-
PDF
- Split View
-
Views
-
Cite
Cite
Geoffrey Schoenbaum, Barry Setlow, Cocaine Makes Actions Insensitive to Outcomes but not Extinction: Implications for Altered Orbitofrontal–Amygdalar Function, Cerebral Cortex, Volume 15, Issue 8, August 2005, Pages 1162–1169, https://doi.org/10.1093/cercor/bhh216
Close - Share Icon Share
Abstract
Addiction is characterized by persistent drug-seeking despite adverse consequences or outcomes. Such persistent behavior may result from drug-induced brain changes that increase the control of behavior by associations between antecedent cues and responses. However, it is equally plausible that brain changes cause a decrease in the control of behavior by the value of likely outcomes. To test whether drug exposure can cause persistent behavior, and to distinguish between these two accounts of such behavior, we tested cocaine-experienced rats in a Pavlovian ‘reinforcer devaluation’ task, which provides independent assessments of the control of behavior by antecedent cues and outcome representations. We found that cocaine exposure caused persistent responding in this setting a month after the last drug treatment, and that this deficit resulted from an inability to use representations of outcome value to guide behavior rather than from changes in stimulus–response learning or response inhibition.
Introduction
Drug addiction is characterized by persistent drug-seeking in the face of adverse consequences or outcomes. Such maladaptive, ‘habit-like’ behavior is thought to reflect drug-induced modifications in learning circuits (Everitt and Wolf, 2002). However, it is not clear, given the widespread effects that addictive drugs have on brain structure and function, which changes are the critical ones. One possibility is that this behavior is the result of drug-induced changes in brain circuits involving the orbitofrontal cortex (OFC), damage to which causes a decrease in the control of behavior by the value of likely outcomes (Hatfield et al., 1996; Bechara et al., 1997; Gallagher et al., 1999; Baxter et al., 2000; Parkinson et al., 2001; Pears et al., 2003; Pickens et al., 2003). Long-term cocaine use in humans is associated with structural and functional abnormalities in OFC (Breiter et al., 1997; London et al., 2000; Volkow and Fowler, 2000), and some drug users exhibit cognitive deficits similar to those observed in patients with OFC damage (Bechara et al., 2001). Although the direction of causality in studies of drug addicts is unclear (i.e. pre-existing OFC dysfunction may increase the likelihood of addiction), drug use may promote the maladaptive behavior characteristic of addiction in part by impairing the ability to represent and use likely outcomes to guide behavior. For example, it has recently been reported that oral cocaine seeking in rats is not affected when the sucrose solution containing the cocaine is made aversive by pairing with illness (Miles et al., 2003). Another possibility is that such behavior reflects changes in circuits that mediate learning in other associative domains. For example, drug exposure may affect control of stimulus–response learning mediated in part by neural circuits including the striatum (Berke and Hyman, 2000; Packard and Knowlton, 2002). By this proposal, the maladaptive behavior that characterizes addiction results from increases in the strength of associations between antecedent cues and responses.
To test whether drug exposure causes a long-lasting and general increase in maladaptive behavior and to distinguish the associative basis of any such effect, we gave rats daily injections of cocaine or saline for 2 weeks using a dose regimen similar to that employed to demonstrate molecular and structural changes in corticolimbic circuits (Pierce et al., 1995, 1996; Toda et al., 2002; Trantham et al., 2002; Bowers and Kalivas, 2003; Martin et al., 2004) and then, after a 3 week withdrawal period, tested them in a Pavlovian ‘reinforcer devaluation’ task (Holland and Rescorla, 1975; Pickens et al., 2003). Food-deprived rats were trained to associate a light cue with a rewarding food outcome. The value of the food outcome was then reduced or ‘devalued’ by pairing it with illness in half the rats. Subsequently, all rats were presented with the cue again in a 16-trial extinction session. We compared conditioned responding in ‘devalued’ and ‘non-devalued’ rats to determine whether cocaine treatment altered the spontaneous decrease in responding to the cue normally caused by reinforcer devaluation. This decrease reflects the ability to learn about and use representations of the value of likely outcomes to guide responding (Holland and Rescorla, 1975). We also examined the change in conditioned responding from the beginning to the end of the extinction session in each group. Unlike the comparison between ‘devalued’ and ‘non-devalued’ rats, this measure reflects new learning during the session and provides an assessment of the strength or persistence of associations between antecedent cues and responses (Rescorla, 1993, 1996, 1997; Delamater, 1996). We found that cocaine-exposed rats were unable to modify conditioned responding as a result of devaluation but showed normal extinction learning. This deficit was observed over a month after the last cocaine treatment, suggesting that cocaine exposure can cause maladaptive behavior due to a long-lasting and selective impairment in the ability to encode and/or use information about outcome value to guide behavior.
Materials and Methods
Animal testing was conducted at Johns Hopkins University and conformed to university and NIH guidelines; data were analyzed at the University of Maryland School of Medicine and at Texas A&M University.
Subjects
Forty male Long–Evans rats (300–350 g), obtained from Charles River Laboratories (Wilmington, MA) served as subjects. Rats were housed individually on a 12 h light/12 h dark cycle (lights on at 8 a.m.) with ad libitum access to food and water except during behavioral testing where noted below. During such testing, the rats were food deprived to 85% of their free-feeding weight. All testing was performed during the light phase of the cycle. Of the 40 rats that began the study, one rat in the saline-treated group and two rats in the cocaine-treated group failed to consume the food pellets during the taste aversion training. In addition, one rat in the cocaine-treated group failed to learn the Pavlovian conditioning task. These rats were excluded from the study. Data from the remaining 36 rats are presented below, divided into saline-treated (9 paired, 10 unpaired) and cocaine-treated (8 paired, 9 unpaired).
