-
PDF
- Split View
-
Views
-
Cite
Cite
Conor Liston, Richard Watts, Nim Tottenham, Matthew C. Davidson, Sumit Niogi, Aziz M. Ulug, B.J. Casey, Frontostriatal Microstructure Modulates Efficient Recruitment of Cognitive Control, Cerebral Cortex, Volume 16, Issue 4, April 2006, Pages 553–560, https://doi.org/10.1093/cercor/bhj003
Close - Share Icon Share
Abstract
Many studies have linked activity in a frontostriatal network with the capacity to suppress inappropriate thoughts and actions, but relatively few have examined the role of connectivity between these structures. Here, we use diffusion tensor imaging to assess frontostriatal connectivity in 21 subjects (ages 7–31 years). Fifteen subjects were tested on a go/no-go task, where they responded with a button press to a visual stimulus and inhibited a response to a second infrequent stimulus. An automated fiber tracking algorithm was used to delineate white matter fibers adjacent to ventral prefrontal cortex and the striatum, and the corticospinal tract, which was not expected to contribute to control per se. Diffusion in frontostriatal and corticospinal tracts became more restricted with age. This shift was paralleled by an increase in efficiency of task performance. Frontostriatal radial diffusivities predicted faster reaction times, independent of age and accuracy, and this correlation grew stronger for trials expected to require greater control. This was not observed in the corticospinal tract. On trials matched for speed of task performance, adults were significantly more accurate, and accuracies were correlated with frontostriatal, but not corticospinal, diffusivities. These findings suggest that frontostriatal connectivity may contribute to developmental and individual differences in the efficient recruitment of cognitive control.
Introduction
Cognitive control — the ability to suppress inappropriate or irrelevant sensory or motor representations and to strengthen others — is a fundamental component of normal cognition (Kahneman et al., 1983; Cohen and Servan-Schreiber, 1992). Cognitive control is mediated by a neural circuit linking prefrontal cortex and the striatum (Casey et al., 1997b, 2002b; Miller and Cohen, 2001; Koechlin et al., 2003). Deficits in cognitive control, which have been linked to disorders including attention-deficit/hyperactivity disorder (ADHD), schizophrenia, obsessive-compulsive disorder and anxiety disorders, are associated with abnormalities in these structures (Cohen and Servan-Schreiber, 1992; Casey et al., 2002b; Durston et al., 2003b; Bishop et al., 2004). For example, imaging studies have identified hypofrontality, abnormalities in structure and function of the basal ganglia, and delayed frontal maturation in ADHD (Castellanos et al., 1996; Casey et al., 1997a; Vaidya et al., 1998; Rubia et al., 1999, 2000; Durtson et al., 2003b).
Development of frontostriatal circuitry is protracted; synaptic pruning and myelination of prefrontal fibers as measured histologically (Conel, 1939–1963; Yakovlev and LeCours, 1967; Huttenlocher, 1979) and indexed by imaging methods (Sowell et al., 1999, 2003, 2004; Klingberg et al., 1999; Paus et al., 2001; Giedd, 2004; Gogtay et al., 2004) proceeds slowly throughout late childhood and adolescence. Concomitantly, children's capacity for cognitive control develops across the first decade with younger children more susceptible to interference on a variety of tasks in this domain (Casey et al., 1997b, 2002a; Munakata and Yerys, 2001; Brophy et al., 2002; Diamond, 2002; Hughes, 2002;Durston et al., 2003a). It is generally assumed that protracted prefrontal maturation contributes to enhanced performance, but prefrontal connectivity is difficult to assess in vivo. Links between task performance and development of this circuit have been examined using morphometric and fMRI techniques. Children recruit distinct and often larger, more diffuse frontostriatal regions when performing cognitive control tasks (Casey et al., 1997a,b, 2002a; Luna et al., 2001; Bunge et al., 2002). How development and refinement of projections to and from these regions may contribute to enhanced control remains an important question. In the present study, we used diffusion tensor imaging (DTI) to examine the development of frontostriatal connectivity and its contribution to performance of a cognitive control task.
DTI is a relatively new technique that can detect changes in white matter microstructure based on properties of diffusion (Pierpaoli et al., 1996; Le Bihan et al., 2003). Diffusion of water in white matter tracts is affected by myelin and the orientation and regularity of fibers. Water diffuses more readily in parallel to a tract than perpendicular to it, a property termed anisotropic diffusion. Anisotropic diffusion in three dimensions can be characterized in terms of a 3 × 3 symmetric tensor model. Magnetic resonance images can be sensitized to water diffusion to yield a solution to the diffusion tensor, from which variables describing the magnitude and anisotropy of diffusion can be derived (Pierpaoli et al., 1996). These variables can be used as a measure of myelination and white matter microstructure in vivo (Song et al., 2003), and to investigate prefrontal connectivity in normal maturation (Klingberg et al., 1999, 2000; Nagy et al., 2004) and pathological states (Lim et al., 1999). DTI-based fiber tracking algorithms can be applied to delineate white matter tracts automatically and reliably (Conturo et al., 1999; Mori et al., 1999; Basser et al., 2000).
