of motion stimuli (Shiffrar and Freyd 1990; Bertenthal and Pinto 1994; Ahlström et al. 1997; Shiffrar et al. 1997; Neri et al. 1998; Grossman and Blake 1999). During the past decades, many studies have sought to elucidate the neural mechanisms underlying these specificities. Many investigations, that used point-light displays as stimuli (Johansson 1973), identified regions in the superior temporal sulcus, cerebellum, amygdala, premotor cortex, fusiform face area, and lateral occipital complex that are sensitive to motion-defined bodily movements (e.g., Bonda et al. 1996; Grossman et al. 2000; Grèzes et al. 2001; Vaina et al. 2001; Grossman and Blake 2002; Servos et al. 2002; Beauchamp et al. 2003; Saygin et al. 2004; Peuskens et al. 2005). Further imaging studies that used videotaped sequences of human movements suggested that brain areas which are involved in planning, preparation, and execution of movements are also activated during action observation (Decety et al. 1994; Grafton et al. 1996; Rizzolatti et al. 1996; Iacoboni et al. 1999; Nishitani and Hari 2000; Buccino et al. 2001; for a review, see Grèzes and Decety 2001; Rizzolatti and Craighero 2004). Finally, recent studies have also identified specific areas in the occipital cortex (extrastriate body area and fusiform body area) that are selectively activated by static and dynamic displays depicting human bodies (Downing et al. 2001; Peelen and Downing 2005; Peelen et al. 2006).
Notably, human actions are characterized not only by the presence of a moving body but also by the compliance of the performed movements with characteristic kinematic laws of motion. A well-established example is the so-called “two-thirds power law,” that describes the characteristic dependency of the speed of human movements on the geometrical properties of the motion path. This empirical law states that when tracing a curvilinear path, the angular velocity A and the curvature C of the hand trajectory are related through an exponential law, that is A = kCβ, where β∼2/3 and k is a piecewise constant coefficient called the velocity gain factor (Lacquaniti et al. 1983). This law can be expressed in a mathematically equivalent manner in terms of the tangential velocity V, that is, V = kCβ. In this formulation, which will be used here, a value of the exponent β = −1/3 gives precisely the two-thirds power law. The two-thirds power law is a ubiquitous feature of movement generation that characterizes drawing movements (Lacquaniti et al. 1983; Viviani and Flash 1995), eye movements (de'Sperati and Viviani 1997), speech-related movements (Tasko and Westbury 2004), and human locomotion along curved paths (Hicheur et al. 2005). A notable characteristic of this law is that it holds not only in movement production but also in motion perception. Indeed, psychophysical and brain imaging studies with simple motion displays showed that human visual perception is biased toward stimuli complying with the two-thirds power law (Viviani and Stucchi 1992; Kandel et al. 2000; Flach et al. 2004; Levit-Binnun et al. 2006; Dayan et al. 2007). This bias is a complex phenomenon whose mechanisms are still not fully understood. For example, a recent psychophysical experiment by Bidet-Ildei et al. (2006) found different behavioral performances in judging whether a visual stimulus complied or not with the two-thirds power law depending on whether the stimulus was a simple motion display or a point-light display depicting a person moving.
The neuronal structures that are specifically sensitive to the characteristic kinematic laws of motion of the human body during action observation are still unknown. Previous neuroimaging investigations have contrasted the observation of natural as opposed to unnatural actions. For example by comparing human and robot movements (Tai et al. 2004; Gazzola et al. 2007) or biomechanically possible versus impossible movements (Stevens et al. 2000; Costantini et al. 2005). However, these manipulations affected human motion in a global manner and typically confounded changes of the kinematic characteristics of the movement with changes in the associated body shape or configuration. In particular, to our best knowledge, no brain imaging study in the literature has investigated, in a well-controlled manner, how the neural substrates of the visual processing of bodily motion are influenced by those laws that reflect the underlying constraints governing human motor production (e.g., the two-thirds power law).
The goal of the present study is to investigate the neuronal substrates specifically subserving the visual processing of kinematic invariants during action observation while at the same time carefully controlling for body shape cues across different conditions. A prerequisite for achieving this goal is a set of stimuli in which body motion is controlled independently from body shape. Videotaped natural sequences cannot provide such a dissociation because humans cannot produce movements that violate the two-thirds power law even after extensive training (Viviani et al. 1987). Thus, in order to achieve a complete control of form and motion features, we used methods from computer graphics. We used a professional rendering software package to animate a human-like avatar. Similar stimuli have been already used to investigate patterns of brain activity in response to well-controlled changes of high- and low-level characteristics of human movements (Pelphrey et al. 2003, 2004). In our experiments, we presented the subjects with a computer-generated character that executed exactly the same movements, derived from motion-captured data, but with different types of kinematic characteristics that either complied with or violated the two-thirds power law. In this way, the body shape of the avatar was kept constant across all conditions, and only the kinematics of the joint trajectories was changed. In a functional magnetic resonance imaging experiment, we then recorded and compared the blood oxygen level–dependent (BOLD) responses of human subjects while observing movements that were either compatible or incompatible with normal kinematic laws of human movements.
Materials and Methods
Participants
Fourteen subjects (7 females and 7 males) mean age = 26±3 years (mean ± standard deviation), participated in the experiment. All of them were right-handed and had normal or corrected-to-normal vision. Participants gave their written informed consent and were paid for their participation in the experiment. All the procedures were approved by the ethics committee of the University Clinic of Tübingen.