Apparatus
All testing took place in a set of eight individual training chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls, clear acrylic sides and top, and a floor made of 0.48 cm stainless steel rods spaced 1.9 cm apart. A dimly illuminated food cup was recessed in the center of one end wall. An infrared photocell placed just inside the food cup was polled (1 kHz) by computer circuitry. Each chamber was enclosed in a sound-resistant shell. A 6 W house light, which was used as the conditioned stimulus (CS), was mounted on the inside wall of the shell, 10 cm above the experimental chamber and even with the end wall opposite the food cup. Ventilation fans provided masking noise (70 dB). Constant dim illumination was provided by a 6 W lamp behind a dense red lens mounted on the ceiling of the shell. A TV camera was mounted within each shell to provide a view of the chamber; the output from each camera was digitized, merged into one of two composite images of four of the chambers, displayed on a monitor and recorded on videotape. Behavioral data from the videotapes are not reported here.
Cocaine Sensitization
Table 1 shows an outline of the experimental procedures. Before any behavioral training, rats were sensitized to cocaine. The day before the start of the sensitization regimen, the rats were placed into the training chambers for 1 h. Based on activity during this 1 h session, the rats were divided into two groups with similar activity levels. Over the next 14 days, one group (n = 20) received daily injections of cocaine HCl (30 mg/kg); the other group (n = 20) received similar volume injections of a 0.9% saline solution. This dose is similar to that employed by other labs to study molecular and structural brain changes associated with psychomotor sensitization (Pierce et al., 1995, 1996; Toda et al., 2002; Trantham et al., 2002; Bowers and Kalivas, 2003; Martin et al., 2004). After each injection, the rats were immediately placed into the training chambers, and activity was again monitored for 1 h, using overhead activity monitors (Coulbourn Instruments) mounted in the ceilings of the chambers. At the end of the 14 day treatment regimen, the rats were left in their home cages for an additional 21 days before behavioral training was begun.
Outline of experimental procedures
| Group . | Cocaine sensitization . | Conditioning . | CTAx2 . | Devaluation probe . | CTA tests . | Sensitization test . |
|---|---|---|---|---|---|---|
| Saline/devalued | Saline | Light → food | Food → LiCl | Light | Food | Cocaine |
| Saline/non-devalued | Saline | Light → Food | Food, LiCl | Light | Food | Cocaine |
| Cocaine/devalued | Cocaine | Light → food | Food → LiCl | Light | Food | Cocaine |
| Cocaine/non-devalued | Cocaine | Light → food | Food, LiCl | Light | Food | Cocaine |
| Group . | Cocaine sensitization . | Conditioning . | CTAx2 . | Devaluation probe . | CTA tests . | Sensitization test . |
|---|---|---|---|---|---|---|
| Saline/devalued | Saline | Light → food | Food → LiCl | Light | Food | Cocaine |
| Saline/non-devalued | Saline | Light → Food | Food, LiCl | Light | Food | Cocaine |
| Cocaine/devalued | Cocaine | Light → food | Food → LiCl | Light | Food | Cocaine |
| Cocaine/non-devalued | Cocaine | Light → food | Food, LiCl | Light | Food | Cocaine |
CTA, conditioned taste aversion; LiCl, lithium chloride; →, paired presentations; , unpaired presentations.
Outline of experimental procedures
| Group . | Cocaine sensitization . | Conditioning . | CTAx2 . | Devaluation probe . | CTA tests . | Sensitization test . |
|---|---|---|---|---|---|---|
| Saline/devalued | Saline | Light → food | Food → LiCl | Light | Food | Cocaine |
| Saline/non-devalued | Saline | Light → Food | Food, LiCl | Light | Food | Cocaine |
| Cocaine/devalued | Cocaine | Light → food | Food → LiCl | Light | Food | Cocaine |
| Cocaine/non-devalued | Cocaine | Light → food | Food, LiCl | Light | Food | Cocaine |
| Group . | Cocaine sensitization . | Conditioning . | CTAx2 . | Devaluation probe . | CTA tests . | Sensitization test . |
|---|---|---|---|---|---|---|
| Saline/devalued | Saline | Light → food | Food → LiCl | Light | Food | Cocaine |
| Saline/non-devalued | Saline | Light → Food | Food, LiCl | Light | Food | Cocaine |
| Cocaine/devalued | Cocaine | Light → food | Food → LiCl | Light | Food | Cocaine |
| Cocaine/non-devalued | Cocaine | Light → food | Food, LiCl | Light | Food | Cocaine |
CTA, conditioned taste aversion; LiCl, lithium chloride; →, paired presentations; , unpaired presentations.
Behavioral Training Procedures
At the end of the 21 day withdrawal period and before training began, the rats were food deprived to 85% of their baseline free-feeding weights. The rats were kept at this weight for the remainder of the experiment. Rats were first trained to eat from the recessed food cup in a single 64 min shaping session that included 16 deliveries of the unconditioned stimulus (US), which consisted of two 45 mg Noyes food pellets (P.J. Noyes, Manchester, NH). Next, the rats received light–food conditioning (Table 1) in eight daily 64 min sessions. There were 16 trials in each session, consisting of a 10 s presentation of the house light conditioned stimulus (CS) followed immediately by US delivery (two 45 mg food pellets). The inter-trial interval was 4 ± 2 min. Although an explicitly unpaired CS (CS–) was not used in this experiment, prior work employing unpaired control CSs using the same apparatus and conditioning procedures used here (Hatfield et al., 1996; Setlow et al., 2002; Lindgren et al., 2003) has demonstrated that the increased responding to the light CS paired with food does indeed result from associative learning (i.e. it is not a consequence of pseudoconditioning).