Here we used DTI to assess how variations in connectivity within frontostriatal circuitry may contribute to developmental and individual differences in performance of a go/no-go task, where subjects responded with a button press to repeated presentations of a visual stimulus (‘go’ trials) but inhibited this response when presented with a second, distinctive and infrequent stimulus (‘no-go’ trials). Although accuracies on no-go trials are the conventional means of indexing cognitive control in this paradigm, most children in the present study performed at a ceiling level of accuracy but required much longer response latencies to attain this level of performance. As such, control was indexed by analyzing reaction times to different trial types while controlling for speed of processing. Certain trials were expected to require greater control because they were more frequently associated with multiple conflicting responses, in accord with theoretical accounts of cognitive control (Botvinick et al., 2001; Miller and Cohen, 2001). Subsets of ‘no-go’ trials from each group were also matched for preceding response times to assess whether frontostriatal maturation contributes to enhanced accuracies when subjects are responding quickly, as described in more detail below.
Materials and Methods
Subjects
Eleven right-handed adults (five males, six females; ages 18–31 years, mean ± SD = 24 ± 4.1 years) and ten right-handed children (six males, four females; ages 7–14 years, mean 9 ± 2.7 years) were scanned. Fifteen of these subjects (nine children, ages 7–15 years; six adults, ages 18–31 years) were tested on a go/no-go task, described below. All subjects were screened for contraindications for MRI, and written informed consent was obtained from subjects before scanning.
Behavioral Paradigm
Prior to scanning, subjects were tested on a go/no-go task where they responded with a button press to repeated presentations of a visual stimulus (‘go’ trials) but inhibited this response when presented with a second, distinctive and infrequent stimulus (‘no-go’ trials). Visual stimuli were distinctive images of Pokemon cartoon characters as described in Durston et al. (2002b). The stimulus duration was 500 ms, with an interstimulus interval of 3500 ms. Each subject performed 114 trials, 28 of which were no-go trials, when the subject was instructed to refrain from responding. Twenty-four no-go trials were preceded by one, three or five go trials, arranged in a pseudorandomized sequence. To minimize strategic learning of trial sequences, the remaining four no-go trials served as foils and were preceded by two or four go trials. Reaction times and accuracies were recorded automatically using E-Prime version 1.0 (Psychology Software Tools).
Image Acquisition
Subjects were first acclimated to the MRI environment in a simulator. Next, T2-weighted echo planar images (30 slices, 5 mm, 0 skip, TR = 2000 ms, TE = 40 ms, flip angle = 90°, FOV = 22) and 32 diffusion scans obtained using a single-shot, multi-slice echo-planar diffusion tensor pulse sequence (each 30 slices, 5 mm, TR = 5000 ms, TE = min, FOV = 22, δ = 25.62ms, Δ = 44.97ms, b = 820 s/mm2) were acquired for all 21 subjects on a GE 1.5T scanner.
DTI Analysis
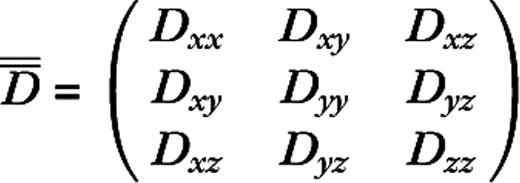
Diagonalization of the tensor yields three voxel-specific eigenvalues or diffusivities (λ1 > λ2 > λ3), which can be manipulated to yield several theoretically useful variables, three of which are applied here. The apparent diffusion coefficient (ADC) is defined as the arithmetic mean of the three eigenvalues and approximates the magnitude of water diffusion in a given voxel. Radial diffusivity (λ⊥), defined as the arithmetic mean of the two smaller eigenvalues, describes motion perpendicular to the direction of greatest diffusion. In mouse models of retinal ischemia, λ⊥ has been shown to be maximally sensitive to changes in myelination (Song et al., 2003). Lastly, relative anisotropy (RA) approximates the degree to which water diffuses preferentially in one direction and is defined as the standard deviation of the three eigenvalues normalized by ADC (Pierpaolis et al., 1996). RA, which is generally higher in white matter than in gray matter, was used here in an automated fiber tracking algorithm described below.