Visual Stimuli
Smooth curvilinear hand and arm movements of a human subject were recorded using a Vicon 612 motion capture system (Vicon Motion Systems Ltd, Oxford, UK) with 7 cameras. The temporal sampling rate was 120 Hz, and the spatial error was less than 1 mm. The recorded movements were smooth, curvilinear, and roughly resembled the 3 trigrams “lll”, “lle”, and “leo” written in the air with both hands. These movements were chosen because previous studies have shown that hand movements during the reproduction of these types of stimuli comply with the two-thirds power law (Kandel et al. 2000). For the purpose of the experiment, we selected 2 repetitions of each of the 3 movements. The average duration of the movements was 2.61 ± 0.26 s (see Supplementary Table S1).
Data were preprocessed using commercial software by Vicon. Movements were executed relatively slowly, and most of the power was thus concentrated at low temporal frequencies. The power spectra plotted in Supplementary Figure S3 clearly show that frequencies above 2 Hz contained only a negligible part of the power of the movement. Thus, similar to previous studies from other groups (Schaal and Sternad 2001; Hicheur et al. 2005), high-frequency noise was removed by low passing the recorded trajectories with a 5th order Butterworth low-pass filter with a cutoff frequency of 3.6 Hz.
For the recorded movements, the best power-law approximation of the relationship between the tangential velocity and curvature of the hands’ trajectories yielded a value of −0.3 ± 0.02, which is in agreement with previous results (Viviani and Flash 1995; Kandel et al. 2000; see Supplementary Fig. S1 and Supplementary Tables S1 and Supplementary Data for a complete listing of the kinematic characteristics of the stimuli). Estimates of the exponent of the power law were largely insensitive to changes of the cutoff frequency up to a value of approximately 8 Hz. In order to change the exponent of the power law by an amount Δ, the tangential velocity V of each hand was multiplied by the factor CΔ, where C represents the local curvature of the hand path. In this manner, the new power law relating velocity and curvature was, on average, Vnew = kCβ+Δ. For the purpose of our experiment, we set Δ = 0.6, which yielded a final value of the exponent of 0.32 ± 0.03. The value Δ = 0.6 was selected based on pilot psychophysical experiments showing that such perturbation of the two-thirds power law yielded biological motion stimuli that were reported by the subjects as markedly unnatural (see Supplementary Fig. S2). In order to ensure high spatial and temporal sampling, both the tangential velocity and the curvature of the left and right-hand trajectories were computed from the polynomial coefficients obtained from the fitting of a cubic spline. The velocity profile Vnew was integrated to obtain the temporal evolution of the hand's position. We then computed a time-warping function that, when applied to the original movement, ensured that the new hand movement fulfilled the new kinematic profile. This computational step yielded 2 warping functions, one for each hand. Each of these warping functions was then applied to all the joints in the corresponding arm. The remaining points in the body not belonging to either arms were characterized by very small movements, and their trajectories were transformed using an average of the 2 warping functions. The characteristics of the hand trajectories before and after the perturbation of the kinematics for 1 of the 6 selected movements are shown in Supplementary Figure S1, whereas the kinematic characteristics for all the 6 movements are shown in Supplementary Tables S1 and Supplementary Data. Both the original and the “perturbed” stimuli had a sampling rate of 120 Hz.
Stimuli with normal and perturbed kinematics were uniformly rescaled in time such that each stimulus lasted for 3 s, were imported into a commercial software (3D Studio Max 9, Autodesk) and were used to animate a commercial avatar model. 3D Studio Max imports motion capturing data by first fitting them to a body model and then resampling them at a rate of 30 samples per second.
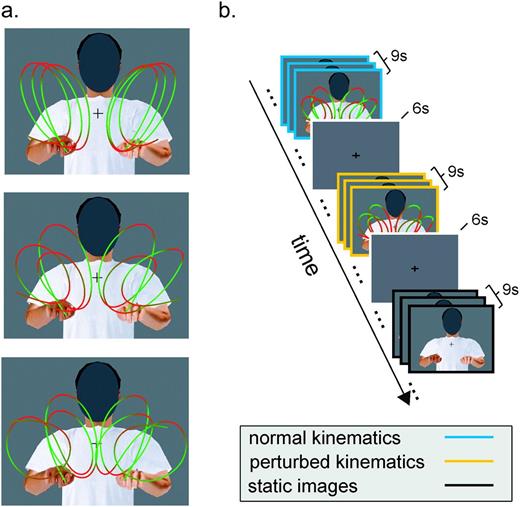
Two types of stimuli were created: “normal” stimuli in which the avatar moved following the original motion-captured trajectories and “perturbed” stimuli in which the avatar moved following the time-warped trajectories. The face of the avatars were masked with a gray ellipsoid in order to prevent additional activations of cerebral structures involved in the processing of faces. The scenes were then rendered in order to generate a set of 3 s movies in AVI format (524 × 412 pixels at 30 frames/s) used for the experiments. An additional set of stimuli (“static”) was created by extracting at regular time interval 6 snapshots from the “lll” animation.