At the end of light–food conditioning, the rats in each group were assigned to ‘devalued’ and ‘non-devalued’ groups for taste aversion training (Table 1). Activity on the last day of drug treatment and the level of conditioned responding at the food cup on the last day of conditioning were balanced across the devalued and non-devalued groups in each treatment condition. Taste aversion training took place in the animals' home cages over 4 days. On the first and third days of aversion training, the rats in the devalued group received 10 min access to a ceramic bowl containing 100 Noyes food pellets (45 mg each). Immediately after this 10 min period, the ceramic bowl was removed and rats in both the devalued and non-devalued groups received an injection of 0.3 M LiCl solution (5 ml/kg i.P.). On the second and fourth days of aversion training, the rats in the non-devalued group received 10 min access to a ceramic bowl containing 100 Noyes food pellets; no injections were given on these days. In this design, both groups of rats received the same exposure to the food pellets and LiCl-induced illness, but only the rats in the devalued group experienced illness in conjunction with the food consumption.
After taste aversion training, the rats underwent the devaluation probe test (Table 1). This 64 min session consisted of 16 presentations of the 10 s house light in the absence of food delivery. Six hours later, the rats received a chamber consumption test, consisting of 10 min access to 50 food pellets (those used as the US) placed in the food cup of the experimental chamber, in order to assess the level of generalization of the taste aversion from the home cage to the experimental chamber (CTA tests, Table 1). Finally, on the next day of testing, the rats received, in their home cages, 10 min access to a ceramic bowl containing 100 food pellets, as in the taste aversion training (CTA tests, Table 1).
Confirmation of Sensitization
After completion of behavioral testing, all rats were challenged with ascending doses of cocaine to confirm the persistence of psychomotor sensitization in the cocaine-treated rats. For this test, all rats in both groups received i.P. injections of 0.9% saline and 7.5, 15.0 and 30.0 mg/kg cocaine HCl, in that order. Immediately after each injection, the rats were placed in the training chambers, and locomotor activity was monitored for 1 h as before.
Response Measures
Locomotor activity in each 1 h session after drug injection was averaged across 5 min blocks, and the mean activity in each group across sessions was compared. During the confirmation of sensitization after behavioral testing, locomotor activity was measured similarly. The measure of appetitive conditioning to the house light CS was the percentage of time the rat spent with its head in the food cup during the 10 s CS presentation, as indicated by disruption of a photocell beam. In addition, a baseline level of food cup entry was assessed during the 5 s interval immediately prior to CS presentation. Although several behaviors become conditioned to the light CS as a consequence of its pairing with food (Holland, 1977), prior work has found that percent time in food cup is the most sensitive to LiCl-induced devaluation (Holland and Straub, 1979). In addition, other measures of responding at the food cup (frequency of food cup entries and latency to enter after CS onset) are very strongly correlated with percent time in food cup.
Consumption of food pellets in the home cage was determined by weighing the pellets remaining in the bowl after 10 min. Consumption in the experimental chamber test was determined by weighing the pellets remaining in the food cup and spilled into the tray beneath the floor after 10 min. Note that the amount of food available to the rats differed between the home cage and the experimental chamber for these tests; in the home cage the rats were presented with 4.5 g of food whereas in the box test they were presented with 2.25 g. In all cases, data were compared by analysis of variance (ANOVA) with planned comparisons where appropriate (P < 0.05).
Results
Cocaine Sensitization
Prior to any behavioral training, the rats received 14 daily injections of cocaine (30 mg/kg i.p.) or saline vehicle. After each daily injection, the rats were placed in plexiglas chambers for one h to monitor locomotor activity. There were no differences in activity among the four groups initially, but cocaine-treated groups (both devalued and non-devalued) exhibited increased locomotor activity during drug treatment; activity levels were similar within each treatment condition for devalued and non-devalued groups. Consistent with this description, a three-factor ANOVA (treatment×devaluation×session) showed significant main effects of treatment [F(1,32) = 95.2, P < 0.001] and session [F(14,448) = 3.01, P < 0.001] and a significant interaction between these two factors [F(14,448) = 5.86, P < 0.001], but no effect nor any interactions involving devaluation condition (Fs < 1.10, NS). A direct comparison of activity on the final day of cocaine treatment showed that although cocaine-treated rats differed significantly from saline-treated rats [F(1,32) = 46.7, P < 0.001], there were no differences between rats in the to-be-devalued and non-devalued groups in either treatment (Fs < 1, NS).
Post-sensitization Light–Food Conditioning
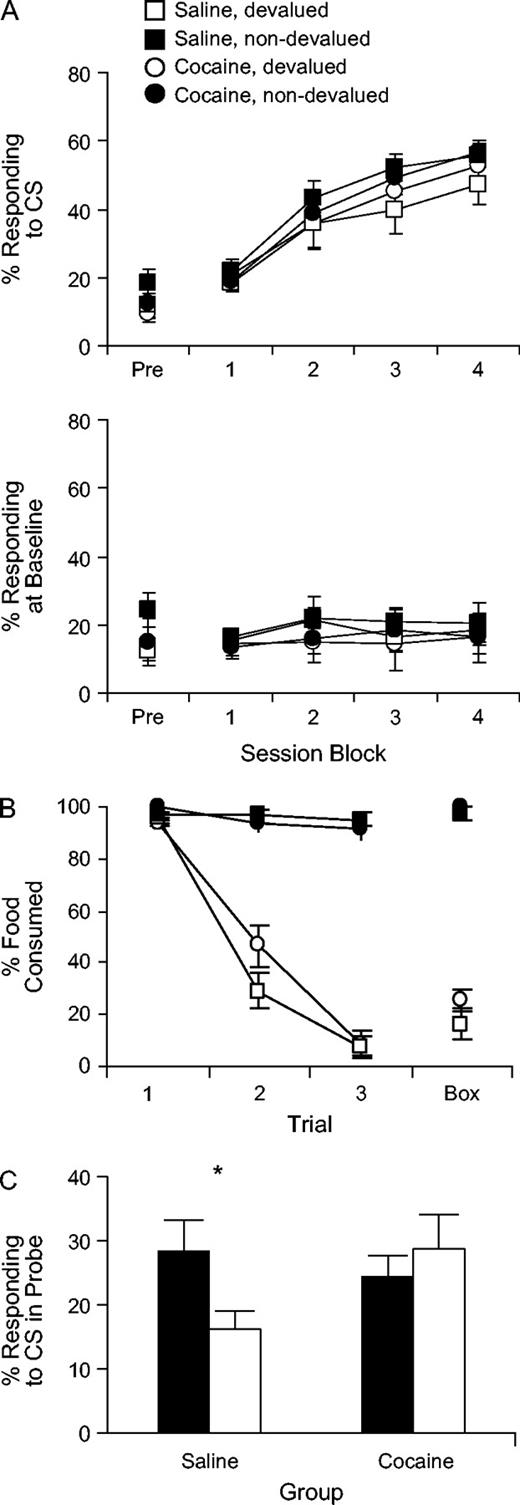
Light–food conditioning began 21 days after the end of sensitization. As illustrated in the top panel of Figure 1A, all groups increased conditioned responding over the course of training (measured as the percentage of time spent in the food cup during light presentation), and conditioned responding was similar among groups. A three-factor ANOVA (treatment × devaluation × session block) of food cup responding during the light CS revealed a significant main effect of session [F(3,93) = 140, P < 0.001], but no effects or interactions involving treatment or devaluation condition (Fs < 2.01, NS). A direct comparison of responding on the final day of conditioning by two-factor ANOVA confirmed that there were no differences among the groups in conditioned responding either during the light cue or at baseline before the light cue was presented (Table 2; CS: Fs < 0.66, NS; baseline: Fs < 2.3, NS).