Fiber Tracking
Automated fiber tractography has been used by several groups to delineate anatomic white matter tracts (e.g. Conturo et al., 1999; Pierpaoli et al., 2001; Lee et al., 2003, 2004; Lehéricy et al., 2004a,b). Here, a fiber tracking algorithm similar to those developed elsewhere (Conturo et al., 1999; Mori et al., 1999; Basser et al., 2000) and reviewed in Watts et al. (2003) was used to delineate frontostriatal white matter fibers and the corticospinal tract. Starting from a manually selected set of seed points, the algorithm extends a tract in the direction of greatest diffusion (λ1) in the seed voxel until it encounters a neighboring voxel. Here, the direction of the tract changes abruptly to match the direction of greatest diffusion (λ1) in the second voxel. This process is reiterated until the algorithm encounters a voxel where the RA is below an empirically derived threshold (RA > 0.155), and the tract is terminated.
Functional MRI studies have shown that performance of the go/no-go task engages a network of frontostriatal structures including ventral prefrontal cortex and the caudate nucleus (Casey et al., 1997b; Durston et al., 2002a). As such, the present study focused on projections from prefrontal cortex to the striatum and used three sets of seed points (Fig. 1) representing left and right lateral prefrontal and ventromedial prefrontal white matter, adjacent to regions of activation in Brodman's areas 44 and 47 observed in previous fMRI studies (Casey et al., 1997b; Durston et al., 2002a,b). Each lateral prefrontal seed spanned approximately 300 voxels or 4.3 cm3 of tissue, and the medial prefrontal set, representing both left and right medial prefrontal tissue, spanned ∼600 voxels, or 8.7 cm3 of tissue. Delineation of the corticospinal tract tract used two sets of seed points, each ∼80 voxels, or 1.2 cm3, representing a segment of the posterior limb of the internal capsule. These fiber tracts defined regions of interest (ROIs) for further analysis. Voxels with radial diffusivities in the highest 10% of each ROI were excluded to avoid analysis of adjacent grey matter.
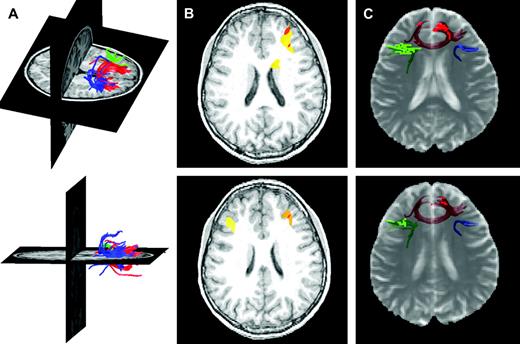
Frontostriatal fiber tracking results. (A) Three-dimensional rendering of frontostriatal fiber tracts superimposed on T1-weighted anatomical images for a typical subject. Blue, green and red tracts are derived from seed points in the right lateral, left lateral and medial prefrontal cortex, respectively. (B) Functional MRI activations from a separate study employing the go/no-go task used here superimposed on representative T1-weighted axial slices for a typical subject. These activations motivated seed point selection and are adjacent to the tracts represented in (C). (C) Frontostriatal fiber tracts cross-registered with semitransparent T2-weighted echo planar images for the same subject. Voxels through which the fiber tracts pass are highlighted in each slice, emphasizing the proximity to the cortical regions defined in (B).
Selection of an appropriate anisotropy threshold is critical for generating reliable fiber tracts. In the present study, large regions of prefrontal white matter and regions of gray matter representing prefrontal cortex, caudate, and putamen, were manually sampled from T2-weighted echo planar images. The RA > 0.155 threshold generally included over 90% of white matter voxels and excluded ∼85% of gray matter voxels.
Manual Region of Interest Analysis
For comparison with the fiber tracking results, regions of interest representing prefrontal white matter anterior to the head of the caudate nucleus in five consecutive axial slices, adjacent to the seed points described above, were manually defined on T2-weighted echo planar images. These ROIs spanned an average of 920 voxels (SD = 156), or 13.3 cm3 of tissue. Voxels with signal intensities in the highest 10% of each ROI were excluded to avoid analysis of adjacent grey matter.
Results
DTI Results
Figure 1 depicts the prefrontal fiber tracts delineated by the tracking algorithm with fMRI data from this task for comparison. For each subject, a region of interest was defined by the tracking algorithm. Fiber tracking ROI volumes were not significantly different in adults compared to children (t = −0.754, P = 0.470). Nor were they correlated with age (r = −0.212, P = 0.369), average radial diffusivity (r = −0.090, P = 0.706) or average relative anisotropy (r = 0.347, P = 0.134).