Experimental Design
During each scanning session, stimuli were displayed in 9 s blocks, interleaved with 6 s periods of fixation. Eight blocks were included for each of the 3 experimental conditions resulting in a total of 24 blocks. Each block contained either 3 successive animations belonging to only 1 of the 2 dynamic conditions (normal or perturbed) or 3 static posture images. The blocks were presented in a one-back counterbalanced manner. Each scanning session lasted a total of 390 s and was performed 3 times by each subject.
To control for eye movements, subjects were instructed to carefully maintain fixation on a cross that was located at the center of the visual display throughout the entire session. Subjects were asked to perform a one-back task while watching the stimuli. When 2 identical stimuli (or static postures, in the static condition) appeared in succession, subjects were required to press a button in the response box. Instances of 2 consecutive identical stimuli were included in 50% of the blocks evenly distributed across the 3 conditions. Overall, subjects were able to perform the one-back task very well (97.6%, 95.2%, and 97.6% for the normal, perturbed and static conditions, respectively), and there were no significant differences among the 3 conditions with respect to this task (due to the violation of normality assumptions we used a Kruskal–Wallis test: χ2 = 1.17, P = 0.56).
Stimulus delivery and response acquisition were controlled using the Psychophysics toolbox for MATLAB (Brainard 1997; Pelli 1997). Stimuli were projected with a LCD projector onto a tangent screen positioned behind the subject and viewed through a tilted mirror. Responses were collected with an Magnetic Resonance Imaging (MRI)-compatible response box.
Imaging Setup
The experiment was performed using a 3-T Siemens Tim Trio scanner (Siemens, Erlangen, Germany), equipped with a resonant gradient echo-planar imaging (EPI) system. T2*-weighted functional images (time repetition [TR] = 3 s, time echo [TE] = 35 ms) sensitive to the BOLD contrast were acquired in gradient EPI sequences. Forty-four slices (3-mm height) were acquired for complete coverage of the cerebral and cerebellar cortex in descending order at 3 × 3 × 3 mm isotropic resolution (flip angle 90°, field of view [FOV] 192 mm, image matrix 64 × 64). Anatomical scans used the 3D gradient echo T1-weighted Siemens magnetization-prepared rapid acquisition echo-gradient sequence (time to inversion 1.1 s, TR 2.3 s, TE 2.92 ms) acquiring 176 slices in the sagittal plane at 1 × 1 × 1 mm isotropic resolution (flip angle 8°, FOV 256 mm, image matrix 256 × 256).
Data Analysis
Image preprocessing and statistical analysis were performed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). Functional image volumes were first unwrapped and spatially realigned to the first image in the series to correct participants’ head motion over time. Images were then corrected for slice timing differences by temporally interpolating the voxel time courses in each slice to align to the time of acquisition of the middle slice, spatially normalized and smoothed with a 8-mm full width half maximum isotropic Gaussian kernel. Structural T1-weighted images for each subject were also spatially normalized to the Montreal Neurological Institute (MNI) space using the T1 template brain of SPM5.
Statistical analysis was based on the general linear model (GLM; Friston et al. 1995) and consisted of 2 levels of analysis. At the first level, the time series of each voxel for each subject and experimental session were fitted with a design matrix, having a regressor for each of the 3 experimental conditions (normal, perturbed, and static). The regressors were modeled as boxcar functions convolved with the canonical hemodynamic response function. For each regressor, the baseline was defined by setting the value of the associated weights to zero. However, it is worth emphasizing that we obtained virtually identical results when baseline was defined as the weight of the regressor modeling the fixation periods. At the second level, a random-effect GLM was applied on the individual parameter estimates obtained from the first-level analysis. Contrasts were thresholded at P < 0.001 at the voxel level and corrected for multiple comparisons, with a threshold of P < 0.05, based on the spatial extent of the activated clusters. The method used for cluster size thresholding was that implemented in SPM5, which is based on Gaussian random field theory (Friston et al. 1994, 1996; Hayasaka and Nichols 2003). Plotting of the contrasts of interest was performed by importing the results into MRICroN.
Eye Movements
In order to exclude the possibility that observed patterns of BOLD response during observation of normal and perturbed stimuli might be related to different oculomotor behavior in the 2 conditions, we conducted a control experiment. The eye movements of 4 subjects were recorded monocularly using an MRI-compatible pupilometry eye tracking system (iView × MRI, SensoMotoric Instruments GmbH, Germany) inside the scanner under the same experimental conditions of the main experiment. Possible differences were assessed by means of t-test analysis on, respectively, the number and amplitude of saccades in the 3 conditions (n = normal, p = perturbed, and s = static). No statistically significant differences were found in any of the pairwise comparisons across conditions both in the number (tnp = 0.76, pnp = 0.50; tns = −1.72, pns = 0.18; tps = −1.56, pps = 0.22) and amplitudes of saccades (tnp = −0.98, pnp = 0.40; tns = −1.48, pns = 0.24; tps = 0.01, pps = 0.99).
