-
PDF
- Split View
-
Views
-
Cite
Cite
Markus Hoeren, Dorothee Kümmerer, Tobias Bormann, Lena Beume, Vera M. Ludwig, Magnus-Sebastian Vry, Irina Mader, Michel Rijntjes, Christoph P. Kaller, Cornelius Weiller, Neural bases of imitation and pantomime in acute stroke patients: distinct streams for praxis, Brain, Volume 137, Issue 10, October 2014, Pages 2796–2810, https://doi.org/10.1093/brain/awu203
Close - Share Icon Share
Abstract
Apraxia is a cognitive disorder of skilled movements that characteristically affects the ability to imitate meaningless gestures, or to pantomime the use of tools. Despite substantial research, the neural underpinnings of imitation and pantomime have remained debated. An influential model states that higher motor functions are supported by different processing streams. A dorso-dorsal stream may mediate movements based on physical object properties, like reaching or grasping, whereas skilled tool use or pantomime rely on action representations stored within a ventro-dorsal stream. However, given variable results of past studies, the role of the two streams for imitation of meaningless gestures has remained uncertain, and the importance of the ventro-dorsal stream for pantomime of tool use has been questioned. To clarify the involvement of ventral and dorsal streams in imitation and pantomime, we performed voxel-based lesion–symptom mapping in a sample of 96 consecutive left-hemisphere stroke patients (mean age ± SD, 63.4 ± 14.8 years, 56 male). Patients were examined in the acute phase after ischaemic stroke (after a mean of 5.3, maximum 10 days) to avoid interference of brain reorganization with a reliable lesion–symptom mapping as best as possible. Patients were asked to imitate 20 meaningless hand and finger postures, and to pantomime the use of 14 common tools depicted as line drawings. Following the distinction between movement engrams and action semantics, pantomime errors were characterized as either movement or content errors, respectively. Whereas movement errors referred to incorrect spatio-temporal features of overall recognizable movements, content errors reflected an inability to associate tools with their prototypical actions. Both imitation and pantomime deficits were associated with lesions within the lateral occipitotemporal cortex, posterior inferior parietal lobule, posterior intraparietal sulcus and superior parietal lobule. However, the areas specifically related to the dorso-dorsal stream, i.e. posterior intraparietal sulcus and superior parietal lobule, were more strongly associated with imitation. Conversely, in contrast to imitation, pantomime deficits were associated with ventro-dorsal regions such as the supramarginal gyrus, as well as brain structures counted to the ventral stream, such as the extreme capsule. Ventral stream involvement was especially clear for content errors which were related to anterior temporal damage. However, movement errors were not consistently associated with a specific lesion location. In summary, our results indicate that imitation mainly relies on the dorso-dorsal stream for visuo-motor conversion and on-line movement control. Conversely, pantomime additionally requires ventro-dorsal and ventral streams for access to stored action engrams and retrieval of tool-action relationships.
Introduction
Deficits in the ability to pantomime the use of tools or imitate meaningless gestures are key features of apraxia, a cognitive disorder of skilled movements not explained by lower-level impairments such as paresis or ataxia (Rothi et al., 1991; Cubelli et al., 2000; Leiguarda and Marsden, 2000; Goldenberg, 2009). However, despite more than a century of research, the exact localizations of brain lesions leading to different forms of apraxic deficits are still debated.
A recent model proposes that higher motor functions are supported by distinct dorso-dorsal and ventro-dorsal processing streams (Buxbaum and Kalénine, 2010; Binkofski and Buxbaum, 2012). Anatomically, first based on data from macaques (Rizzolatti and Matelli, 2003) and later corroborated by data from humans (for review see Binkofski and Buxbaum, 2012), the dorso-dorsal stream is thought to consist of projections from visual areas like area V6 to regions within the intraparietal sulcus and superior parietal lobule, and from there to the dorsal premotor cortex. Conversely, the ventro-dorsal stream originates from area MT/V5+ and traverses through inferior parietal lobule (IPL) to the ventral premotor cortex. Functionally, the dorso-dorsal stream is suggested to maintain on-line sensorimotor representations of the postural alignment of different body parts, and to convert physical object properties such as location or size into appropriate motor commands for reaching. Optic ataxia, characterized by misreaching (Karnath and Perenin, 2005), is considered the hallmark deficit ensuing damage to the dorso-dorsal stream. The ventro-dorsal stream, on the other hand, is thought to contain long-term representations of skilled actions. Disruptions may lead to impairments in pantomime of tool use and actual tool use (Binkofski and Buxbaum, 2012).
In contrast to object-associated actions, however, the involvement of the two streams in imitation of meaningless gestures (henceforth: imitation) remains a matter of debate, as different studies found an involvement of either dorso-dorsal and ventro-dorsal areas (Hermsdörfer et al., 2001; Heiser et al., 2003; Peigneux et al., 2004; Chaminade et al., 2005; Iacoboni, 2005; Molnar-Szakacs et al., 2005; Mühlau et al., 2005; Goldenberg and Karnath, 2006; Tessari et al., 2007; Molenberghs et al., 2010; Buxbaum et al., 2014). Furthermore, behavioural and anatomical dissociations between meaningless hand- or finger postures cast doubt on the assumption of one single pathway for imitation (Goldenberg, 1996, 1999; Goldenberg and Karnath, 2006).
In addition, the association of pantomime deficits with the inferior parietal lobule, a core region of the ventro-dorsal stream has been questioned (Goldenberg, 2009, 2013), as lesion studies instead indicated a prominent role of the inferior frontal gyrus (Goldenberg et al., 2007) or the posterior temporal lobe and extrastriate cortex (Buxbaum et al., 2014) for pantomime. Moreover, the involvement of the different streams in conceptual aspects of pantomime and other tool-associated actions has remained unclear (Heilman et al., 1997). Some authors proposed that incorrect object-action associations, such as combing one’s hair with a toothbrush, result from damage to action representations stored in ventro-dorsal areas (Buxbaum, 2001; Buxbaum and Kalénine, 2010; Binkofski and Buxbaum, 2012); others, however, claimed that the ventral stream, originally proposed to mediate the perceptual identification of objects (Goodale and Milner, 1992), is crucial for the selection of actions while the dorsal stream is only needed for the implementation of these actions (Milner and Goodale, 2008). The latter view may be concordant with the growing recognition of functionally distinct ventral and dorsal networks for different aspects of cognitive domains like language (Saur et al., 2008; Kümmerer et al., 2013), action recognition and imagination (Vry et al., 2012; Hoeren et al., 2013), or attention (Umarova et al., 2010). These reports have led to the concept of a functionally more broadly defined ventral stream (Weiller et al., 2009, 2011; Rijntjes et al., 2012) that anatomically, may also comprise superior and middle temporal regions, ventrolateral prefrontal cortex (Saur et al., 2008; Rauschecker and Scott, 2009), as well as other areas connected by the extreme capsule or the uncinate fascicle (Rijntjes et al., 2012 for review).
Several methodological challenges may have contributed to this unresolved debate. In functional neuroimaging studies, the identification of areas critical to a neuropsychological function may be hampered by frequent coactivation of areas not essential to the task (Rorden and Karnath, 2004). In lesions studies with subacute or chronic patients (Goldenberg and Karnath, 2006; Goldenberg et al., 2007; Manuel et al., 2013; Buxbaum et al., 2014), a shift of functions to other areas (Raineteau and Schwab, 2001; Saur et al., 2006) and spatial deformations within the affected hemisphere may impede precise lesion–symptom mapping (Rorden and Karnath, 2004). Moreover, advanced voxel-based methods for lesion analysis have only been developed in recent years, and require relatively large numbers of patients as well as sophisticated methods for lesion delineation (Rorden et al., 2007). Possibly for those reasons, only few studies investigating apraxic syndromes have used voxel-based statistics (Goldenberg, 2009; Manuel et al., 2013; Mengotti et al., 2013), resorting to descriptive comparisons such as lesion subtraction (Buxbaum et al., 2005; Goldenberg and Karnath, 2006; Goldenberg et al., 2007; Tessari et al., 2007).