The values of variables derived from the diffusion tensor were averaged across all voxels through which the tracts passed. Diffusion of water perpendicular to axons (λ⊥) was significantly lower in adults than in children (t = 3.329, P < 0.004) and was negatively correlated with age (r = −0.639, P < 0.002).
Fiber tracking results did not differ significantly from the diffusion measures yielded by the manually defined ROIs. Average diffusivities (ADC) and relative anisotropies (RA) did not differ significantly (t = 0.222, P = 0.83; t = 1.054, P = 0.30), and measures of ADC and RA yielded by the two methods were highly correlated (r = 0.968, P < 0.001; r = 0.646, P < 0.002).
Behavioral Results
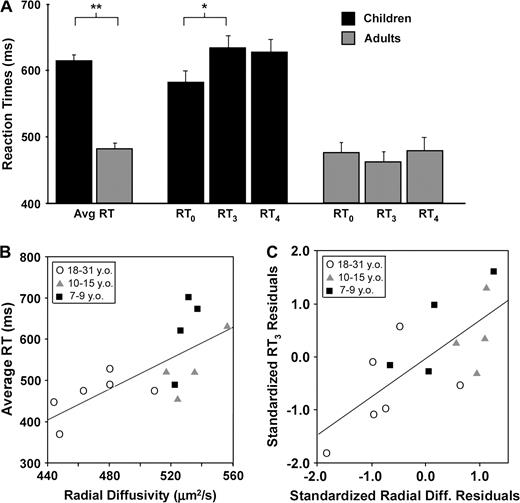
Mean accuracies on no-go trials were not significantly different between age groups (t = 0.07, P = 0.947), but children performed the task more slowly. Children's reaction times averaged across go trials were 32% slower than those of adults (t = 20.1, P < 0.001), and age was correlated with faster reaction times (r = −0.63, P < 0.012). Children's reaction times to go trials were modulated by preceding context (Fig. 2A). Initial go trials (RT0) (i.e. those preceded immediately by a no-go trial) were significantly faster than trials preceded by three consecutive go trials (RT3; t = 3.80, P < 0.011). Reaction times to trials preceded by one, two, or four go trials (RT1, RT2, RT4) were also significantly slower than RT0 (t = 3.21 − 3.76, P < 0.05) but did not differ significantly from one another. In contrast, adults performed all go trials equally quickly. This effect dominated group differences in reaction times: mean RT3 was 30% slower in children than adults (t = 4.64, P < 0.001), but this trend did not reach significance for RT0 (t = 2.09, P = 0.06), and the difference between these trial types (RT3–0 = RT3 − RT0) was significantly larger in children than adults (t = 2.40, P < 0.034). Thus, while children and adults responded to initial go trials equally quickly, children were significantly slower to respond to subsequent go trials.
Restricted frontostriatal radial diffusivity is associated with faster reaction times, and this effect is modulated by trial type. (A) Children were significantly slower than adults to respond to go trials (t = 20.1, P < 0.001). Children were also significantly slower to respond to go trials preceded by three or four consecutive go trials (RT3) than intial go trials (RT0: t = 3.80, P < 0.011), while adults responded equally quickly to all trial types. **P < 0.01; *P < 0.05. (B) Restricted frontostriatal diffusion was correlated with faster average reaction times across all subjects (n = 14, r = 0.71, P < 0.005). This correlation was stronger for right (r = 0.80) than left frontostriatal fibers (r = 0.59), in accord with fMRI studies implicating right frontostriatal circuitry in response inhibition. (C) Demand for cognitive control should be highest on trials preceded by three consecutive go trials, since this trial type was least predictive of the correct response. Here, frontostriatal radial diffusivity predicted RT3 independently of RT0. Standardized residuals after regressing RT3 on RT0 were plotted against standardized residuals after regressing radial diffusivity on RT0 yielding a visual representation of the partial correlation of radial diffusivity and RT3 while controlling for RT0 (r = 0.73, P < 0.005).
Group differences in accuracy were also assessed across a subset of no-go trials matched for speed. Each no-go trial was classified according to the mean RT across the three go trials preceding it. A subset of ∼115 no-go trials was identified for each group such that the mean RT across preceding go trials was equivalent in both groups (mean RT = 490 ms, range 440–600 ms). (This subset did not include any trials from either the fastest adult or the slowest child, so analysis of speed-matched accuracies included 13 subjects.) When matched for speed of task performance, adults were 13% more accurate than children in withholding a response to the nontarget (no-go) trial (t = 2.370, P < 0.037).