Food-cup responding and taste aversion learning in cocaine-treated and saline-treated rats. (A) Percent of time spent in the food cup during presentation of the light CS (top panel) and at baseline during a pre-CS period (bottom panel) in a single pre-conditioning session and during conditioning. During conditioning, data are shown in two-session blocks. (B) Food consumption in the home cage during taste aversion training and testing (‘1–3’) and during the test conducted in the training chamber (‘box’). Consumption is shown as the percentage of presented food that was consumed. Note that the third food consumption test and the test conducted in the training chamber occurred after the extinction probe test. (C) Percent of time spent in the food cup during presentation of the light CS in the extinction probe test after devaluation. Bars show mean responding during the 16 light presentations in the session. Filled bars indicate ‘non-devalued’ groups, and open bars indicate ‘devalued’ groups.
Mean (± SEM) conditioned responding on the last day of conditioning (day 8) and on the devaluation probe test
| Group . | Day 8 . | . | Probe . | . | ||
|---|---|---|---|---|---|---|
. | CS . | Baseline . | CS . | Baseline . | ||
| Saline/devalued | 50.6 ± 5.6 | 25.0 ± 5.8 | 16.2 ± 3.0 | 4.07 ± 2.1 | ||
| Saline/non-devalued | 55.9 ± 5.1 | 19.6 ± 5.3 | 28.8 ± 4.5 | 4.78 ± 1.5 | ||
| Cocaine/devalued | 56.1 ± 5.3 | 14.3 ± 4.9 | 28.7 ± 5.7 | 10.2 ± 3.0 | ||
| Cocaine/non-devalued | 57.9 ± 3.5 | 16.0 ± 3.3 | 24.3 ± 3.5 | 8.52 ± 2.5 | ||
| Group . | Day 8 . | . | Probe . | . | ||
|---|---|---|---|---|---|---|
. | CS . | Baseline . | CS . | Baseline . | ||
| Saline/devalued | 50.6 ± 5.6 | 25.0 ± 5.8 | 16.2 ± 3.0 | 4.07 ± 2.1 | ||
| Saline/non-devalued | 55.9 ± 5.1 | 19.6 ± 5.3 | 28.8 ± 4.5 | 4.78 ± 1.5 | ||
| Cocaine/devalued | 56.1 ± 5.3 | 14.3 ± 4.9 | 28.7 ± 5.7 | 10.2 ± 3.0 | ||
| Cocaine/non-devalued | 57.9 ± 3.5 | 16.0 ± 3.3 | 24.3 ± 3.5 | 8.52 ± 2.5 | ||
Entries are percentage time in the food cup during the light conditioned stimulus (CS) and in the 5 s prior to each CS delivery (baseline).
Mean (± SEM) conditioned responding on the last day of conditioning (day 8) and on the devaluation probe test
| Group . | Day 8 . | . | Probe . | . | ||
|---|---|---|---|---|---|---|
. | CS . | Baseline . | CS . | Baseline . | ||
| Saline/devalued | 50.6 ± 5.6 | 25.0 ± 5.8 | 16.2 ± 3.0 | 4.07 ± 2.1 | ||
| Saline/non-devalued | 55.9 ± 5.1 | 19.6 ± 5.3 | 28.8 ± 4.5 | 4.78 ± 1.5 | ||
| Cocaine/devalued | 56.1 ± 5.3 | 14.3 ± 4.9 | 28.7 ± 5.7 | 10.2 ± 3.0 | ||
| Cocaine/non-devalued | 57.9 ± 3.5 | 16.0 ± 3.3 | 24.3 ± 3.5 | 8.52 ± 2.5 | ||
| Group . | Day 8 . | . | Probe . | . | ||
|---|---|---|---|---|---|---|
. | CS . | Baseline . | CS . | Baseline . | ||
| Saline/devalued | 50.6 ± 5.6 | 25.0 ± 5.8 | 16.2 ± 3.0 | 4.07 ± 2.1 | ||
| Saline/non-devalued | 55.9 ± 5.1 | 19.6 ± 5.3 | 28.8 ± 4.5 | 4.78 ± 1.5 | ||
| Cocaine/devalued | 56.1 ± 5.3 | 14.3 ± 4.9 | 28.7 ± 5.7 | 10.2 ± 3.0 | ||
| Cocaine/non-devalued | 57.9 ± 3.5 | 16.0 ± 3.3 | 24.3 ± 3.5 | 8.52 ± 2.5 | ||
Entries are percentage time in the food cup during the light conditioned stimulus (CS) and in the 5 s prior to each CS delivery (baseline).