To overcome these limitations, we conducted a prospective study with 102 acute left hemisphere stroke patients. To our knowledge, this is the first study to investigate pantomime and imitation using voxel-based lesion–symptom mapping (VLSM) in acute stroke patients. Patients were tested as early as possible, i.e. within a mean interval of 5.3 days (maximum 10) after stroke to evade effects of brain reorganization. Lesions were carefully mapped on diffusion-weighted magnetic resonance images obtained within the first 9 days post-stroke to avoid anatomical alterations as much as possible. Patients underwent testing for imitation of hand and finger postures, as well as a picture-based test for pantomime of tool use. Reflecting the concept of separate neural correlates for motor engrams and action semantics (Rothi et al., 1991), pantomime errors were grouped into the two categories of movement errors and content errors (Heilman et al., 1997). As imitation of meaningless gestures relies on intact postural representations of the different body parts and requires the conversion of visual input into motor commands without access to stored action engrams, we hypothesized that imitation would mainly rely on the dorso-dorsal stream, thus possibly extending the concept of the dorso-dorsal stream to account not only for optic ataxia, but also for imitation deficits. Conversely, we postulated that pantomime, while also requiring dorso-dorsal functions (Buxbaum et al., 2000), additionally involves motor engrams stored in ventro-dorsal areas, as well as, possibly, semantic knowledge processed in the ventral stream. In particular, we postulated an association of ventro-dorsal damage with movement errors indicating a disruption of the motor engram (Binkofski and Buxbaum, 2012), whereas content errors, reflecting the inability to correctly associate tools with their prototypical actions, may be associated with areas and their connections in the ventral stream (Milner and Goodale, 2008).
Materials and methods
Patients
Patients were consecutively recruited from the Stroke Unit of the Department of Neurology at the University Medical Centre of Freiburg, Germany. During a screening-period of 2.5 years (February 2011 to October 2013) we identified 364 patients with embolic left hemisphere stroke. From this cohort, 260 patients were excluded due to the following reasons: (i) age >90 years (n = 8); (ii) reduced general health status (n = 24); (iii) previous infarcts (n = 71); (iv) pre-existing structural brain changes (e.g. severe brain atrophy, extensive white matter changes, previous brain injury) (n = 58); (vi) major cognitive impairment (n = 15); (vii) haemodynamic alterations (e.g. carotid occlusion with insufficient collateralization) (n = 1); and (viii) other reasons, e.g. contraindications for MRI, compliance issues, or technical problems (n = 83). The remaining 104 patients received neuropsychological testing. Of these, we had to exclude two patients due to excessive sleepiness during the examination, and six patients were excluded from further analyses due to object agnosia (see below). Extra- and intracranial ultrasound examinations and available magnetic resonance or CT angiograms were reviewed to ensure sufficient cerebral blood flow at the time of behavioural testing in all cases. In total, here we report data of 96 included patients. Taking into account their overall health status, patients were tested as soon as possible after admission, mean ± standard deviation (SD) 5.3 ± 1.9 days post-stroke (min 2, max 10 days). Of the 96 patients included, all underwent imitation testing and 95 completed testing for pantomime of tool use. Full written consent was obtained from all patients and control subjects. In cases of severe aphasia, detailed information was given to the patient’s relatives or the legal guardian. The study was approved by local ethics authorities.
Behavioural testing
Testing procedures
All patients were tested by one of three specially trained occupational therapists with extensive experience in working with stroke patients. For scoring, performances of 86/96 patients were videotaped and evaluated separately by two raters (M.H. and V.M.L.). V.M.L. was blind to location and extent of the stroke. The items which had been scored differently by the two raters were reviewed jointly by both raters and a consensus rating was established that was used for subsequent analyses. The remaining 10 patients who either declined being recorded on video or could not be filmed due to technical reasons were scored directly by the examining occupational therapist. All examiners were familiarized with the scoring system before starting the study.
Imitation of meaningless gestures
For imitation of meaningless hand and finger postures, an adaptation of a previously published test was used (Goldenberg and Strauss, 2002; Goldenberg and Karnath, 2006). The test could be performed with minimal or even without verbal instructions, and comprised two subtests, one with 10 positions of the hand relative to the head with invariant finger position (ImiHand) and one with 10 finger postures (ImiFinger). The gestures were presented by the examiner with the contralateral hand compared to the patient, i.e. ‘like a mirror’. Patients always used the left hand for imitation of meaningless gestures. According to Goldenberg’s original instructions, the examiner finishes his demonstration before the patient starts imitating (Goldenberg, 2001; Goldenberg and Strauss, 2002). To reduce working memory load and effect of attention that both may be affected in acute stroke patients, in this study, the examiners kept the hand or finger position until the patient had reached his final position. For each item, two points were scored for correct imitation, one point was given if the patient reached the correct posture after a second demonstration of the posture (Goldenberg and Strauss, 2002; Goldenberg and Karnath, 2006). Inter-rater reliability in terms of rank correlations (Kendall’s τ) was good to excellent for both ImiHand (τ = 0.800) and ImiFinger (τ = 0.846). Normative data were obtained from 30 elderly subjects (Supplementary material).
Pantomime of tool use
Pantomime was tested using a modified version of the test developed by Bartolo et al. (2008), which could also be performed with minimal or even without verbal instructions. Patients were asked to mime the use of 14 tools depicted as line drawings. For aphasic patients, the task was repeatedly demonstrated by means of two example items until the patient had understood the instructions. The drawing of the current item was kept in view for the entire time while the patient pantomimed its use. Items were scored in all cases in which the patient produced an overall recognizable movement. In case aphasic patients showed no clear response or an unrecognizable movement, the item was scored if the patient responded with overall recognizable pantomimes to other test items, or, alternatively, if the instructions of the other tests (imitation, Birmingham Object Recognition Battery subtest 11, Corsi, see below) were readily understood. In this respect, our approach followed the study by Heilman et al. (1997) who used a tool-to-silhouette matching task to rule out object agnosia, and an imitative block-stacking test to ascertain that the patients could follow non-verbal commands. Similarly to a previous study (Goldenberg et al., 2007), patients were prompted to use the left hand if paresis or fine motor coordination (which were tested beforehand) interfered with performance. Even though right hand use may constitute a confounding factor when comparing pantomime with imitation of meaningless gestures (performed with the left hand, see above), this strategy was adopted as some patients, probably being highly accustomed to using the right hand for everyday actions, insist on using the right hand for pantomime. See Supplementary material for normative data and rationale for modifications to the original test.
Error classification and scoring
Each item was marked as either correct (1 point) or incorrect (0 points). Incorrect items were grouped into two categories. First, ‘Content errors’ included semantic errors (i.e. categorically wrong movements such as hammering upon the presentation of a knife), no response, and non-recognizable movements (e.g. amorphous, hesitant back-and forth or side-to-side movements without resemblance to the tool-associated action) (Heilman et al., 1997). ‘Movement errors’ referred to overall recognizable actions that were flawed in terms of the hand configuration (e.g. using a whole hand grip when writing with a pen), orientation (e.g. cutting side-to-side instead of back-and-forth), distance (e.g. too little distance between hand and table when ironing), or movement (e.g. not moving the fingers when playing the piano). Three different scores were calculated (maximum 14 points for each score): the number of correct items (PantoComplete), the number of items without content error (Content score), and the number of items without movement error (Movement score). Inter-rater reliability was good to excellent for total pantomime scores (τ = 0.800) and the number of content errors (τ = 0.824) but borderline for the number of movement errors (τ = 0.680).
Additional tests
Additional tests included the Corsi block tapping test for short-term and working memory (Kessels et al., 2000, 2008), subtest 11 of the Birmingham Object Recognition Battery for object recognition (Riddoch and Humphreys, 1993), and three paper-based neglect tests, i.e. the Bells Test (Gauthier et al., 1989), Albert’s line cancellation test (Albert, 1973) and the Ota test (Ota et al., 2001). Of 96 patients, 92 completed the Token Test of the Aachen Aphasia Battery (Huber et al., 1984); of the remaining four patients, two were rapidly discharged and two were unable to complete testing.
MRI
For a detailed description of the number and modality of images obtained, see the online Supplementary material. In sum, lesions were mapped on MRI scans obtained on average 1.9 days after symptom onset (SD 2.8, min 0 max 9 days). MRI scans were obtained on either a 3 T Trio scanner, or on a 1.5 T Avanto scanner (Siemens). For the diffusion-weighted imaging obtained in 95 patients, we used a standard sequence (23 slices, matrix = 128 × 128 pixel, voxel size = 1.8 × 1.8 × 5 mm, repetition time = 3.1 s, echo time = 79 ms, flip angle = 90°, six diffusion-encoding gradient directions with a b-factor of 1000 s/mm2). All patients received FLAIR images (repetition time = 9000 ms, echo time = 93.0 ms, flip angle = 140°, matrix 200 × 256 pixel, voxel size = 0.94 × 0.94 × 5.00 mm, 23 slices). As a prerequisite for spatial normalization, a high-resolution T1 anatomical scan was obtained from 91 patients (repetition time = 2200 ms, echto time = 2.15 ms, flip angle = 12°, matrix = 256 × 256 pixel, voxel size = 1 × 1 × 1 mm, 176 slices).