Correlations between Diffusion Measures and Behavior
To investigate the relation between frontostriatal connectivity and enhanced performance, average radial diffusivities for individual subjects were correlated with measures of reaction time and accuracy. For all correlations, outliers were defined as data points that differed from the group mean by at least two standard deviations on either variable. No more than one outlier was excluded from any correlation, as noted below.
Decreased radial diffusivities averaged across frontostriatal fibers were associated with faster reaction times across all ages (Fig. 2B: n = 14, r = 0.71, P < 0.005). This correlation was stronger in fibers projecting from right ventral prefrontal cortex (r = 0.80, P < 0.001) than for those projecting from left ventral prefrontal cortex (r = 0.59, P < 0.028) or ventromedial prefrontal cortex (r = 0.69, P < 0.007). Restricted radial diffusivities also predicted faster reaction times independent of age and accuracy, as the correlation remained significant with these variables partialed out (r = 0.60, P < 0.041). Relative anisotropy, which is often used as a measure of myelination (Klingberg et al., 1999, 2000; Lim et al., 1999) and was used here for fiber tracking, showed a similar trend (n = 14, r = −0.522, P < 0.055).
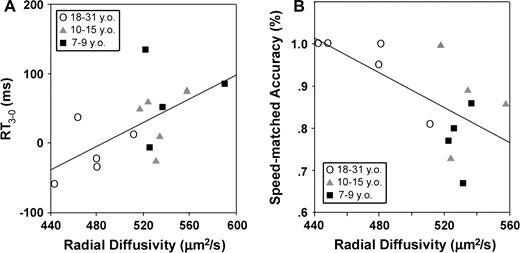
This relation was modulated by trial type, with λ⊥ more tightly correlated with RT3 (n = 14, r = 0.87, P < 0.001) than RT0 (n = 14, r = 0.71, P < 0.004). Comparison of the Fisher Z-transformed correlation coefficients (see Meng et al., 1992) confirmed that they were significantly different (Z = 1.67, P < 0.047), indicating that λ⊥ was a stronger predictor of RT3 than of RT0. The partial correlation between λ⊥ and RT3 remained significant after controlling for a correlation with RT0 (Fig. 2C: r = 0.73, P < 0.005) but not vice versa (r = −0.25, P = 0.414). λ⊥ was also correlated with RT3–0 and with speed-matched accuracies, with restricted diffusion predicting smaller RT adjustments to later go trials (Fig. 3A: n = 14, r = 0.61, P < 0.021) and higher average accuracies when matched for speed of task performance (Fig. 3B: n = 13, r = −0.65, P < 0.016).
Restricted frontostriatal diffusion predicted (A) smaller adjustments to later go trials (RT3–0 = RT3 − RT0; n = 14, r = 0.61, P < 0.021) and (B) higher accuracies to no-go trials when matched for speed (n = 13, r = −0.65, P < 0.016).
An analysis of children only (n = 9) revealed similar trends. Restricted radial diffusivities predicted faster average reaction times (r = 0.84, P < 0.004) independent of age and accuracy (r = 0.86, P < 0.01), and λ⊥ predicted RT3 (r = 0.91, P < 0.001) significantly more strongly than RT0 (r = 0.80, P < 0.01), though the latter effect may be exaggerated by an outlier. However, after excluding this outlier (n = 8), λ⊥ still predicted RT3 (but not RT0) at a level approaching significance (r = 0.64, P = 0.086), and this trend was independent of correlations with age and RT0 (r = 0.78, P = 0.069).
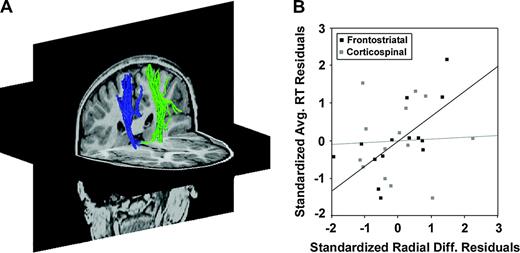
Finally, these correlations were specific to the frontostriatal circuit. For comparison, a segment of the corticospinal tract, which was not expected to have any direct relation to behavioral inhibition per se, was delineated. This tract extended from the posterior limb of the internal capsule superiorly into the centrum semiovale (Fig. 4A). Corticospinal λ⊥ was 8% lower in adults than in children (t = 2.49, P < 0.026). A weaker trend toward faster reaction times with decreasing λ⊥ was observed (n = 14, r = 0.59, P < 0.027), but this trend was attributable to a strong correlation with radial diffusivities in the frontostriatal loops (r = 0.72, P < 0.004). Controlling for this association revealed no unique relation between reaction times and corticospinal λ⊥ (Fig. 4B: r = 0.05, P = 0.878). Conversely, a partial correlation between average reaction time and prefrontal λ⊥ remained significant after controlling for associations with corticospinal λ⊥ (Fig. 4B: r = 0.66, P < 0.014). The relation between corticospinal λ⊥ and reaction time was not modulated by trial type, with weak and equivalent correlations found for RT0 and RT3 (Z = 1.04, P = 0.159), and corticospinal λ⊥ correlated with neither RT3–0 (n = 14, r = 0.38, P = 0.180) nor speed-matched accuracies (n = 13, r = −0.23, P = 0.452).