By contrast, a three-factor ANOVA (treatment × devaluation × session block) of food cup responding at baseline during the 8 days of conditioning showed no significant effect nor any interactions with treatment (Fig. 1A, bottom panel); indeed, this analysis revealed no significant effects at all (Fs < 1.3, NS), suggesting that the rats did not develop conditioned responses at the food cup driven by the training context. This absence of any conditioned responding at baseline suggests that the particular paradigm and training materials used here was successful in promoting conditioning to the light CS. This is consistent with other published reports using the same or similar paradigm and apparatus, which included either explicitely unpaired groups or a CS– for comparison and showed associative conditioning to the CS+ (Hatfield et al., 1996; Setlow et al., 2002; Lindgren et al., 2003).
Taste Aversion Training
The results from taste aversion training are shown in Figure 1B. Training caused an equivalent reduction in food consumption in both cocaine- and saline-treated rats, whereas neither of the non-devalued control groups showed a reduction in consumption. A three-factor ANOVA (treatment × devaluation × trial) revealed a significant effect of devaluation condition [F(1,32) = 307, P < 0.001] and trial [F(2,64) = 374, P < 0.001], and a significant interaction between devaluation condition and trial [F(2,64) = 139, P < 0.001]. Moreover, the taste aversion was not context-specific, in that it transferred readily from the home cage, where it was established, to the experimental chamber (Fig. 1B, ‘Box’); that transfer was unaffected by the cocaine treatment. A two-factor ANOVA (treatment × devaluation) of consumption in the training chamber found a significant effect of devaluation condition [F(1,32) = 447, P < 0.001], but no other significant main effects or interactions (Fs < 2.51, NS).
Devaluation/Extinction Probe Test
The primary results from the devaluation probe test are shown in Figure 1C and Table 2. Saline-treated, devalued rats showed reduced food-cup responding during light presentation compared to the saline-treated, non-devalued rats; this difference was not present in the cocaine-treated rats (Fig. 1C). A two-factor ANOVA (treatment × devaluation) of responding at the food cup during the light CS confirmed this observation, revealing a significant interaction between treatment and devaluation condition [F(1,32) = 4.50, P < 0.05]. Post-hoc comparisons indicated that saline-treated devalued rats responded significantly less to the light cue than saline-treated non-devalued rats (P = 0.028), whereas responding of cocaine-treated devalued and non-devalued rats did not differ (P = 0.45). Responding was also greater in the cocaine-treated devalued rats than in the saline-treated devalued rats (P = 0.038). Thus, responding to the light cue during the probe test was sensitive to changes in the value of the food in saline-treated rats but not in cocaine-treated rats.
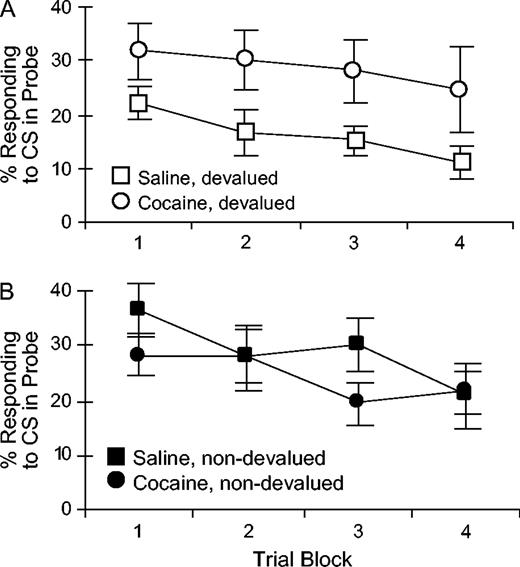
When the probe test was divided into four four-trial blocks, there was also a significant main effect of trial block on conditioned responding during extinction [F(3,96) = 6.57, P < 0.001], reflecting decreased responding to the light across these non-rewarded trials. Importantly, there were no interactions between trial block and treatment or devaluation condition (Fs < 1.2, NS). The absence of any effects or interactions with treatment indicates that the decrease in responding across the extinction session was similar in the saline and cocaine-treated rats. These data are shown in Figure 2A for devalued rats and Figure 2B for non-devalued rats. The parallel extinction curves for cocaine- and saline-treated rats show that, despite the cocaine-treated rats' inability to spontaneously decrease responding to the light cue as a result of devaluation, they were able to extinguish or withhold responding normally as a consequence of new learning in the non-rewarded probe test session.
Extinction of conditioned responding to the light CS, shown as the percent of time spent in the food cup, across the 16-trial extinction probe test in ‘devalued’ (A) and ‘non-devalued’ (B) groups. Data are shown in 4-trial blocks.
Interestingly a two-factor ANOVA (treatment × devaluation) comparing responding to the food cup at baseline during the probe test (Table 2) revealed that there was no main effect of devaluation nor any interaction (Fs < 0.4, NS). Thus devaluation had no effect on responding at baseline in either the cocaine- or saline-treated rats. The absence of any effect of devaluation is notable since it confirms that the devaluation effect on responding to the light CS in saline-treated controls was specific and did not reflect a general impact of devaluation on general activity in the box or on responding at the food cup that was not driven by a representation of the food (recall that baseline responding did not condition). Tthere was a main effect of treatment such that the cocaine-treated rats responded to the food cup slightly more than the saline-treated controls at baseline [during the inter-trial intervals, F(1,32) = 5.32, P < 0.05]. This increase was revealed as a result of a somewhat larger decline in baseline responding in the saline-treated rats from the end of conditioning to the probe test (Table 2). Since baseline responding does not appear to reflect learning, we might conclude that cocaine-treated rats were essentially less likely to decrease their general activity in the boxes as a result of the week-long break in training, perhaps as a result of the sensitization with cocaine. Whatever the cause, it must be stressed that this modest increase in responding was observed only at baseline and not during presentation of the light cue (compare saline-and cocaine-treated non-devalued groups), and it was present at baseline in both the devalued and non-devalued rats treated with cocaine. Thus, its presence does not compromise the effect of cocaine treatment on responding to the light cue after devaluation of the associated food.