Lesion analysis
First, a rough delineation of the diffusion-weighted imaged lesion was performed using a customized region-of-interest toolbox implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Individual intensity thresholds were applied to find the best match between the binary lesion map and the diffusion-restricted brain tissue. Subsequently, the lesions maps were inspected in MRIcron (http://www.cabiatl.com/mrico/mricon/stats.html) and manually adjusted if necessary. In one case, no diffusion weighted image was available and the lesion was drawn directly onto a FLAIR image.
For spatial normalization of the lesion maps, the underlying diffusion-weighted imaging scan (or FLAIR image) was co-registered to the anatomical T1 scan (n = 91). High-resolution T1 scans were segmented using the VBM8 toolbox (r435; http://dbm.neuro.uni-jena.de/vbm/download/). Deformation field parameters for nonlinear normalization into the stereotactic Montreal Neurological Institute (MNI) standard space were then computed using the DARTEL (diffeomorphic anatomical registration through exponentiated lie algebra; Ashburner, 2007) approach implemented in VBM8. Normalization quality of lesion maps was visually checked by M.H. In five cases in which no T1 scan of sufficient quality was available, parameters for normalization were obtained using FLAIR images. As smaller diffusion restricted areas may be indiscernible in chronic MRI scans or CT scans, our approach of mapping lesions on acute diffusion weighted images may contribute significantly to a more precise determination of lesion locations compared to previous studies (Buxbaum et al., 2014).
For VLSM, we used the non-parametric statistics implemented in MRIcron (Rorden et al., 2007). Specifically, we performed the Brunner-Munzel test, a rank test for continuous behavioural variables and binary images to identify lesioned voxels associated with deficits in specific tests. The resulting maps display voxels with a significant difference in the distribution of the behavioural measure depending on whether the voxel was lesioned. Only voxels affected in at least five patients were included into the analysis. To avoid inflated z-scores in voxels with <10 subjects in either the lesion or no-lesion groups, we used a recent version of NPM (Non-Parametric Mapping, the statistical package included with MRIcron; version 12/12/2012) that uses a permutation-derived correction (Medina et al., 2010).
Separate Brunner-Munzel analyses were performed for the overall pantomime and imitation scores (PantoComplete and ImiComplete) as well as for the respective subtests (ImiHand, ImiFinger, Content and Movement scores). Different approaches were pursued to single out areas specifically associated with different subtests. First, similar to other studies (Kalénine et al., 2010; Kümmerer et al., 2013), separate logistic regression analyses were computed for pairs of (sub)scores using one score as predictor variable and the other score as covariate and vice versa. This approach allows for the analysis of the variance in one score while controlling for the variance in another score. Pairs included ImiHand versus ImiFinger, Content versus Movement scores and PantoComplete versus ImiComplete. Furthermore, to account for the correlation of lesion volume with pantomime and imitation deficits (see below), separate analyses were performed for each subscore with lesion volume as covariate.
However, the regression analyses described above do not allow one to test whether a lesion in a given voxel leads to significantly stronger impairments in one versus another task. Thus, to more directly explore the areas of the brain where damage predicts a significant difference between imitation and pantomime subscores (ImiHand versus ImiFinger, Content versus Movement scores), separate Brunner-Munzel analyses were performed with the respective score differences (ImiHand-ImiFinger and Content-Movement and vice versa, respectively). To single out regions significantly associated with differences between overall imitation and pantomime scores (ImiComplete and PantoComplete), scores were first converted to per cent correct before calculating score differences to account for the different score ranges (0–40 points for ImiComplete, 0–14 points for PantoComplete). To exclude that using the differences of raw scores (as used for score differences between Movement and Content subscores, as well as for differences between ImiHand and ImiFinger), or per cent correct transformed scores (used for differences between ImiComplete and PantoComplete) may have biased the results in terms of false negative or false positive findings, additional control analyses concerned an alternative standardization approach by transforming the different scores to z-scores. However, given that the results patterns using differences from raw/per cent-correct transformed and z-transformed scores were highly similar and thus independent of the applied standardization approach, results for z-transformed scores are not shown.
Following the convention established by previous studies (Karnath et al., 2011; Kümmerer et al., 2013), the statistical threshold for the analyses with overall scores of pantomime and imitation as well as subscores and subscore differences for pantomime was set to P < 0.01 [using a false discovery rate correction (FDR) for multiple comparisons]. As for ImiFinger, no voxels survived the FDR corrected P < 0.01 threshold, results for the imitation subtests ImiHand and ImiFinger (but not the results for the overall imitation scores, ImiComplete) are displayed at an FDR corrected threshold of P < 0.05 for comparability.
For each statistical results map calculated as outlined above, the number of voxels within the 90 different areas of the Automated Anatomical Labelling atlas (AAL-Atlas) (Tzourio-Mazoyer et al., 2002) were calculated using SPM8 and expressed for each anatomical region as percentage of the volume of the anatomical area, and as percentage and of the total volume of the statistical map.
Results are displayed on an in-house average template of 50 non-linearly normalized T1 scans from a sample of healthy subjects who had participated in other studies in our lab (age, mean ± SD 47 ± 20.75, range 22–84 years; 25 male).
Results
Demographic and behavioural results
Demographic and behavioural data of the included patients are given in Table 1. Of the 102 patients, six scored below cut-off in the object recognition test and, given that our pantomime test required intact object recognition, were excluded from further analysis. Only one patient showed symptoms of neglect at the time of testing for apraxia; this patient scored above cut-off for pantomime and imitation. Thirty-five patients were aphasic as defined by the Token Test (Huber et al., 1984).
Demographic data and general clinical scores
| . | Mean . | SD . | Min . | Max . |
|---|---|---|---|---|
| Age (years) | 63 | 15 | 26 | 85 |
| Sex (female/male) | 40 / 56 | |||
| Infarct volume (ml) | 22.3 | 35.9 | 0.2 | 243.0 |
| NIHSS on admission | 6.6 | 5.4 | 0 | 24 |
| NIHSS on discharge | 2.4 | 2.5 | 0 | 12 |
| Right arm motor NIHSS on testing | 0.0 | 0.0 | 0 | 0 |
| mRS on discharge | 1.8 | 1.1 | 0 | 4 |
| Barthel index on discharge | 86 | 27 | −25 | 100 |
| Thrombolysis (none / iv / bridging or mechanical) | 54/34/8 | |||
| Apraxia test scores | ||||
| ImiComplete | 36.5 | 4.4 | 11 | 40 |
| ImiFinger | 18.4 | 2.1 | 7 | 20 |
| ImiHand | 18.1 | 2.9 | 4 | 20 |
| PantoComplete | 10.8 | 3.7 | 0 | 14 |
| Content score | 12.7 | 3.1 | 0 | 14 |
| Movement score | 12.1 | 2.1 | 3 | 14 |
| Other test scores | ||||
| Corsi span forward | 4.7 | 1.2 | 2 | 7 |
| Corsi span backwards | 4.6 | 1.4 | 0 | 7 |
| BORB-11 | 31.3 | 1.1 | 28 | 32 |
| Token Test percentile rank | 81.7 | 28.1 | 2 | 99 |
| . | Mean . | SD . | Min . | Max . |
|---|---|---|---|---|
| Age (years) | 63 | 15 | 26 | 85 |
| Sex (female/male) | 40 / 56 | |||
| Infarct volume (ml) | 22.3 | 35.9 | 0.2 | 243.0 |
| NIHSS on admission | 6.6 | 5.4 | 0 | 24 |
| NIHSS on discharge | 2.4 | 2.5 | 0 | 12 |
| Right arm motor NIHSS on testing | 0.0 | 0.0 | 0 | 0 |
| mRS on discharge | 1.8 | 1.1 | 0 | 4 |
| Barthel index on discharge | 86 | 27 | −25 | 100 |
| Thrombolysis (none / iv / bridging or mechanical) | 54/34/8 | |||
| Apraxia test scores | ||||
| ImiComplete | 36.5 | 4.4 | 11 | 40 |
| ImiFinger | 18.4 | 2.1 | 7 | 20 |
| ImiHand | 18.1 | 2.9 | 4 | 20 |
| PantoComplete | 10.8 | 3.7 | 0 | 14 |
| Content score | 12.7 | 3.1 | 0 | 14 |
| Movement score | 12.1 | 2.1 | 3 | 14 |
| Other test scores | ||||
| Corsi span forward | 4.7 | 1.2 | 2 | 7 |
| Corsi span backwards | 4.6 | 1.4 | 0 | 7 |
| BORB-11 | 31.3 | 1.1 | 28 | 32 |
| Token Test percentile rank | 81.7 | 28.1 | 2 | 99 |
ImiComplete, overall score for imitation (maximum 40 points); ImiFinger and ImiHand, subscores for imitation of finger and hand postures, respectively (maximum 20 points); PantoComplete, overall score for pantomime (maximum 14 points), Content and Movement scores, pantomime subscores for content and movement errors (maximum 14 points).