Reaction times are not correlated with radial diffusivities in the corticospinal tract after accounting for association with frontostriatal radial diffusivities. (A) Left (green) and right (blue) corticospinal fiber tracts superimposed on axial and coronal T1-weighted anatomical images for a typical subject. (B) Frontostriatal radial diffusivity (λ⊥) predicted average reaction times independent of corticospinal radial diffusivity, but not vice versa. In black, standardized residuals after regressing average reaction time on corticospinal λ⊥ were plotted against standardized residuals after regressing frontostriatal λ⊥ on corticospinal λ⊥, yielding a visual representation of the partial correlation of frontostriatal λ⊥ and average RT after controlling for corticospinal λ⊥ (r = 0.66, P < 0.014). In contrast, corticospinal λ⊥ did not predict average RT after controlling for associations with frontostriatal λ⊥ (grey data points: r = 0.05, P = 0.878) and was not associated with RT adjustments or speed matched accuracies (not shown).
Discussion
Radial diffusivities along frontostriatal fibers were more restricted in adults than in children. Shifts in diffusivity were paralleled by developmental changes in performance on the go/no-go task, and individual differences in frontostriatal, but not corticospinal, diffusivities predicted individual differences in performance independent of age. Collectively, these results provide evidence to indicate that maturation of frontostriatal connectivity, as indexed by DTI measures, contributes to a developing capacity for cognitive control.
Age-associated decreases in radial diffusivities probably reflect an ongoing process of myelination. Although assessments of radial diffusivity do not measure myelin directly, previous work suggests that λ⊥ is sensitive to changes in myelination. In a mouse model of retinal ischemia, in which retinal neurons undergo first axonal degeneration and then demyelination, increases in λ⊥ were associated with histologically verified demyelination but not with the axonal degeneration that precedes it (Song et al., 2003). Relative anisotropy and fractional anisotropy have been used more extensively as a measure of white matter microstructure (Klingberg et al., 1999, 2000; Lim et al., 1999; Nagy et al., 2004). Here we focused on radial diffusivity, a slightly different measure, to minimize confounds associated with selection of voxels by fiber tractography, which relied on RA values. Regardless, higher relative anisotropies were also associated with faster reaction times. Combined with previous studies indicating that prefrontal white matter matures slowly during childhood and adolescence (Yakovlev and LeCours, 1967; Klingberg et al., 1999; Sowell et al., 1999; Paus et al., 2001), it is likely that the increasingly restricted diffusion observed here reflects, at least in part, ongoing myelination of frontostriatal tracts.
This maturation of frontostriatal tracts was associated with enhanced task performance. Reaction times averaged across trials were significantly faster in adults than children (t = 20.1, P < 0.001), and restricted diffusion was associated with faster RTs (r = 0.71, P < 0.005). Other groups have demonstrated correlations between restricted diffusion and speed of processing (e.g. Madden et al., 2004). However, several factors indicate that our result reflects more than a general enhancement of speed of processing, which would predict equivalent enhancements of RTs across all trial types. Instead, group differences in RTs and correlations with radial diffusivity were significantly modulated by trial type. Theoretical accounts of cognitive control posit a greater need for control when a stimulus is associated with multiple conflicting responses (Botvinick et al., 2001; Miller and Cohen, 2001). In the go/no-go paradigm, the need for control on initial go trials was minimal because a no-go trial was never followed consecutively by a second no-go trial: the correct response to any trial following a no-go trial was always a button press. Conversely, on trials preceded by three consecutive go trials, the need for control should be maximal because this trial type was least predictive of the correct response: 51% of trials preceded by three consecutive go trials were no-go trials. All other trial types were associated with one response (go or no-go) on at least 70% of trials. Accordingly, children were significantly slower to respond to trials preceded by three go trials than to initial go trials (t = 3.80, P < 0.011) and showed a larger slowing effect than adults (t = 2.40, P < 0.034). Moreover, frontostriatal radial diffusivity was a significantly stronger predictor of RT3 than RT0 and predicted RT3 independent of predicting RT0, which is not consistent with a simple speed-of-processing interpretation. Instead, these results suggest that maturation of frontostriatal white matter contributes to a more facile recruitment of cognitive control resources above and beyond its contribution to faster processing overall.