Results
In this study, we investigated the neuronal correlates of the two-thirds power law during visual perception of human movements. For this purpose, we compared BOLD responses of human subjects while observing computer-generated human-like avatars performing curvilinear movements simultaneously with both hands in the air. Two main conditions were investigated (Fig. 1). In the first condition (normal), visual stimuli were rendered using the original hand trajectories derived from motion-captured data, and thus the movements complied with the two-thirds power law. In the second condition (perturbed), we time warped the original trajectories in order to invert the normal relationship between the tangential velocity and the curvature (for details, see “Materials and Methods”). That is, contrary to the normal behavior, the hands increased their velocity at points of high curvature and decreased their velocity at points of low curvature. It is worth emphasizing that the normal and perturbed stimulus classes differed only in the temporal characteristics of the movements, whereas the global spatial features of joint trajectories, and in particular, the instantaneous joint configurations were identical in the 2 cases. A third condition (static) consisted of static snapshots extracted from the presented movies.
Biological motion stimuli and experimental paradigm. (a) Stimuli consisted of computer-generated animations depicting a person executing 3 types of curvilinear movements (roughly resembling the trigrams lll, lee, and leo) with both hands in the air. Hand trajectories were obtained by motion capturing of a human subject. In one set of stimuli (normal), the movements of the animated avatar were the original recorded movements and thus complied with the normal human kinematic laws of motion. A second set of stimuli (perturbed) was created from the first one by means of nonlinear time warping in order to destroy compliance with the two-thirds power law. The different colors along the trajectories represent the instantaneous hand velocity, with hues of red indicating points of lower velocity and hues of green indicating points of higher velocity. A third set of stimuli consisted of static snapshots of the animations (static). In all stimuli, the face was occluded to minimize face-related neuronal activity. Each stimulus lasted for 3 s. (b) Example of an experimental session. Each scanning session consisted of a sequence of trials of 9 s during which 3 visual stimuli were shown consecutively followed by a 6 s fixation interval. During each 9 s trial, only stimuli of one type (normal, perturbed, or static) were shown. Trials were presented in a counterbalanced manner.
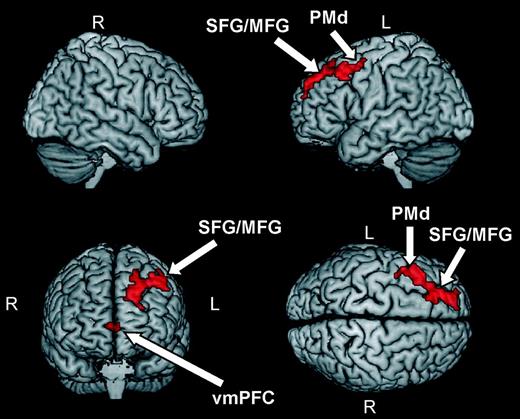
As shown in Figure 2 and Table 1, 2 groups of areas in the frontal lobes exhibited significantly higher BOLD responses during observation of stimuli that were compatible with the two-thirds power law compared with stimuli that violated this motor invariant. The first group of areas was located in the left hemisphere and ranged from the dorsal part of premotor cortex (BA 6), extending frontally to the middle and frontal gyri (BA 8 and BA 9). The second focus of activity was located bilaterally, although with a stronger response in the left hemisphere, in the medial frontal cortex (BA 10, BA 32). Our analysis revealed no areas showing significantly increased activation for perturbed compared with normal stimuli. This result suggests the presence in the frontal lobe of a network of areas, mostly lateralized to the left hemisphere, exhibiting higher activity during observation of human movements complying with normal kinematic laws of motion.
Foci of activity for the contrasts “normal > perturbed” and “perturbed > normal”, respectively
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z- score |
| Normal > Perturbed | |||||
| Left middle frontal gyrus | 6, 8 | −42 | 24 | 45 | 4.23 |
| Left superior frontal gyrus | 9 | −21 | 45 | 39 | 4.17 |
| Left middle frontal gyrus | 8, 9 | −24 | 33 | 39 | 3.93 |
| Right medial frontal gyrus | 10 | 6 | 60 | 3 | 3.68 |
| Left medial frontal gyrus | 10 | −3 | 57 | 3 | 3.66 |
| Left anterior cingulate gyrus | 32 | 0 | 45 | 3 | 3.58 |
| Perturbed > Normal | |||||
| No activations were obtained | |||||
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z- score |
| Normal > Perturbed | |||||
| Left middle frontal gyrus | 6, 8 | −42 | 24 | 45 | 4.23 |
| Left superior frontal gyrus | 9 | −21 | 45 | 39 | 4.17 |
| Left middle frontal gyrus | 8, 9 | −24 | 33 | 39 | 3.93 |
| Right medial frontal gyrus | 10 | 6 | 60 | 3 | 3.68 |
| Left medial frontal gyrus | 10 | −3 | 57 | 3 | 3.66 |
| Left anterior cingulate gyrus | 32 | 0 | 45 | 3 | 3.58 |
| Perturbed > Normal | |||||
| No activations were obtained | |||||
Note: Activations were thresholded at P < 0.001 (uncorrected) at the single-voxel level and at P < 0.05 (corrected) at the cluster level.