BORB-11 = subtest 11 of the Birmingham Object Recognition Battery (maximum 32 points); NIHSS = National Institutes of Health Stroke Scale; mRS = modified Rankin Scale.
Demographic data and general clinical scores
| . | Mean . | SD . | Min . | Max . |
|---|---|---|---|---|
| Age (years) | 63 | 15 | 26 | 85 |
| Sex (female/male) | 40 / 56 | |||
| Infarct volume (ml) | 22.3 | 35.9 | 0.2 | 243.0 |
| NIHSS on admission | 6.6 | 5.4 | 0 | 24 |
| NIHSS on discharge | 2.4 | 2.5 | 0 | 12 |
| Right arm motor NIHSS on testing | 0.0 | 0.0 | 0 | 0 |
| mRS on discharge | 1.8 | 1.1 | 0 | 4 |
| Barthel index on discharge | 86 | 27 | −25 | 100 |
| Thrombolysis (none / iv / bridging or mechanical) | 54/34/8 | |||
| Apraxia test scores | ||||
| ImiComplete | 36.5 | 4.4 | 11 | 40 |
| ImiFinger | 18.4 | 2.1 | 7 | 20 |
| ImiHand | 18.1 | 2.9 | 4 | 20 |
| PantoComplete | 10.8 | 3.7 | 0 | 14 |
| Content score | 12.7 | 3.1 | 0 | 14 |
| Movement score | 12.1 | 2.1 | 3 | 14 |
| Other test scores | ||||
| Corsi span forward | 4.7 | 1.2 | 2 | 7 |
| Corsi span backwards | 4.6 | 1.4 | 0 | 7 |
| BORB-11 | 31.3 | 1.1 | 28 | 32 |
| Token Test percentile rank | 81.7 | 28.1 | 2 | 99 |
| . | Mean . | SD . | Min . | Max . |
|---|---|---|---|---|
| Age (years) | 63 | 15 | 26 | 85 |
| Sex (female/male) | 40 / 56 | |||
| Infarct volume (ml) | 22.3 | 35.9 | 0.2 | 243.0 |
| NIHSS on admission | 6.6 | 5.4 | 0 | 24 |
| NIHSS on discharge | 2.4 | 2.5 | 0 | 12 |
| Right arm motor NIHSS on testing | 0.0 | 0.0 | 0 | 0 |
| mRS on discharge | 1.8 | 1.1 | 0 | 4 |
| Barthel index on discharge | 86 | 27 | −25 | 100 |
| Thrombolysis (none / iv / bridging or mechanical) | 54/34/8 | |||
| Apraxia test scores | ||||
| ImiComplete | 36.5 | 4.4 | 11 | 40 |
| ImiFinger | 18.4 | 2.1 | 7 | 20 |
| ImiHand | 18.1 | 2.9 | 4 | 20 |
| PantoComplete | 10.8 | 3.7 | 0 | 14 |
| Content score | 12.7 | 3.1 | 0 | 14 |
| Movement score | 12.1 | 2.1 | 3 | 14 |
| Other test scores | ||||
| Corsi span forward | 4.7 | 1.2 | 2 | 7 |
| Corsi span backwards | 4.6 | 1.4 | 0 | 7 |
| BORB-11 | 31.3 | 1.1 | 28 | 32 |
| Token Test percentile rank | 81.7 | 28.1 | 2 | 99 |
ImiComplete, overall score for imitation (maximum 40 points); ImiFinger and ImiHand, subscores for imitation of finger and hand postures, respectively (maximum 20 points); PantoComplete, overall score for pantomime (maximum 14 points), Content and Movement scores, pantomime subscores for content and movement errors (maximum 14 points).
BORB-11 = subtest 11 of the Birmingham Object Recognition Battery (maximum 32 points); NIHSS = National Institutes of Health Stroke Scale; mRS = modified Rankin Scale.
Imitation and pantomime
Forty-four patients scored below cut-off in at least one subtest of imitation or pantomime. For imitation of meaningless gestures, 21 had a deficit in imitation of hand postures, 22 showed defective imitation of finger postures (cut-off score for either subtest 18/20 points); for pantomime, 27 patients scored below cut-off (11/14 points). Eighteen patients showed an isolated imitation deficit (three ImiHand deficit only; 11 ImiFinger deficit only; four combined ImiHand and ImiFinger deficit), 10 had an isolated pantomime deficit, and 16 patients had a combined imitation and pantomime deficits (nine pantomime and imiHand deficit; two pantomime and ImiFinger; five pantomime, ImiHand and ImiFinger). For correlations between scores, see Table 2.
Rank correlations between test scores (Kendall’s τ)
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| ImiComplete | 1 | 0.742** | 0.755** | 0.484** | 0.485** | 0.289** |
| ImiHand | 0.742** | 1 | 0.401** | 0.474** | 0.434** | 0.257** |
| ImiFinger | 0.755** | 0.401** | 1 | 0.342** | 0.333** | 0.214** |
| PantoComplete | 0.484** | 0.474** | 0.342** | 1 | 0.623** | 0.730** |
| Content score | 0.458** | 0.434** | 0.333** | 0.623** | 1 | 0.205* |
| Movement score | 0.289* | 0.257** | 0.214** | 0.730** | 0.205* | 1 |
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| ImiComplete | 1 | 0.742** | 0.755** | 0.484** | 0.485** | 0.289** |
| ImiHand | 0.742** | 1 | 0.401** | 0.474** | 0.434** | 0.257** |
| ImiFinger | 0.755** | 0.401** | 1 | 0.342** | 0.333** | 0.214** |
| PantoComplete | 0.484** | 0.474** | 0.342** | 1 | 0.623** | 0.730** |
| Content score | 0.458** | 0.434** | 0.333** | 0.623** | 1 | 0.205* |
| Movement score | 0.289* | 0.257** | 0.214** | 0.730** | 0.205* | 1 |
*P < 0.05; **P < 0.01.
Rank correlations between test scores (Kendall’s τ)
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| ImiComplete | 1 | 0.742** | 0.755** | 0.484** | 0.485** | 0.289** |
| ImiHand | 0.742** | 1 | 0.401** | 0.474** | 0.434** | 0.257** |
| ImiFinger | 0.755** | 0.401** | 1 | 0.342** | 0.333** | 0.214** |
| PantoComplete | 0.484** | 0.474** | 0.342** | 1 | 0.623** | 0.730** |
| Content score | 0.458** | 0.434** | 0.333** | 0.623** | 1 | 0.205* |
| Movement score | 0.289* | 0.257** | 0.214** | 0.730** | 0.205* | 1 |
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| ImiComplete | 1 | 0.742** | 0.755** | 0.484** | 0.485** | 0.289** |
| ImiHand | 0.742** | 1 | 0.401** | 0.474** | 0.434** | 0.257** |
| ImiFinger | 0.755** | 0.401** | 1 | 0.342** | 0.333** | 0.214** |
| PantoComplete | 0.484** | 0.474** | 0.342** | 1 | 0.623** | 0.730** |
| Content score | 0.458** | 0.434** | 0.333** | 0.623** | 1 | 0.205* |
| Movement score | 0.289* | 0.257** | 0.214** | 0.730** | 0.205* | 1 |
*P < 0.05; **P < 0.01.