Analysis of reaction time differences and speed-matched accuracies lend further support to this conclusion. Restricted frontostriatal diffusivities were associated not just with faster reaction times but also with smaller RT costs in responding to later go trials relative to initial go trials (r = 0.61, P < 0.021). Notably, group differences in accuracies to no-go trials were not observed. Although accuracies to no-go trials are the conventional means of indexing control in this paradigm, it should be noted that reaction times are a common measure of control in related paradigms, including the classic Stroop task and a variety of interference suppression paradigms, among others (e.g. Kerns et al., 2004; Fan et al., 2003). In the present study, most children performed at a ceiling level of accuracy but required much longer response latencies to attain this level of performance. To assess whether frontostriatal maturation contributes not only to greater efficiency but also to higher accuracies when subjects are responding quickly, subsets of no-go trials from each group were matched for preceding response times. On these trials, adults performed significantly more accurately (t = 2.37, P < 0.037), and restricted diffusion was associated with higher accuracy (r = −0.65, P < 0.016). Together, these findings suggest that when control needs to be implemented, it is implemented more reliably and with greater efficiency as frontostriatal white matter matures.
It should be noted that developmental differences in vigilance, an attentional process that complements cognitive control and depends on frontostriatal circuitry, may also contribute to the effects observed here. This hypothesis cannot be ruled out, but it cannot fully account for the pattern of results either. Impaired vigilance should cause steady increases in reaction times across blocks of go trials in children more than adults; however, this was not observed: RT1 was no faster than RT3 (t = 0.08, P = 0.937) or RT4 (t = 0.49, P = 0.627). Instead, RT0 was significantly faster than all other trial types, but no other trial types differed significantly from each other. Interestingly, mean RTs tended to vary with the predictive value of the trial type, such that RT3 and RT1, with predictive values of 51 and 71%, respectively, were ∼15 ms slower than RT2 and RT4, with predictive values of 90 and 80%, respectively, though these trends did not reach significance. This suggests that vigilance may contribute to the modulation of reaction time by trial type but seems inadequate to account fully for the distribution of reaction times, which appear sensitive to task demands for greater or lesser control.
Importantly, associations between restricted diffusion and enhanced control were not evident in the corticospinal tract, which supports a specific relation between frontostriatal maturation and behavioral performance on this task. Restricted corticospinal diffusion predicted faster average reaction times, but this is probably attributable in part to correlations between frontostriatal and corticospinal diffusivities. Frontostriatal λ⊥ predicted faster reaction times independently of corticospinal λ⊥ but not vice versa, and the relation between corticospinal λ⊥ and RT was not modulated by trial type, which is more consistent with a general speed-of-processing effect than a specific contribution to cognitive control. Nor was corticospinal λ⊥ related to speed-matched accuracies or to RT costs in responding to later go trials. These results are consistent with functional imaging findings and theoretical accounts that assign to prefrontal and striatal structures the role of selecting an appropriate motor response from a set of conflicting responses while the corticospinal tract conducts signals that execute this response (e.g. Miller and Cohen, 2001; Casey et al., 2002a). This interpretation is bolstered by differences in laterality. The relation between λ⊥ and RT was stronger in fibers projecting from right ventral prefrontal cortex (r = 0.8) than in those projecting from the left ventral prefrontal cortex (r = 059), which is in accord with previous work demonstrating that behavioral inhibition engages predominantly right frontostriatal circuitry (Casey et al., 1997b; Durston et al., 2002a).
Although automated fiber tractography has been used elsewhere for delineation of anatomic white matter tracts (Conturo et al., 1999; Pierpaoli et al., 2001; Lee et al., 2003, 2004; Lehéricy et al., 2004b), use of this methodology for selecting a region of interest does have its limitations. A selection bias may arise if path geometries are significantly affected by group differences in diffusion properties. However, an analysis of fiber tract ROI volumes revealed no group differences in this study. Nor were tract volumes correlated with radial diffusivities or age, and measures of diffusion yielded by manually defined ROIs were highly correlated with fiber tract values. These findings suggest that a potential selection bias did not significantly confound the results presented here. Another limitation of diffusion tensor-based fiber tracking is that it cannot adequately represent voxels containing fibers with a range of orientations. In these voxels, the tracking algorithm predicts an average of the crossed fibers, and shifts in radial diffusivity may not parallel shifts in myelination as closely. Thus, increased axonal regularity may also contribute to decreased radial diffusivity in some voxels. In either case, these findings indicate that refined connectivity is contributing to improvements in cognitive control. Future work will seek to combine this methodology with fMRI and electrophysiological measures to assess more directly how refined connectivity may contribute to the mature, relatively focal patterns of activation and enhanced cognitive control observed in adults relative to children. These methods might also be useful for assessing children with mild to moderate traumatic brain injury, in whom white matter shearing is common but standard clinical MRI sequences are usually non-contributory (Alsop et al., 1996; Huisman et al., 2004), or elderly patients with deficits in frontal lobe function, which may be associated with age-related white matter blebbing (Pfefferbaum et al., 2000).