Relationships between Sensitization and Behavioral Impairments
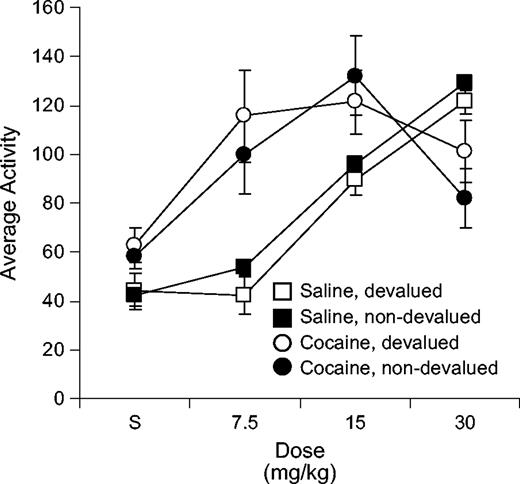
After the completion of behavioral training, rats in both the cocaine- and saline-treated groups received ascending doses of cocaine (saline, 7.5, 15, 30 mg/kg i.p.) to confirm the presence of psychomotor sensitization. Locomotor activity counts from these sessions are presented in Figure 3. Cocaine-treated rats in both the devalued and non-devalued groups showed enhanced locomotor activity relative to saline-treated controls, such that the dose–response curve was shifted to the left. A three-factor ANOVA (treatment × devaluation × dose) revealed a significant main effect of dose [F(3,96) = 38.4, P < 0.001] and a significant interaction between dose and treatment [F(3,96) = 14.4, P = 0.001]. Note that this interaction was significant even when the 30 mg/kg dose was excluded [F(2,64) = 4.89, P < 0.05]. There were no significant effects or interactions involving devaluation condition.
Locomotor activity in cocaine-treated and saline-treated rats during dose-response testing after behavioral training. Mean activity counts per 5 min block are shown for the 1 h session that followed each saline or cocaine injection.
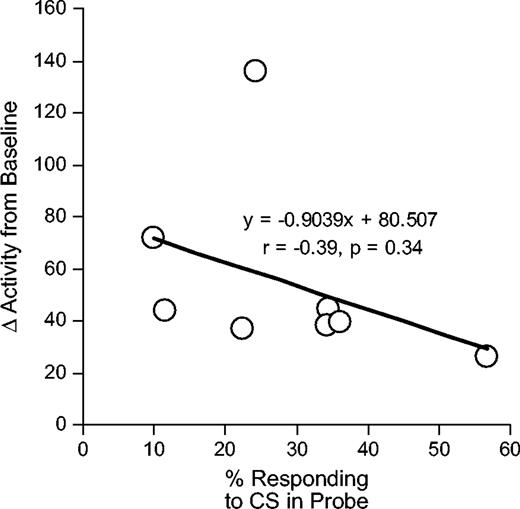
Finally, we compared conditioned responding in the cocaine-treated, devalued rats with locomotor activity in response to the three doses of cocaine minus baseline activity obtained during exposure to the boxes before sensitization at the beginning of the study. These measures were not correlated (r = −0.39, P = 0.34). In other words, the persistent responding present in the cocaine-treated rats after devaluation bore no relationship to the change in locomotor activity exhibited by these rats in response to ascending doses of cocaine in a testing session administered immediately thereafter. This is evident in Figure 4.
Correlation between the change in locomotor activity as a result of sensitization and responding during presentation of the light CS in the extinction probe test after devaluation in the cocaine-treated, ‘devalued’ rats. The change in locomotor activity was calculated as the average activity induced by the three ascending doses of cocaine in the dose-response testing done after behavioral training minus the activity exhibited by each rat in a single pre-exposure session in the box at the beginning of the study prior to any cocaine treatment. Note that this calculation was also performed for each cocaine dose individually, as well as by using saline exposure during sensitization testing as the baseline. In no case was any significant correlation observed between sensitization and conditioned responding.
Discussion
Here we have shown in a simple Pavlovian conditioning paradigm that exposure to cocaine causes a long-lasting impairment in the control of behavior. Rats previously treated with cocaine failed to use information about the perceived value of the outcome to guide their responses to a cue during an extinction probe test administered after devaluation of the associated food reward. As a result, the cocaine-treated rats responded inappropriately during the test, as if out of habit rather than according to the expected outcome or consequence of responding. Such persistent behavior is reminiscent of the behavior of addicts, who are similarly unable to control their drug-seeking behavior despite disastrous consequences. Importantly, our findings suggest that such behavior is not the result of enhanced stimulus–response learning but rather results from an inability to encode, modify or use stimulus–outcome representations. As different brain systems are proposed to mediate learning in different associative domains, these findings have important implications for understanding the neural circuitry critical to the loss of behavioral control that characterizes addiction.
Before considering those implications, however, several features of these data deserve comment. First, the effect of cocaine treatment on responding after devaluation did not reflect a general learning impairment, as acquisition of both conditioned responding and taste aversion were normal. Thus saline- and cocaine-treated rats both acquired a conditioned response to the food cup at the same rate and of the same intensity, and both were able to inhibit food consumption as a result of pairing with illness. In addition, cocaine-treated rats were able to extinguish responding normally, again showing that they did not suffer from a general inability to inhibit responding.