There were 67 patients with at least one movement error, and 27 patients with at least one content error. Of the patients with content errors, 25 patients displayed non-recognizable movements, 10 showed no-response-errors, and one patient showed a semantic error. All 27 patients with content errors indicated their ability to understand the test instructions by means of at least partial success in other tests. Thus, all patients in this group had at least 28 points in the Birmingham object matching test, and were able to correctly imitate some hand and finger postures (minimum 4/20 points in ImiHand, 7/20 in ImiFinger). Moreover, only three patients failed the pantomime task completely (0 points), thus indicating by means of at least one correctly or at least overall recognizably executed item that test instructions were understood.
Correlations with clinical and demographic data
Table 3 gives an overview over the rank correlations between test performances and possible confounding factors. On the whole, there were weak to moderate correlations of imitation and pantomime scores with infarct volume, degree of overall neurological impairment as well as object-recognition and short-term memory scores. Sex differences were observed neither for PantoComplete, or number or type of errors (Mann-Whitney U-Tests, lowest P > 0.677), nor for ImiHand or ImiFinger (lowest P > 0.507). Patients who used the left hand for pantomime (n = 24) compared to patients who used the right hand (n = 71) performed significantly worse on PantoComplete (Mann-Whitney U test, P = 0.003; mean scores ± SD, 8.7 ± 4.5 versus 11.6 ± 3.2); however, while the number of content errors was also significantly greater in the left hand group (3.0 ± 4.4 versus 0.7 ± 2.4, P = 0.001), the differences in the number of movement errors did not reach significance (P = 0.15). Moreover, patients who used the left hand also performed worse on ImiHand (17.1 ± 3.7 versus 18.5 ± 2.5, P = 0.01), had more extensive lesions (infarct volume 44.0 ± 57.3 versus 15.0 ± 21.2 ml, P = 0.004), and, consecutively, were more impaired with respect to right upper extremity motor function (National Institutes of Health Stroke Scale value 1.4 ± 1.3 versus 0.2 ± 0.7, P < 0.001), as well as overall (P < 0.004 for National Institutes of Health Stroke Scale on admission and discharge, and modified Rankin Scale on discharge).
Rank correlations between test scores, and demographic and clinical data (Kendall’s τ)
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| Infarct volume | −0.260** | −0.308** | −0.144 | −0.376** | −0.375** | −0.203** |
| Age | −0.281** | −0.188* | −0.324** | −0.118 | −0.119 | −0.075 |
| NIHSS on admission | −0.230** | −0.265** | −0.154* | −0.265** | −0.294** | −0.127 |
| NIHSS on discharge | −0.227** | −0.305** | −0.108 | −0.214** | −0.352** | −0.055 |
| mRS on discharge | −0.257** | −0.346** | −0.118 | −0.226** | −0.309** | −0.062 |
| Barthel score on discharge | 0.226** | 0.294** | 0.150 | 0.316** | −0.334** | 0.135 |
| Right arm motor NIHSS | −0.061 | −0.133 | −0.002 | −0.125 | −0.213 | 0.017 |
| Token-Test percentile rank | 0.289 | 0.303 | 0.156 | 0.401** | 0.469** | 0.184 |
| BORB-11 | 0.285** | 0.222* | 0.212* | 0.355** | 0.365** | 0.161 |
| Corsi span forward | 0.335** | 0.244** | 0.279** | 0.240** | 0.284** | 0.059 |
| Corsi span backwards | 0.381** | 0.224** | 0.419** | 0.213** | 0.204* | 0.091 |
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| Infarct volume | −0.260** | −0.308** | −0.144 | −0.376** | −0.375** | −0.203** |
| Age | −0.281** | −0.188* | −0.324** | −0.118 | −0.119 | −0.075 |
| NIHSS on admission | −0.230** | −0.265** | −0.154* | −0.265** | −0.294** | −0.127 |
| NIHSS on discharge | −0.227** | −0.305** | −0.108 | −0.214** | −0.352** | −0.055 |
| mRS on discharge | −0.257** | −0.346** | −0.118 | −0.226** | −0.309** | −0.062 |
| Barthel score on discharge | 0.226** | 0.294** | 0.150 | 0.316** | −0.334** | 0.135 |
| Right arm motor NIHSS | −0.061 | −0.133 | −0.002 | −0.125 | −0.213 | 0.017 |
| Token-Test percentile rank | 0.289 | 0.303 | 0.156 | 0.401** | 0.469** | 0.184 |
| BORB-11 | 0.285** | 0.222* | 0.212* | 0.355** | 0.365** | 0.161 |
| Corsi span forward | 0.335** | 0.244** | 0.279** | 0.240** | 0.284** | 0.059 |
| Corsi span backwards | 0.381** | 0.224** | 0.419** | 0.213** | 0.204* | 0.091 |
*P < 0.05; **P < 0.01.
BORB-11 = subtest 11 of the Birmingham Object Recognition Battery; NIHSS = National Institutes of Health Stroke Scale.
Rank correlations between test scores, and demographic and clinical data (Kendall’s τ)
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| Infarct volume | −0.260** | −0.308** | −0.144 | −0.376** | −0.375** | −0.203** |
| Age | −0.281** | −0.188* | −0.324** | −0.118 | −0.119 | −0.075 |
| NIHSS on admission | −0.230** | −0.265** | −0.154* | −0.265** | −0.294** | −0.127 |
| NIHSS on discharge | −0.227** | −0.305** | −0.108 | −0.214** | −0.352** | −0.055 |
| mRS on discharge | −0.257** | −0.346** | −0.118 | −0.226** | −0.309** | −0.062 |
| Barthel score on discharge | 0.226** | 0.294** | 0.150 | 0.316** | −0.334** | 0.135 |
| Right arm motor NIHSS | −0.061 | −0.133 | −0.002 | −0.125 | −0.213 | 0.017 |
| Token-Test percentile rank | 0.289 | 0.303 | 0.156 | 0.401** | 0.469** | 0.184 |
| BORB-11 | 0.285** | 0.222* | 0.212* | 0.355** | 0.365** | 0.161 |
| Corsi span forward | 0.335** | 0.244** | 0.279** | 0.240** | 0.284** | 0.059 |
| Corsi span backwards | 0.381** | 0.224** | 0.419** | 0.213** | 0.204* | 0.091 |
| . | ImiComplete . | ImiHand . | ImiFinger . | PantoComplete . | Content score . | Movement score . |
|---|---|---|---|---|---|---|
| Infarct volume | −0.260** | −0.308** | −0.144 | −0.376** | −0.375** | −0.203** |
| Age | −0.281** | −0.188* | −0.324** | −0.118 | −0.119 | −0.075 |
| NIHSS on admission | −0.230** | −0.265** | −0.154* | −0.265** | −0.294** | −0.127 |
| NIHSS on discharge | −0.227** | −0.305** | −0.108 | −0.214** | −0.352** | −0.055 |
| mRS on discharge | −0.257** | −0.346** | −0.118 | −0.226** | −0.309** | −0.062 |
| Barthel score on discharge | 0.226** | 0.294** | 0.150 | 0.316** | −0.334** | 0.135 |
| Right arm motor NIHSS | −0.061 | −0.133 | −0.002 | −0.125 | −0.213 | 0.017 |
| Token-Test percentile rank | 0.289 | 0.303 | 0.156 | 0.401** | 0.469** | 0.184 |
| BORB-11 | 0.285** | 0.222* | 0.212* | 0.355** | 0.365** | 0.161 |
| Corsi span forward | 0.335** | 0.244** | 0.279** | 0.240** | 0.284** | 0.059 |
| Corsi span backwards | 0.381** | 0.224** | 0.419** | 0.213** | 0.204* | 0.091 |
*P < 0.05; **P < 0.01.
BORB-11 = subtest 11 of the Birmingham Object Recognition Battery; NIHSS = National Institutes of Health Stroke Scale.
Lesion analysis
Lesion distribution
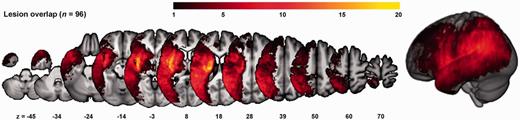
The overlap of the binary normalized lesion maps of the 96 patients with intact object recognition that were included in the main analysis is displayed in Fig. 1. As in a previous study (Kümmerer et al., 2013), maximum lesion overlap (24/96 patients) was found in subcortical areas. The lesion density within inferior frontal gyrus was similar to the IPL.
Overlap of the binarized lesions of the 96 patients included in the main analysis. The colour bar indicates the degree of overlap of lesions, e.g. bright yellow values indicate that in 20 of 96 subjects, tissue was affected by stroke.