Foci of activity for the contrasts “normal > perturbed” and “perturbed > normal”, respectively
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z- score |
| Normal > Perturbed | |||||
| Left middle frontal gyrus | 6, 8 | −42 | 24 | 45 | 4.23 |
| Left superior frontal gyrus | 9 | −21 | 45 | 39 | 4.17 |
| Left middle frontal gyrus | 8, 9 | −24 | 33 | 39 | 3.93 |
| Right medial frontal gyrus | 10 | 6 | 60 | 3 | 3.68 |
| Left medial frontal gyrus | 10 | −3 | 57 | 3 | 3.66 |
| Left anterior cingulate gyrus | 32 | 0 | 45 | 3 | 3.58 |
| Perturbed > Normal | |||||
| No activations were obtained | |||||
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z- score |
| Normal > Perturbed | |||||
| Left middle frontal gyrus | 6, 8 | −42 | 24 | 45 | 4.23 |
| Left superior frontal gyrus | 9 | −21 | 45 | 39 | 4.17 |
| Left middle frontal gyrus | 8, 9 | −24 | 33 | 39 | 3.93 |
| Right medial frontal gyrus | 10 | 6 | 60 | 3 | 3.68 |
| Left medial frontal gyrus | 10 | −3 | 57 | 3 | 3.66 |
| Left anterior cingulate gyrus | 32 | 0 | 45 | 3 | 3.58 |
| Perturbed > Normal | |||||
| No activations were obtained | |||||
Note: Activations were thresholded at P < 0.001 (uncorrected) at the single-voxel level and at P < 0.05 (corrected) at the cluster level.
Foci of activity when contrasting BOLD responses in trials containing a button press with trials containing no button press
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z-score |
| Button press > No button press | |||||
| Left insula | 13 | −39 | 9 | 15 | 5.1 |
| Left medial frontal gyrus | 6 | −3 | −12 | 51 | 4.44 |
| Left pre- and postcentral gyrus | 2, 4 | −54 | −24 | 54 | 4.4 |
| Right cerebellum anterior lobe | 24 | −54 | −21 | 4.32 | |
| Right inferior parietal lobule | 40 | 66 | −36 | 24 | 4.28 |
| Left cerebellum anterior lobe | −27 | −48 | −21 | 4.22 | |
| Right precentral gyrus | 44 | 48 | 3 | 9 | 4.1 |
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z-score |
| Button press > No button press | |||||
| Left insula | 13 | −39 | 9 | 15 | 5.1 |
| Left medial frontal gyrus | 6 | −3 | −12 | 51 | 4.44 |
| Left pre- and postcentral gyrus | 2, 4 | −54 | −24 | 54 | 4.4 |
| Right cerebellum anterior lobe | 24 | −54 | −21 | 4.32 | |
| Right inferior parietal lobule | 40 | 66 | −36 | 24 | 4.28 |
| Left cerebellum anterior lobe | −27 | −48 | −21 | 4.22 | |
| Right precentral gyrus | 44 | 48 | 3 | 9 | 4.1 |
Note: Activations were thresholded at P < 0.001 (uncorrected) at the single-voxel level and at P < 0.05 (corrected) at the cluster level.
Foci of activity when contrasting BOLD responses in trials containing a button press with trials containing no button press
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z-score |
| Button press > No button press | |||||
| Left insula | 13 | −39 | 9 | 15 | 5.1 |
| Left medial frontal gyrus | 6 | −3 | −12 | 51 | 4.44 |
| Left pre- and postcentral gyrus | 2, 4 | −54 | −24 | 54 | 4.4 |
| Right cerebellum anterior lobe | 24 | −54 | −21 | 4.32 | |
| Right inferior parietal lobule | 40 | 66 | −36 | 24 | 4.28 |
| Left cerebellum anterior lobe | −27 | −48 | −21 | 4.22 | |
| Right precentral gyrus | 44 | 48 | 3 | 9 | 4.1 |
| MNI coordinates | |||||
| Brain areas | Brodmann area | X | Y | Z | z-score |
| Button press > No button press | |||||
| Left insula | 13 | −39 | 9 | 15 | 5.1 |
| Left medial frontal gyrus | 6 | −3 | −12 | 51 | 4.44 |
| Left pre- and postcentral gyrus | 2, 4 | −54 | −24 | 54 | 4.4 |
| Right cerebellum anterior lobe | 24 | −54 | −21 | 4.32 | |
| Right inferior parietal lobule | 40 | 66 | −36 | 24 | 4.28 |
| Left cerebellum anterior lobe | −27 | −48 | −21 | 4.22 | |
| Right precentral gyrus | 44 | 48 | 3 | 9 | 4.1 |
Note: Activations were thresholded at P < 0.001 (uncorrected) at the single-voxel level and at P < 0.05 (corrected) at the cluster level.
Brain areas showing higher BOLD responses during observation of human movements complying with normal kinematic laws of motion (see Table 1). Activations were thresholded at P < 0.001 (uncorrected) at the single-voxel level and at P < 0.05 (corrected) at the cluster level. Abbreviations: superior frontal gyrus (SFG), middle frontal gyrus (MFG), premotor dorsal (PMd), and ventromedial prefrontal cortex (vmPFC).