Although a number of researchers have reported enhanced appetitive conditioned responding after exposure to psychostimulants using similar measures (Harmer and Phillips, 1998; Taylor and Jentsch, 2001; Phillips et al., 2002), we did not see such an enhancement in conditioned responding in our data, either during training or during the probe test (although there were changes in unconditioned baseline responding to the food cup). There are many reasons that might explain this discrepancy with these published reports, particularly since the effect reported in these studies is often relatively modest. For example, it may be that cocaine is less apt to promote increased conditioned approach than amphetamine, which is more often associated with such enhancement. Alternatively it may be that this effect on responding is not observed with longer treatment regimens or after a longer period of withdrawal, both variables which differed between our study and these earlier reports. Whatever the explanation, none of these prior studies identified the associative basis of the enhancement. Thus, although each claimed to have demonstrated enhanced stimulus-reward learning, they did not probe the behavior in the drug-treated rats to determine if it was in fact sensitive to manipulation of the value of the reward. As a result, their findings are not specifically at odds with our result. For example, enhanced stimulus–outcome encoding may occur as a result of cocaine exposure, leading to increased responding in some settings, and yet cocaine-exposed rats may be unable to subsequently modify these associative representations to reflect the current value of the outcome as in our study. Such an effect might reflect changes in interactions between brain systems mediating stimulus–outcome learning, which we discuss below. Alternatively, enhanced responding in these studies may reflect enhancements in other associative processes, which have been linked to psychostimulant exposure (Wyvell and Berridge, 2001).
Cocaine Makes Actions Insensitive to Outcomes but not Extinction
The present results show that simple exposure to cocaine can cause a relatively long-lasting and general (i.e. outside of drug-seeking) increase in persistent responding in the face of adverse consequences. In addition, these data distinguish between two possible accounts of the underlying associative basis of such behavior. One possible account of this behavior is that brain changes caused by drug exposure increase the strength of stimulus–response associations, thereby allowing antecedent cues to exert an overwhelming influence on behavior. Our data do not support this account, since cocaine treatment did not affect the rate at which the rats acquired or extinguished conditioned responding.
Extinction learning is particularly interesting in this regard because extinction training similar to that applied in the devaluation probe session has been shown to cause modifications in associations between antecedent cues and responses rather than changes in knowledge regarding predicted outcomes (Rescorla, 1993, 1996, 1997; Delamater, 1996). For example, rats trained to associate cues with food outcomes show the same decline in conditioned responding after devaluation for both extinguished and non-extinguished cues, indicating that extinction leaves stimulus–outcome associations preserved (Rescorla, 1996). Furthermore, extinction training does not affect responses that are not available during extinction, even when the responses are all trained with a single outcome (Rescorla, 1997). This intuitively obvious result shows that extinction reflects changes in associations between cues and specific responses. According to these data, increases in the strength or persistence of associations between cues and responses should result in prolonged extinction. The fact that the cocaine-treated rats showed normal extinction suggests that these mechanisms are not affected by cocaine exposure. This result is consistent with findings that learning mediated by dorsal striatum, which is thought to involve stimulus–response associations, is unaffected in rats receiving ongoing exposure to cocaine (Udo et al., 2004).
Instead, our data support an alternative account that the maladaptive behavior that characterizes addiction is due, in part, to an inability to guide behavior according to expected outcomes or consequences. Saline-treated control rats that received taste aversion training to devalue the food outcome exhibited a spontaneous decrease in conditioned responding during the probe test session. In contrast, cocaine-treated rats failed to decrease their conditioned responding. Thus, prior cocaine exposure caused an inability to encode, modify and/or use a representation of the outcome value to guide responding to the light cue in the task.
Of course our results do not directly address the effects of cocaine (or other drug) exposure by other routes, under different dosing regimens or after different periods of withdrawal. Each of these variables have been reported to impact the effects of cocaine on various parameters (Robinson et al., 1998; Grimm et al., 2001; Robinson et al., 2002). Indeed, there is evidence in monkeys trained to self-administer cocaine for 100 days that there is a progressive impact on metabolic activity in the dorsal striatum (Porrino et al., 2004), so it is possible that we would see effects on stimulus–response learning if cocaine were administered for a much longer period of time. Moreover, contingent drug administration may be associated with somewhat unique effects compared with non-contingent administration (Robinson et al., 2002; Ben-Shahar et al., 2004). However, it is of note that our data are consistent with and extend recent reports that cocaine seeking in rats is insensitive to adverse consequences (Miles et al., 2003; Vanderschuren and Everitt, 2004). In these reports drug was delivered contingent upon behavior and at doses and time scales that differed substantially from our study, and yet they report conceptually similar behavioral deficits. Miles et al. (2003) showed that rats trained to respond for a cocaine-laced sucrose solution failed to decrease responding when the solution was devalued by pairing with illness, and Vanderschuren and Everitt (2004) reported that rats given prolonged training to self-administer cocaine continue responding in the face of presentation of an aversive conditioned stimulus. The current study extends these reports in several ways. First, we have demonstrated that non-contingent cocaine exposure affects the ability of rats to use information about consequences to guide their behavior. Second, we show that this effect occurs in a Pavlovian setting for normal learning materials (lights, food), which are not associated with drug exposure. Third, we show that this effect occurs over a month after the last cocaine treatment. Fourth, we show that this effect does not correlate with psychomotor sensitization, as indexed by locomotor activity. Fifth, we show that this effect is specifically unrelated to an ability to withhold or extinguish responding.
Cocaine and the Orbitofrontal–Amygdalar Circuit
The deficit reported here after cocaine exposure is similar to that observed in OFC-lesioned monkeys on a comparable task (Izquierdo et al., 2004) and identical to that observed in this task in rats after OFC lesions (Gallagher et al., 1999; Pickens et al., 2003). In these two studies, conducted using the same procedures employed here, we showed that rats given neurotoxic lesions of OFC either before or after conditioning failed to decrease responding during the extinction probe test when compared to lesioned, ‘non-devalued rats’. Although it was not shown in these studies, when data from the probe test was divided into blocks as we have done here, this deficit was observed despite normal extinction learning during the probe test. For example, analysis of the probe test data in Pickens et al. (2003) revealed a significant main effect of trial block on conditioned responding during extinction [F(3,90) = 4.88, P < 0.01], reflecting decreased responding to the light across these non-rewarded trials, but there was no interaction between trial block and lesion (Fs < 1, NS), indicating that the decrease in responding was similar in control and OFC-lesioned rats.