Imitation
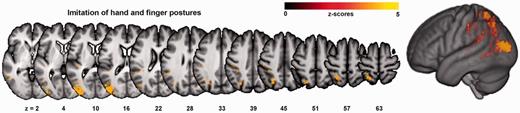
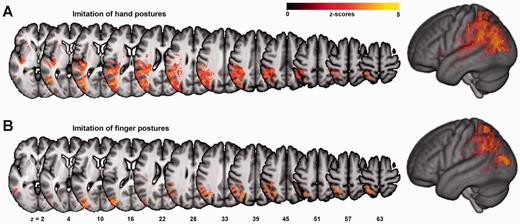
Results are depicted in Figs 2 and 3 and listed in detail in Supplementary Table 1. For ImiComplete, significant voxels were mainly found within lateral occipito-temporal cortex (LOTC), superior parietal lobule (SPL), as well as around the posterior intraparietal sulcus (IPS). As no regions were significantly associated with ImiFinger at a threshold of P < 0.01 FDR corrected, both ImiHand and ImiFinger are displayed at a lowered threshold of P < 0.05 FDR corrected to allow for a comparison of these two imitation modalities. Both ImiHand and ImiFinger were associated with a larger proportion of predominantly posterior IPL areas such as angular gyrus. Compared with ImiFinger, ImiHand showed less SPL involvement. The subscore difference ImiHand − ImiFinger and the corresponding logistic regression analysis indicated an association of LOTC damage with the behavioural pattern of relatively greater impairments of ImiHand compared with ImiFinger; no significant results were found for the reverse difference. Specific regions for imitation of meaningless gestures were found neither in the analyses with the score difference ImiComplete − PantoComplete, nor in the corresponding logistic regression analysis. However, the association of imitation with SPL and posterior IPS seemed more pronounced compared to pantomime, as for pantomime, an involvement of the SPL became apparent only after lowering the statistical threshold to P < 0.05 FDR corrected (data not shown).
VLSM map for the combined score for imitation of hand and finger postures (ImiComplete). Colour bars indicate z-scores. Reported results are thresholded at P < 0.01, FDR corrected.
VLSM maps for subtest for the imitation of finger postures (ImiFinger, A), and hand postures (ImiHand, B). Colour bars indicate z-scores. Note that these results, in contrast to the other analyses, are plotted at a lowered threshold of P < 0.05, FDR corrected.
Pantomime
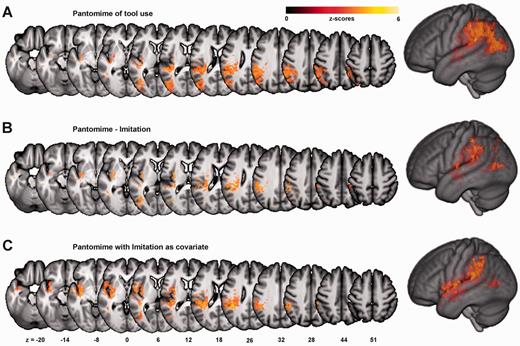
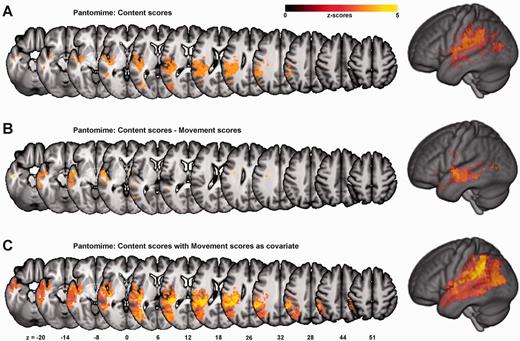
PantoComplete was mainly related to IPL, LOTC, as well as insula and extreme capsule (Fig. 4 and Supplementary Table 2). The score difference PantoComplete − ImiComplete and the logistic regression analysis for PantoComplete with ImiComplete as covariate indicated a greater importance of LOTC areas slightly anterior to the cluster found for ImiComplete, anterior IPL (mainly supramarginal gyrus), as well as insula and extreme capsule for pantomiming relative to imitation (Fig. 4 and Supplementary Table 2).
Results for pantomime. VLSM maps are shown for overall pantomime scores (PantoComplete, A), and for areas specific for pantomime in contrast to imitation, i.e. for the score difference PantoComplete − ImiComplete after conversion to percent correct (B), as well as the regression analysis of PantoComplete with ImiComplete as covariate (C). Colour bars indicate z-scores. Reported results are thresholded at P < 0.01, FDR corrected.
Specific regions for different error categories
The main finding of the analyses for the Content score with and without Movement scores as covariate, as well as the score difference Content − Movement was a greater extent of significant voxels within the temporal lobe compared to PantoComplete (Fig. 5 and Supplementary Table 3 for details). Analyses for different subtypes of content errors yielded significant results for non-recognizable movements and no-response errors; while associated lesion locations were overall similar to the results for Content scores, the extent of significant voxels in the inferior parietal lobule was greater for non-recognizable movements whereas for no-response errors, the association with temporal regions seemed more pronounced (Supplementary Fig. 1). No significant results were found in the analyses for Movement scores with and without Content scores as covariate, or Movement − Content. Different movement error subtypes (e.g. hand configuration errors) were not consistently associated with a specific lesion location.
Specific areas for content errors. VLSM maps for the analysis with Content scores alone (A), for the analysis with the subscore-difference Content − Movement (B), and the regression analysis for Content scores with Movement scores as covariate (C). Colour bars indicate z-scores. Reported results are thresholded at P < 0.01, FDR corrected.
Other analyses
There were no significant voxels for the logistic regression analyses for sub- and total pantomime and imitation scores when lesion size was added as a covariate.
Region of interest analysis within the frontal lobe
To further explore the lack of associations with frontal lesions, VLSM analyses of total scores and subscores were repeated with a mask combining the regions from the Automated Anatomical Labelling atlas (AAL-Atlas) (Tzourio-Mazoyer et al., 2002) for pars opercularis, triangularis and orbitalis of the inferior frontal gyrus as well as middle frontal and precentral gyri. Thus, sensitivity was increased by means of a less stringent false discovery rate correction. Additionally lowering the statistical threshold to P < 0.05 FDR corrected, only few significant voxels emerged for ImiHand and ImiComplete around the inferior frontal junction zone, and, for pantomime, within the inferior frontal gyrus (Supplementary Figs 2 and Supplementary Data).
Discussion
Using VLSM, we investigated the hypothesis that imitation of meaningless gestures depends mainly on the dorso-dorsal stream while pantomime of tool use additionally involves ventro-dorsal and ventral streams due to additional requirements on stored action representations, and semantic knowledge of tool-action associations, respectively. By performing separate analyses for the pantomime error categories Movement and Content, we strove to further disentangle ventro-dorsal and ventral streams. To our knowledge, our study features the largest cohort of stroke patients prospectively investigated with reliably-scored pantomime and imitation tests (but see also Manuel et al., 2013; Buxbaum et al., 2014 for other large samples), and it is the first to report VLSM data from acute stroke patients.
In addition to LOTC, which likely provides visual input to several streams (see below), the overall ability to imitate meaningless hand and finger postures was mainly associated with regions implicated in the dorso-dorsal stream (Buxbaum and Kalénine, 2010; Binkofski and Buxbaum, 2012), such as posterior IPS, and SPL (Fig. 2 and Supplementary Table 1). Overall pantomime abilities also relied on areas within the dorso-dorsal stream, but were additionally related to ventro-dorsal stream regions such as the anterior IPL and posterior middle temporal gyrus (Daprati and Sirigu, 2006; Binkofski and Buxbaum, 2012), as well as regions previously assigned to a ventral processing stream, i.e. fibres traversing the extreme capsule (Weiller et al., 2011; Rijntjes et al., 2012; Vry et al., 2012; Hoeren et al., 2013) (Fig. 4 and Supplementary Table 2). The involvement of the ventral stream in pantomime was highlighted further by the specific relation of content errors with anterior temporal lobe damage (Fig. 5 and Supplementary Table 3). However, no areas were significantly associated with movement errors. To our knowledge, our study is the first study to describe lesion locations significantly associated with content errors during pantomime of tool use. Moreover, while previously, damage to the dorso-dorsal stream was mainly linked to the occurrence of optic ataxia (Daprati and Sirigu, 2006; Kalénine et al., 2010; Binkofski and Buxbaum, 2012), our data suggest that the concept of the dorso-dorsal stream should be expanded to account for the association between imitation deficits and damage to posterior IPS and SPL.