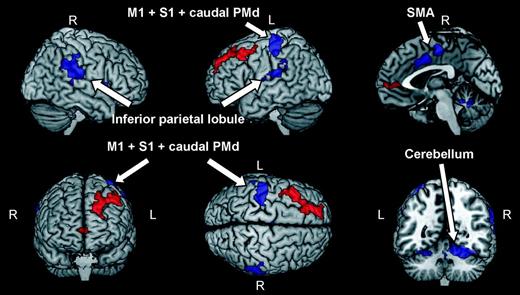
A second analysis of both behavioral and brain imaging results was conducted in order to exclude that activation patterns in Figure 2 might be, at least partially, related to motor responses. In our experimental paradigm subjects had to report, by pressing a button with the right hand, the occurrences of two subsequent identical stimuli. This simple one-back task was included in order to keep the subjects’ level of attention as constant as possible throughout the experiment. The occurrences of button presses were counterbalanced across conditions. However, a higher degree of activity of the left motor and premotor areas, possibly overlapping with foci of activity associated with the main contrast in Figure 2, is expected in the case of significant differences across conditions in the number of button presses. This can be ruled out for 2 reasons. First, as reported in the Materials and Methods section, subjects’ responses in the one-back task were monitored during scanning, and no statistically significant difference was found across conditions. Second, in order to isolate the effect of button presses, we contrasted BOLD responses of sessions in which 2 subsequent identical stimuli occurred with those of sessions in which stimuli were all different. As shown in Figure 3 and Table 2, button presses selectively activated regions in the left motor, supplementary motor, and somatosensory cortex (BA 4, BA 3, and BA 6) as well as the cerebellum and the insula. These areas are routinely activated during execution of hand movements (Rizzolatti et al. 1996; Buccino et al. 2004). Notably, all motor and premotor areas, activated in sessions containing button presses lay more posterior than activations found in the main experiment, and activation patterns in the 2 cases were strongly separated. This result is in line with a recent suggestion by Picard and Strick (2001) that the human dorsal premotor cortex can be divided into a caudal part (PMd proper), more involved in motor preparation and generation, and a rostral part (Pre-PMd) more involved in cognitive processes. In our experiment, areas in dorsal premotor cortex differentially responding during observation of movements complying with the two-thirds power law lay entirely within the Pre-PMd.
Brain areas showing higher BOLD response during trials containing button presses versus trials containing no button press (blue regions). Activations were thresholded at P < 0.001 (uncorrected) at the single-voxel level and at P < 0.05 (corrected) at the cluster level. Abbreviations: primary motor cortex (M1), dorsal premotor cortex (PMd), primary sensory cortex (S1), and supplementary motor area (SMA). Brain areas that in the main experiment exhibited higher level of BOLD response for human movements complying with the two-thirds power law are plotted in red for comparison purposes (see also Fig. 2).
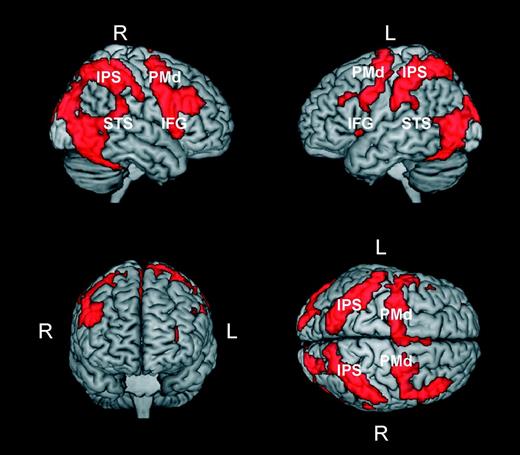
In a third analysis, we sought to identify areas that were similarly activated during the observation of bodily movements independently of whether or not they complied with normal kinematic laws of motion. For this purpose, we identified voxels that were common to the 2 contrasts “normal > baseline” and “perturbed > baseline.” Results of this analysis are presented in Figure 4 and show that the observation of a moving body, independently of its kinematics, produced a widespread pattern of activity. This pattern encompassed frontal, parietal, and temporo-occipital regions that are typically activated during action observation and perception of biological motion (Grossman et al. 2000; Grèzes and Decety 2001; Grèzes et al. 2001; Vaina et al. 2001; Servos et al. 2002; Beauchamp et al. 2003; Peuskens et al. 2005; Lestou et al. 2008). Notably, it also included the superior temporal sulcus and regions in the inferior parietal lobe and in the inferior frontal gyrus, which are considered the putative human homolog of the monkey mirror neuron system (Buccino et al. 2001; Rizzolatti and Craighero 2004; Iacoboni and Dapretto 2006). This result is in line with previous studies reporting activity in the mirror neuron system during observation of both human and robotic actions (Gazzola et al. 2007; but see Tai et al. 2004) and possible versus impossible movements (Stevens et al. 2000; Costantini et al. 2005). Furthermore, the widespread activation pattern reported in Figure 4 was not caused by the one-back task. Indeed, very similar activations were obtained when activation patterns resulting from the 2 contrasts “normal > static” and “perturbed > static” were intersected (Supplementary Fig. S5a). Because the one-back task was common to the normal, perturbed, and static conditions, the subtraction of this latter condition in the contrasts normal > static and perturbed > static discounts for its possible effects.
Brain areas activated by human movements independent of their compliance with normal kinematic laws of motion. The activation patterns in the figure were obtained by masking the contrast perturbed > baseline with the contrast normal > baseline. Prior to masking, activations in both contrasts were thresholded at P < 0.001 (uncorrected) at the voxel level and at P < 0.05 (corrected) at the cluster level. Abbreviations: PMd, premotor dorsal; IPS, intraparietal sulcus; STS, superior temporal sulcus; IFG, inferior frontal gyrus).