The similarity between the current findings and the effects of OFC lesions in the same paradigm suggests that OFC function may be impaired after cocaine exposure. Such impairment would be consistent with reports that psychostimulant exposure is associated with metabolic/structural abnormalities in OFC (Breiter et al., 1997; London et al., 2000; Volkow and Fowler, 2000; Crombag et al., 2004) and deficits in other OFC-dependent tasks (Bechara et al., 2001; Jentsch et al., 2002; Schoenbaum et al., 2004). Interestingly, it has recently been reported that amphetamine causes a decrease in dendritic spine density in OFC, whereas it increases spine density in other prefrontal regions and subcortical areas (Crombag et al., 2004). If this effect were to generalize to cocaine, it would provide an intuitive mechanism for the effect we have observed.
However, although these behavioral deficits point to an effect of cocaine exposure on OFC-dependent functions, it may be that changes in associated brain regions, such as amygdala or ventral striatum, contribute to these deficits. The amygdala is a particularly interesting candidate in this regard. Neurotoxic lesions of basolateral amygdala (ABL) cause deficits in responding after reward devaluation (Hatfield et al., 1996), and we have found that damage to ABL can selectively disrupt neural encoding of stimulus-outcome associations in OFC (Schoenbaum et al., 2003). Moreover, interactions between ABL and OFC are clearly implicated in phenomena of addiction such as relapse and craving (Kantak et al., 2002; See et al., 2003; Fuchs et al., 2004). For example, lidocaine inactivation of rostral ABL, which is the source of projections to OFC (Krettek and Price, 1977), blocks reinstatement of drug-seeking behavior by cocaine-associated cues (Kantak et al., 2002), and Fuchs et al. (2004) have recently reported that post-training inactivation of the area of OFC that receives ABL afferents also blocks reinstatement of drug-seeking behavior. Interactions between ABL and OFC appear to be particularly crucial for using information about expected outcomes to guide decisions (Baxter et al., 2000; Arana et al., 2003; Pickens et al., 2003; Schoenbaum et al., 2003), particularly as the likely outcomes or consequences of those decisions become more abstract and further removed from predictive cues (Mobini et al., 2002). An effect of cocaine exposure on this integrative function might be particularly detrimental to the ability to utilize knowledge about the longer-term, more abstract and probabilistic negative consequences of drug use, while leaving largely unaffected the ability to respond to the immediate, concrete, less probabilistic, presumably rewarding effects. This description bears an obvious similarity to anecdotal descriptions of drug addiction in which addicts seem to have a partial understanding of the implications of their behavior and yet are unable to apply this limited knowledge to control their behavior (Robinson and Berridge, 2000).
This work was supported by R01-DA015718 to G.S. and by F32-MH12699 to B.S. The authors would like to thank Dr. Michela Gallagher (R01-MH60179) and Dr Peter Holland for their support (R01-MH53667).
References
Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC (
Baxter MG, Parker A, Lindner CCC, Izquierdo AD, Murray EA (
Bechara A, Damasio H, Tranel D, Damasio AR (
Bechara A, Dolan S, Denburg N, Hindes A, Andersen SW, Nathan PE (
Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A (
Berke JD, Hyman SE (
Bowers MS, Kalivas PW (
Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE (
Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE (
Delamater AR (
Everitt BJ, Wolf AP (
Fuchs RA, Evans KA, Parker MP, See RE (
Gallagher M, McMahan RW, Schoenbaum G (
Grimm JW, Hope BT, Wise RA, Shaham Y (
Harmer CJ, Phillips GD (
Hatfield T, Han JS, Conley M, Gallagher M, Holland P (
Holland PC (
Holland PC, Rescorla RA (
Holland PC, Straub JJ (
Izquierdo AD, Suda RK, Murray EA (
Jentsch JD, Olausson P, De La Garza R, Taylor JR (
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (
Krettek JE, Price JL (
Lindgren JL, Gallagher M, Holland PC (
London ED, Ernst M, Grant S, Bonson K, Weinstein A (
Martin YL, Acerbo MJ, Robinson TE (
Miles FJ, Everitt BJ, Dickinson A (
Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM (
Packard MG, Knowlton BJ (
Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC (
Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC (
Phillips GD, Harmer CJ, Hitchcott PK (
Pickens CL, Setlow B, Saddoris MP, Gallagher M, Holland PC, Schoenbaum G (
Pierce RC, Duffy P, Kalivas PW (
Pierce RC, Bell K, Duffy P, Kalivas PW (
Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (
Rescorla RA (
Rescorla RA (
Robinson TE, Berridge KC (
Robinson TE, Browman KE, Crombag HS, Badiani A (
Robinson TE, Gorny G, Savage VR, Kolb B (
Schoenbaum G, Setlow B, Saddoris MP, Gallagher M (
Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B (
See RE, Fuchs RA, Ledford CC, McLaughlin J (
Setlow B, Gallagher M, Holland P (
Taylor JR, Jentsch JD (
Toda S, McGinty JF, Kalivas PW (
Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A (
Udo T, Ugalde F, DiPietro N, Eichenbaum HB, Kantak KM (
Vanderschuren LJMJ, Everitt BJ (
Volkow ND, Fowler JS (
Author notes
1Department of Anatomy and Neurobiology, University of Maryland School of Medicine, 20 Penn Street, HSF-2 S251, Baltimore, MD 21201, USA, 2Department of Psychiatry, University of Maryland School of Medicine, 701 West Pratt Street, Suite 388, Baltimore, MD 21201, and 3Department of Psychology, Texas A&M University, College Station, TX 77843-4235, USA