Imitation relies on the dorso-dorsal stream
Imitation of meaningless gestures has been suggested to involve several distinct processes, including the maintenance of the body schema dynamically keeping track of the spatial relations between different body parts (Heilman et al., 1986; Buxbaum et al., 2000; Schwoebel and Coslett, 2005; de Vignemont, 2010), as well as the construction of a higher-level representation of the perceived gesture (Goldenberg, 1995; Goldenberg and Hagmann, 1997) that may be mapped onto the body schema (de Vignemont, 2010). In addition to LOTC, which may rather constitute an area providing higher order visual information to several streams, our results highlight the specific importance of dorso-dorsal stream areas, in particular posterior IPS and SPL for the processes involved in imitation (Fig. 2 and Supplementary Table 1). These results are consistent with a number of previous reports reports (e.g. Buxbaum, 2001; Koski et al., 2003; Mühlau et al., 2005; Rumiati et al., 2005; Menz et al., 2009; Vanbellingen et al., 2014), but are partly at variance with a recent study on chronic stroke patients that also reported an association between LOTC damage and imitation deficits, but, however, may have lacked sufficient lesion density to detect an association between gesture production deficits and lesions within SPL and IPS (Buxbaum et al., 2014).
Integrating on-line sensory information about the positions of the different body parts with efference copies of motor actions, the body schema generates sensorimotor representations of the body for the guidance of actions (Wolpert et al., 1998; Buxbaum, 2001; Schwoebel and Coslett, 2005; Pellijeff et al., 2006; de Vignemont, 2010). Imitation of meaningless gestures may be particularly vulnerable to a dysfunction of the body schema as unlike pantomime, imitation may not be compensated by stored action representations (Buxbaum et al., 2000). Particularly, SPL may be essential for the maintaining the body schema, as data from monkeys and humans have demonstrated that SPL integrates visual and proprioceptive input about the position of body parts to maintain spatial limb representations (Lacquaniti et al., 1995; Hagura et al., 2007; Seelke et al., 2012). SPL and posterior IPS may also be crucial for the visuospatial transformations involved in mapping observed postures into ‘somatesthetic spatial code’, i.e. the coordinates of the body schema (Heilman et al., 1986; Buxbaum, 2001; Creem-Regehr et al., 2007).
Areas within LOTC likely mediate in the decoding of observed gestures, extracting features like identity or spatial relationships of the body parts involved (Goldenberg, 1995, 2009; Goldenberg and Hagmann, 1997). Thus, extrastriate body area, MT/V5+ and the posterior STS have been associated with the perception of body parts and biological motion, respectively (Downing et al., 2001; Grossman and Blake, 2002; Grill-Spector and Malach, 2004; Iacoboni, 2005; Peelen and Downing, 2007). Together, LOTC areas likely provide input not only to the dorso-dorsal stream, but also to ventro-dorsal and ventral pathways (Gallese, 2007). This view is consistent with the proposal that both dorsal and ventral streams process the same set of visual input for different behavioural goals (Binkofski and Buxbaum, 2012).
In line with previous studies (Hermsdörfer et al., 2001; Mühlau et al., 2005), both ImiHand and ImiFinger were also associated with posterior IPL, albeit at a lower statistical threshold (Fig. 2 and Supplementary Table 1). IPL has been suggested to facilitate imitation by generating more abstract gesture representations defining observed postures as sets of categorical spatial relationships between a limited number of body parts (Goldenberg, 1995, 1999). This ‘body part coding’ (Goldenberg, 2009) is thought to reduce working memory demands and likely requires the integration of higher-order visual information with stored conceptual knowledge about body parts. Consequently, rather than an exclusive dorso-dorsal region, posterior IPL may function as a hub connecting dorsal and ventral streams (see below). The comparatively weak association between imitation deficits and IPL lesions in our study may have resulted from a reduced working memory load as, in contrast to past studies (e.g. Goldenberg and Karnath, 2006) the examiner maintained the target posture until the patient had completed the imitation. Possibly, this modification to the test may also explain the distinctly higher scores (18/20 for ImiHand and ImiFinger compared with ∼15/20 in previous studies, e.g. Goldenberg and Karnath, 2006).
Differences between imitation of hand and finger postures
As no significant voxels were found for ImiFinger at the FDR corrected threshold of P < 0.01 used for all other analyses, both ImiFinger and ImiHand are displayed at P < 0.05 FDR corrected to enable a comparison of the overall pattern of lesion locations associated with deficits in either imitation subtest (Fig. 3). Although a previous study based on lesion overlaps of chronic stroke patients reported a fronto-parietal dissociation between imitation of meaningless hand and finger postures (Goldenberg and Karnath, 2006), our VLSM data along the significant behavioural correlation indicate the importance of the dorso-dorsal stream for both imitation modalities. However, the overall statistically weaker association of ImiFinger with occipito-parietal lesions may be consistent with the proposed additional involvement of other left and right hemisphere regions in ImiFinger (Goldenberg, 1999; Goldenberg and Karnath, 2006). The relatively more pronounced associations of ImiFinger and ImiHand with SPL and IPL, respectively, are in line with a PET study (Hermsdörfer et al., 2001). The significant voxels within LOTC found for the analysis with the difference ImiHand − ImiFinger may have reflected simply the greater overall association of ImiHand with the dorso-dorsal stream areas, or, alternatively, higher demands on visual decoding, e.g. due to the higher number of body parts involved.
Pantomime of tool use involves both dorsal and ventral streams
Several regions associated with pantomime deficits overlapped with those found for imitation (Figs 2, 4 and Supplementary Tables 1 and Supplementary Data). Along with the behavioural correlation, this overlap indicates that that mechanisms mainly related to the dorso-dorsal stream are involved in either task. However, the association of pantomime with specific dorso-dorsal stream areas, i.e. posterior IPS and SPL, was evident only from the VLSM analysis with PantoComplete at a lower statistical threshold, as well as from the absence of imitation-specific areas in the analysis with ImiComplete − PantoComplete and the corresponding regression analysis (see ‘Results’ section). This weaker association is in line with lower demands on the body schema and mechanisms for visuo-motor conversion (Buxbaum et al., 2000; Buxbaum, 2001; de Vignemont, 2010).
With respect to areas involved in several streams, for pantomime, areas within LOTC likely decode the visual features of the tool images (Grill-Spector and Malach, 2004; Taylor and Downing, 2011). Although impossible to discern with VLSM data, the LOTC regions involved likely differ at least partly from those necessary for the perception of hand and finger postures (Rizzolatti and Matelli, 2003; for review see Grill-Spector and Malach, 2004; Taylor and Downing, 2011). Posterior IPL, by contrast, may integrate perceived object features with motor engrams stored within anterior IPL (Buxbaum et al., 2005), and possibly, semantic knowledge about tools and objects (Binder et al., 2009; Hoeren et al., 2013; Seghier, 2013). Thus, posterior IPL may contribute to the selection of the action appropriate for the currently presented tool.
Compared to imitation, pantomime selectively depended on posterior middle temporal gyrus and anterior IPL, as well as insula and extreme capsule (Fig. 4B and C, Supplementary Table 2). Anterior IPL and posterior middle temporal gyrus have been assigned to the ventro-dorsal stream involved in storing motor engrams and knowledge about the manipulation of tools and objects (Kalénine et al., 2010; Binkofski and Buxbaum, 2012). Particularly anterior IPL has been linked to the maintenance of canonical action engrams (Heilman and Rothi, 2003; Buxbaum et al., 2005, 2007; Peeters et al., 2009), also called ‘visuokinaesthetic engrams’ (Heilman et al., 1982), ‘action prototypes’ (Hermsdörfer et al., 2013), or ‘blueprints for movement’ (Rijntjes et al., 1999). Shaped during motor learning (Weisberg et al., 2007), these engrams are thought to specify invariant spatiotemporal features of skilled movements. Upon movement execution, the engrams may be flexibly adapted according to current physical constraints like the shape or size of a tool, or the effector used (Rijntjes et al., 1999; Haaland et al., 2000; Binkofski and Buxbaum, 2012; Hermsdörfer et al., 2012). Although the importance of the IPL for representational action aspects has recently been challenged (Buxbaum et al., 2014), our results corroborate the importance of the IPL for action engrams. Possibly, as Buxbaum et al. (2014) studied chronic patients, the IPL functions relevant to pantomime of tool use had shifted to other areas due to brain reorganization.