Discussion
We investigated the neuronal substrates subserving the processing of motor invariants during action observation. For this purpose, we compared the BOLD responses of 14 subjects while observing human movements complying with or violating the two-thirds power law. We found that human movements complying with the two-thirds power law yielded higher levels of BOLD response in a network of structures encompassing the left dorsal premotor cortex, the middle and superior frontal gyri, and medial frontal cortex. These results have implications for theories of action perception and are important for several reasons.
First, our results represent direct evidence that specific brain structures, mostly lateralized to the left hemisphere, exhibit significantly higher BOLD responses during observation of human movements complying with normal kinematic laws of motion. Our approach complements that of previous studies that investigated possible neuronal correlates of other characteristics of human movements. For example, Hamilton and Grafton (2006) reported that areas in the anterior intraparietal sulcus might represent the goal of observed actions, whereas areas in the inferior frontal cortex might be more involved in coding the action outcome (Hamilton and Grafton 2008). In a similar fashion, Lestou et al. (2008) found that areas in frontal and parietotemporal cortices are differentially modulated by the physical and perceptual similarity, respectively, of observed actions. The picture emerging from these studies is that action observation elicits a higher level of BOLD responses in a widespread network of areas, with different areas encoding different characteristics of the observed action. The present study adds to this picture by providing the first clear evidence that the kinematic laws of observed movements are among the characteristics encoded in this network of areas.
Second, our results suggest a specific role of the left dorsal premotor cortex in the visual processing of the two-thirds power law during action observation. Although several previous studies suggest the involvement of the left premotor cortex in the visual analysis of human movements, a specific function of its dorsal part is suggested by 2 recent studies by Calvo-Merino et al. (2006) and Tai et al. (2004). In these studies, neuronal structures in the left dorsal premotor cortex were differentially activated by the observation of actions that were already in the observer's motor repertoire (Calvo-Merino et al. 2006) or human goal-directed movements when contrasted with the same movements executed by a robot (Tai et al. 2004). Robot movements differ from those of a human with respect to both the body shape features and the kinematic features of the action. Our study carefully matched the body shape of the moving agent across conditions, and thus demonstrates that the left dorsal premotor cortex shows also a clear preference for kinematic invariants of human motor behavior. Thus, in agreement with previous studies, our results show that structures in the premotor cortex are also selectively activated during movement observation. They further suggest that this selective activation critically depends on the degree of compliance of observed movements with normal kinematic laws of human movements.
Third, our results show that, in addition to the left dorsal premotor cortex, large parts of the middle and superior frontal gyri (BA 8 and BA 9) exhibited higher levels of BOLD response to movements complying with the two-thirds power law. Experimental evidence shows that these dorsolateral frontal areas contain high-level visuomotor representations of actions. For example, they are activated by action observation and imitation (Decety et al. 1997; Koski et al. 2003; Buccino et al. 2004), “free willed” (as opposed to cued) finger movements (Hyder et al. 1997), motor learning (Jenkins et al. 1994), action words related to arm movements (Hauk et al. 2004), and attributing an action to others (as opposed to self; Farrer and Frith 2002). The results reported here suggest that kinematic laws of motion form an important component of the representations of actions in the prefrontal cortex, thus providing novel insights into their nature. There are 2 possible caveats to this conclusion. The first caveat is that area BA 8 is known to contain the frontal eye fields, and thus at least some portion of activations in that area might be due to oculomotor behavior. A detailed analysis of the subjects’ eye movements in the scanner evidenced no differences both in the number and amplitude of saccades across different conditions (see Materials and Methods). Because eye movement recordings were performed in separate sessions than the actual scanning, these results do not provide a completely conclusive evidence. However, they strongly suggest that activation patterns in our experiments cannot be explained simply by differences in oculomotor behavior across the different conditions. The second caveat to our conclusions is that areas in the dorsolateral prefrontal cortex were also reported to be selectively activated by memory tasks (Blumenfeld and Ranganath 2006) and in particular by verbal memory tasks (Petrides et al. 1993). Thus, it seems possible that the activation patterns in these regions might be related to memory-specific differences in performing the one-back task and in particular to verbal memory effects given that the movements resembled trigrams. Two reasons make also this explanation unlikely. First, the occurrences of trials requiring feedback from the subjects were carefully counterbalanced across conditions. Second, a detailed analysis of the behavioral responses in the scanner revealed no statistically significant performance difference across conditions (see Materials and Methods). Although making it unlikely, this behavioral evidence does not completely rule out the possibility that the subjects adopted different working memory strategies, that is, verbal versus nonverbal, to perform the one-back task. However, this explanation would predict activation differences in areas known to be involved in language production (e.g., Broca's area; Chein and Fiez 2001; Chein et al. 2002), and we failed to observe such activation differences.
An unexpected finding of our experiment is the selective activation of medial frontal regions during observation of human movements complying with the two-thirds power law. Although not routinely activated during action observation, the ventromedial frontal cortex, albeit a little bit more ventral than in the present study, has been found to be selectively activated during observation of movements already in the observer's motor repertoire in comparison with unpracticed movements (Calvo-Merino et al. 2005). Motivated by studies showing that ventromedial frontal cortex is strongly activated by rewarding stimuli (O'Doherty et al. 2003) and might contribute to social judgments (Decety and Chaminade 2003), Calvo-Merino et al. (2005) hypothesized that its activation in the context of action perception might reflect a higher degree of either “pleasantness” or social engagement of the perceived movements. The experimental paradigm used in our study was not designed to specifically investigate this hypothesis. However, our results motivate further experiments in order to test the possible relationship between the perceived pleasantness of human movements or motion in general and their degree of compliance with the two-thirds power law.