Finally, it is interesting to note that radial diffusivities along frontostriatal fibers predicted individual differences in reaction times independent of age with a relatively modest sample size. Indeed, reaction times were more strongly correlated with right frontostriatal λ⊥ (r = 0.80) than with age (r = −0.63). This study was not designed to examine individual differences within groups, and correlations of eight or nine data points are difficult to interpret. Nonetheless, a post-hoc analysis of nine children demonstrated comparable trends that approached significance, with λ⊥ correlated with RT3 independent of age and RT0 (r = 0.78, P = 0.069). Likewise, Figures 2 and 3 reveal considerable overlap in the data of children of different ages and considerable variability in the diffusivities of fully mature adults. Collectively, these findings suggest not only that enhanced connectivity in frontostriatal fiber tracts contributes to children's developing capacity for cognitive control, but that variability in the myelination and regularity of frontostriatal fibers may contribute to individual differences in subjects matched for age as well. This result adds to a growing body of evidence (Lim et al., 1999; Fredericksen et al., 2002; Ardekani et al., 2003) suggesting that genetic studies of disorders like ADHD and schizophrenia, which traditionally have focused on genes regulating dopaminergic neurotransmission, should also examine the regulation of myelination and axon migration in the prefrontal cortex.
The authors wish to thank the constructive comments of two anonymous reviewers. This work was supported in part by in part by R21 DA15882, R01 MH63255, and P01 MH62196 (Project IV) to B.J.C. C.L. is supported by NIH Medical Scientist Training Program grant GM 07739, a W.M. Keck Foundation Medical Scientist Fellowship, and a Paul and Daisy Sores Fellowship.
References
Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO (
Alsop DC, Murai H, Detre JA, McIntosh TK, Smith DH (
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (
Bishop S, Duncan J, Brett M, Lawrence AD (
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (
Brophy M, Taylor E, Hughes C (
Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD (
Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL (
Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL (
Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL (
Casey BJ, Tottenham N, Fossella J (
Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Vaituzis AC, Dickstein DP, Sarfatti SE, Vauss YC, Snell JW, Lange N, Kaysen D, Krain AL, Ritchie GF, Rajapakse JC, Rapoport JL (
Cohen JD, Servan-Schreiber D (
Conel JL (
Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME (
Diamond A (
Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ (
Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ (
Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, Watts R, Ulug AM, Casey BJ (
Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ (
Fan J, Fossella J, Sommer T, Wu Y, Posner MI (
Fredericksen KA, Cutting LE, Kates WR, Mostofsky SH, Singer HS, Cooper KL, Lanham DC, Denckla MB, Kaufmann WE (
Giedd JN (
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson, PM (
Huisman TAGM, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wu O, Sorensen AG (
Huttenlocher PR (
Kahneman D, Treisman A, Burkell J (
Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (
Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M (
Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA (
Koechlin E, Ody C, Kouneiher F (
Le Bihan D (
Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Chu M, Heo K, Lee BI, Kim DI (
Lee SK, Mori S, Kim DJ, Kim SY, Kim SY, Kim DI (
Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (
Lehéricy S, Ducros M, Krainik A, Francois C, Van de Moortele PF, Ugurbil K, Kim DS (
Lim KO, Hedehus M, Moseley M, deCrespigny A, Sullivan EV, Pfefferbaum A (
Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (
Madden DJ. Whiting WL. Huettel SA. White LE. MacFall JR. Provenzale JM (
Meng XL, Rosenthal R, Rubin DB (
Miller EK, Cohen JD (
Mori S, Crain BJ, Chacko VP, van Zijl PCM (
Munakata Y, Yerys BE (
Nagy Z, Westerberg H, Klingberg T (
Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A (
Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M (
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, DiChiro G (
Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (
Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET (
Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Andrew C, Bullmore ET (
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (
Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (
Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW (
Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD (
Watts R, Liston C, Niogi S, Uluğ AM. (