Although posterior middle temporal gyrus is also involved in the processing of manipulation knowledge (Kellenbach et al., 2003; Tranel et al., 2003; Boronat et al., 2005; Valyear and Culham, 2010), it may also contribute to the integration of semantic knowledge about tools and objects with movement representations in dorsal areas (Willems et al., 2009; Kalénine et al., 2010). Moreover, the importance of posterior middle temporal gyrus for action representations was recently highlighted by a large lesion study on chronic stroke patients (Buxbaum et al., 2014).
The voxels found within insula and subinsular white matter likely reflected damage to a ventral fibre tract traversing the extreme capsule. This tract has been shown to connect areas of the posterior parietal lobe with the anterior inferior frontal gyrus (Vry et al., 2012; Hoeren et al., 2013), and is considered a central component of a domain-independent ventral stream also involved in language (Weiller et al., 2011; Rijntjes et al., 2012). For pantomime, this ventral pathway may facilitate the top–down activation of movement engrams, the selection of distinctive and relevant movement features that ensure a high recognizability of the pantomime, as well as the integration of motor programs with semantic knowledge about the function of tools and objects (Goldenberg et al., 2007; Vry et al., 2012). Similar lesion locations associated with pantomime deficits have been described previously (Goldenberg et al., 2007; Manuel et al., 2013).
Correlates of different pantomime error types: selective importance of the temporal lobe for content errors
Content errors, indicating an inability to correctly associate tools with actions, are thought to result from disturbances within a separate semantic system for action (Rothi et al., 1991; Heilman et al., 1997; Cubelli et al., 2000). When asked to pantomime the use of tools, patients may, thus, display unrecognizable movements, remain in a state of helpless perplexity, or perform actions not usually associated with the tool (e.g. cutting with a spoon) (De Renzi and Lucchelli, 1988; Ochipa et al., 1989, 1992; Heilman et al., 1997).
The association of IPL lesions with content errors is consistent with the view that a complete inability to pantomime the use of a tool may result from a severe disruption of the action engram (De Renzi and Lucchelli, 1988; Heilman et al., 1997). The greater parietal involvement compared to no-response errors indicates that this may in particular apply to non-recognizable movements, which could result from severe spatiotemporal errors rather than from erroneous ‘content’ per se (Supplementary Fig. 1). However, the additional involvement of the anterior temporal lobe, which became particularly apparent when controlling for the number of movement errors as a measure of ventro-dorsal stream dysfunction (Fig. 5B and C), highlights the importance of this region for the action semantic system (Fig. 5 and Supplementary Table 3). Alternatively, content errors may have resulted from more general semantic impairments following lesions in the anterior temporal lobe region (Patterson et al., 2007; Guo et al., 2013). In line with this view, patients with semantic dementia and temporal lobe atrophy have been documented to be unable to demonstrate the prototypical use of tools despite preserved mechanical problem solving abilities (Hodges et al., 1999). However, while in general, severe amodal semantic following stroke seem to be rare (Hillis et al., 1990), no detailed assessment was carried out for the present sample of patients to evaluate the integrity of other aspects of semantic knowledge. In sum, the data confirm the importance of the ventral stream for the selection of appropriate actions (Goodale and Milner, 1992; Milner and Goodale, 2008).
Although in line with our hypothesis, the only weak correlation between content and movement scores (Table 2) points to different underlying mechanisms, no significant result were found in the VLSM-analysis for movement errors. Several reasons may have contributed to this negative finding. First, compared with content errors, the reliable identification of pathological movement features may be more difficult as up to three movement errors could be observed even in healthy control subjects. Secondly, lesion locations associated with movement errors may be more variable compared to content errors, as not only defective movement engrams ensuing damage to the ventro-dorsal stream (Rothi et al., 1991; Cubelli et al., 2000), but also disturbances of dorso-dorsal stream functions like the body schema may lead to movement errors (Buxbaum et al., 2000; Buxbaum, 2001). Consistent with this view, a recent report indicated that errors of the hand or arm posture may predominantly arise from damage to the representational component of the action while errors of amplitude or timing result from disturbances within regions for online movement control (Buxbaum et al., 2014). This dissociation could not be reproduced in the present study as separate analyses for different error types, e.g. hand configuration errors or amplitude/timing errors did not yield significant results. This difference may have resulted from distinct scoring systems or differences in the study populations.
Minor contribution of frontal lobe lesions to pantomime and imitation deficits
A rapid compensation by the contralateral hemisphere may account for the weak association between frontal lesions and praxis deficits (Supplementary Figs 2 and Supplementary Data) that stands in contrast with the findings of several past studies using functional imaging and lesion overlaps (Leiguarda and Marsden, 2000; Goldenberg and Karnath, 2006; Goldenberg et al., 2007). Accordingly, a functional MRI study with aphasic patients with left hemisphere strokes showed a rapid increase in activity mainly in right frontal areas within the first 12.1 days post-stroke (Saur et al., 2006). As we examined the study participants after a mean of 5.3 days, we cannot exclude that some degree of reorganization within the frontal lobes had already taken place at the time of testing.
Limitations
The correlations between test scores and lesion size as well as overall impairment likely resulted from the inclusion of consecutive patients with a large range of lesion sizes, and, consecutively, of overall impairment (Table 3). However, the clear differences between the VLSM results for pantomime and imitation are unlikely to be attributable to these factors, given that confounds more directly related to the tests, such as object recognition were meticulously assessed in each patient.
Our data suggest that rather than being a causative factor for lower pantomime scores, left hand use seemed to be an indicator for greater overall disability and larger lesions, which in turn were more likely to lead to pantomime deficits. Thus, patients who were prompted to use their left hand for pantomime due to right upper extremity paresis displayed a significantly higher number of content errors, but not movement errors. The reverse pattern would be expected if clumsiness due to left hand use had affected the scores. This view is further supported by the lack of score differences of healthy controls using either the right or left hand for pantomime. Moreover, imitation of meaningless hand postures was also significantly more impaired in patients who used their left hand for pantomime, although all patients used their left hand for imitation.
Similarly, as the instructions of all tests could easily be understood non-verbally, the correlation of imitation and pantomime scores with the Token Test rather reflects the joint involvement of brain regions in language and praxis rather than a causal relationship.
Lastly, although studies with acute compared to chronic stroke patients allow for a more accurate lesion delineation, and, moreover, permit avoiding the effects of brain reorganization (Saur et al., 2006), studies with acute patients may also suffer from disadvantages. First, diaschisis, the dysfunction of one or more functionally dependent cortical areas caused by a remote lesion, may affect patients in the acute phase (von Monakow, 1985; Saur et al., 2006; Jarso et al., 2013; Kümmerer et al., 2013). The effects of diaschisis are difficult to assess, yet as these changes are thought to occur within a network of functionally related areas (Price et al., 2001) false positive results, i.e. the detection of areas unrelated to the task, are unlikely. Secondly, acute patients may suffer more intensely from non-specific symptoms such as fatigability or reduced concentration. However, in our study, all patients indicated overall testability by at least partly complete tests for working memory, object recognition and imitation.
Conclusion
In summary, our results indicate that the ability to imitate meaningless gestures largely relies on the dorso-dorsal stream. Thus, our data suggest that damage to the dorso-dorsal stream may not only result in optic ataxia (Daprati and Sirigu, 2006; Binkofski and Buxbaum, 2012), but also imitation deficits. Pantomime of tool use, conversely, requires the interplay of dorso-dorsal, ventro-dorsal and ventral streams to enable the integration of movement engrams with semantic knowledge as well as with mechanisms for on-line control of actions. Our study particularly highlights the significance of the ventral stream for conceptual aspects of pantomime.
Acknowledgements
We thank Hansjörg Mast for assistance in data acquisition. We thank Gabriele Lind, Sarah Höfer and Cornelia Pietschmann for conducting the neuropsychological testing; without their careful and patient examinations, this study would not have been possible. We thank two anonymous reviewers for their suggestions.
Funding
This work was supported by the BrainLinks-BrainTools Cluster of Excellence funded by the German Research Foundation (DFG, grant #EXC1086).
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- IPL
inferior parietal lobule
- IPS
intraparietal sulcus
- LOTC
lateral occipito-temporal cortex
- SPL
superior parietal lobule
- VLSM
voxel-based lesion–symptom mapping