Fourth, the comparison of the present experiment with our previous study (Dayan et al. 2007) suggests possible differences between the processing of biological motion displays and abstract motion stimuli during visual perception of the two-thirds power law. In that study, we compared the BOLD responses during observation of a cloud of dots moving along elliptical trajectories with a motion that either violated or complied with the two-thirds power law. Stimuli complying with this motor invariant differentially activated a widespread network of areas that included several regions involved in action observation (Dayan et al. 2007). In contrast, in the present study, observation of bodily movements complying with the two-thirds power law, when contrasted with movements violating this motor invariant, produced a more focused activation pattern involving only regions in the frontal lobe, mostly lateralized to the left hemisphere. Figure 4 and Supplementary Figure S4 suggest that this difference might be due to the manner in which stimuli violating the two-thirds are processed by the brain in the 2 cases. The 2 figures show brain areas in the 2 studies, respectively, that were equally activated by the presented visual stimuli independently of their kinematics. As is evident from Figure 4, many brain areas involved in action observation were equally active during observation of a moving body, independently of the compliance of its movements with the two-thirds power law. On the contrary, in the case of abstract motion stimuli, only a small subset of this network was equally activated by normal and perturbed stimuli. Thus, the comparison between the normal and the perturbed conditions yielded, in the case of bodily movements, a much more focused activation pattern. A possible reason underlying the widespread activation pattern yielded by computer-generated characters whose movements violate the two-thirds power law is the interaction between 2 factors: body shape and movements resembling those of a human. Indeed, in our experiments, static pictures of the human body were sufficient to activate brain areas largely overlapping with the action observation network above baseline (Supplementary Fig. S5b). Moreover, Supplementary Figure S5a shows that these activations are stronger, in comparison with pictures of static bodies, when the displays contain bodily movements, even when they do not comply with normal kinematic laws of human movements. In summary, taken together with our previous results, the present study reveals that the observation of a moving body, independently of its kinematics, is sufficient to activate many of the brain areas involved in action observation. This appears not to be the case for simple motion displays where stimuli violating the kinematics laws of human movements activated this network of areas in a weaker and much less widespread manner. The present findings and those reported in our earlier study suggest that, in addition to the preference for movements at a constant equi-affine speed or with smooth velocity profiles (see Dayan et al. 2007), activation patterns obtained in the case of simple motion displays complying with the two-thirds power law might be also due to top-down effects, for example, the subjects’ “expectations.”
The fact that specific brain structures are selectively activated by the two-thirds power law suggests that the recognition and discrimination of normal human kinematics have high ecological relevance. For example, under conditions of low visual acuity (for instance, under low illumination or if the stimuli are in the periphery of the visual field), when shapes are poorly recognizable, a rapid analysis of the kinematics of the perceived movement might be instrumental in discriminating biological motion against the background of nonbiological motion, such as the shaking of a tree. Under natural conditions, this mechanism could be potentially important for survival.
In conclusion, our study demonstrates that regions in the left dorsofrontal and premotor cortex that are critical for action recognition, possibly by matching the observed movements with one's own motor programs, are differentially activated by human movements dependent on their compliance with kinematic invariants of human movements. The matching process seems thus to be critically dependent not only on the consistency between the body structure of the observer and actor but also on the consistency with human kinematic laws of motion. Interestingly, in agreement with this proposal, psychophysical data show that differences in spontaneous interference effects between observed human and robotic actions on concurrently executed movements (Kilner et al. 2003) disappeared when the kinematic law of motion of the robotic movements was matched to the human one (Oztop et al. 2005). This result, per se, does not exclude that, in addition to motor expertise, also visual expertise might contribute to our sensitivity to human kinematics (Jastorff et al. 2006). For example, recent psychophysical evidence shows that, under ecologically relevant conditions, visual familiarity plays a role in person identification from point-light displays (Jacobs et al. 2004), a task that relies on a fine analysis of kinematic cues. Indeed, given our extensive visual and motor exposure to the two-thirds power law, it is possible that the recruitment of either type of expertise might depend on the particular task that the subjects have to carry out (Hiris et al. 2005). A third possibility is that visual sensitivity to the two-thirds power law might reflect a specific tuning to fundamental geometric properties of the observed movements. Theoretical results show that the two-thirds power law is equivalent to moving at a constant equi-affine speed (Pollick and Sapiro 1997). Thus, the selective activations found in premotor and dorso-frontal areas during observation of bodily movements complying with the two-thirds power law might reflect visual selectivity for movements that have a simpler description using equi-affine rather than Euclidean metrics (Pollick and Sapiro 1997; Flash and Handzel 2007; Polyakov et al. 2009). Additional experiments are needed in order to fully explore these 3 nonmutually excluding possibilities.
Funding
Deutsche Forschungsgemeinschaft (SFB550-C10); Human Frontier Science Program (RGP0054/2004-C); European Commission (COBOL Grant 043403); the Volkswagenstiftung; and the Hermann and Lilly Schilling Foundation T.F. is an incumbent of the Dr. Hymie Moross professorial chair.
Conflict of Interest: None declared.
References
Author notes
Antonino Casile and Eran Dayan have contributed equally to this work.