-
PDF
- Split View
-
Views
-
Cite
Cite
Michael V. Lombardo, Bhismadev Chakrabarti, Edward T. Bullmore, Susan A. Sadek, Greg Pasco, Sally J. Wheelwright, John Suckling, MRC AIMS Consortium, Simon Baron-Cohen, Atypical neural self-representation in autism, Brain, Volume 133, Issue 2, February 2010, Pages 611–624, https://doi.org/10.1093/brain/awp306
Close - Share Icon Share
Abstract
The ‘self’ is a complex multidimensional construct deeply embedded and in many ways defined by our relations with the social world. Individuals with autism are impaired in both self-referential and other-referential social cognitive processing. Atypical neural representation of the self may be a key to understanding the nature of such impairments. Using functional magnetic resonance imaging we scanned adult males with an autism spectrum condition and age and IQ-matched neurotypical males while they made reflective mentalizing or physical judgements about themselves or the British Queen. Neurotypical individuals preferentially recruit the middle cingulate cortex and ventromedial prefrontal cortex in response to self compared with other-referential processing. In autism, ventromedial prefrontal cortex responded equally to self and other, while middle cingulate cortex responded more to other-mentalizing than self-mentalizing. These atypical responses occur only in areas where self-information is preferentially processed and does not affect areas that preferentially respond to other-referential information. In autism, atypical neural self-representation was also apparent via reduced functional connectivity between ventromedial prefrontal cortex and areas associated with lower level embodied representations, such as ventral premotor and somatosensory cortex. Furthermore, the magnitude of neural self-other distinction in ventromedial prefrontal cortex was strongly related to the magnitude of early childhood social impairments in autism. Individuals whose ventromedial prefrontal cortex made the largest distinction between mentalizing about self and other were least socially impaired in early childhood, while those whose ventromedial prefrontal cortex made little to no distinction between mentalizing about self and other were the most socially impaired in early childhood. These observations reveal that the atypical organization of neural circuitry preferentially coding for self-information is a key mechanism at the heart of both self-referential and social impairments in autism.
Introduction
The self is a complex multidimensional construct key to many disciplines, including psychology, psychiatry, philosophy and neuroscience, among many others. The self is deeply embedded in the social world and is integral to many aspects of social behaviour and cognition (Brewer, 1991; Banaji and Prentice, 1994). One example of this integral relationship between the self and the social world is clearly seen in simulationist theories of social cognition (Goldman, 2006). The main premise of simulation theory is that an understanding of others occurs through the use of privileged access to self-representations. In the general population, evidence from infants (Meltzoff and Brooks, 2008), toddlers (Birch and Bloom, 2003), and adults (Ames, 2004; Epley et al., 2004; Birch and Bloom, 2007) suggests that representations about others are ‘anchored’ in processes centred on self-representations. This simulative anchoring is one of many context-dependent strategies that one learns throughout development when navigating the social world.
Similarly, in the brain, neural representations of self and other recruit largely identical neural circuitry. During low level embodied/simulative processes, areas in anterior insula, middle cingulate cortex, frontal operculum/ventral premotor cortex (FO/PMv) and somatosensory cortex (SI/SII) respond both to our own actions, emotions, and sensations and when we observe others acting or experiencing similar emotional or somatosensory states (Wicker et al., 2003; Keysers et al., 2004; Singer et al., 2004; Blakemore et al., 2005; Gazzola and Keysers, 2009). Additionally, during higher level inference-based processes, the medial prefrontal cortex, posterior cingulate/precuneus, and temporo-parietal junction are recruited both when we reflect on ourselves and others (Ochsner et al., 2005; Amodio and Frith, 2006; Mitchell et al., 2006; Saxe et al., 2006). Emerging evidence also suggests that these two neural circuits for shared representations of self and other interact during high level social cognitive processing (Zaki et al., 2009; Lombardo et al., 2010b) and may set the foundation for how we make sense of the complex social world.
Among this distributed network coding for shared representations, the ventromedial prefrontal cortex (vMPFC) possesses some special characteristics that make it a crucial centre for neural coding of self-representations. The ventromedial prefrontal cortex responds in a preferential manner for information that is self-relevant (Moran et al., 2006) even in the absence of explicit self-referencing (Moran et al., 2009). This preferential tuning for self-relevant information is apparent even when the task is to think about others' impressions of ourselves (Ochsner et al., 2005; D'A;rgembeau et al., 2007; Izuma et al., 2008), during on-line tracking of how our own actions influence others (Hampton et al., 2008), or when thinking about others who simply share some variance with oneself, such as similar or close others (Ochsner et al., 2005; Mitchell et al., 2006; Jenkins et al., 2008; Mobbs et al., 2009). In preferentially responding to self-relevant information, the ventromedial prefrontal cortex distinguishes self-referential from other-referential processing (particularly for familiar but non-close others) (Craik et al., 1999; Kelley et al., 2002; Vogeley et al., 2004; David et al., 2006; Pfeifer et al., 2007). These attributes of the ventromedial prefrontal cortex make it a key neural mechanism that distinguishes self from other, specifically through the coding of self-information. This neural distinction between self and other enables us to appreciate the similarities and differences between our own and others' minds (Brewer, 1991; Amodio and Frith, 2006) and is critical since accurate mentalizing/empathizing and appropriate social behaviour hinges upon the subtle flexibility in employing context-dependent strategies that may either use the self as the anchor point for modelling others’ minds, but also in instances where de-centring from the self is crucial (Epley et al., 2004).
Individuals with autism spectrum conditions display marked difficulties in reciprocal social interaction. However, while clinically important, focusing exclusively on the interpersonal difficulties in autism may overshadow the importance of the self in underlying such difficulties. Historically, the self has always been integral in defining autism. The word ‘autism’ derives from the Greek word ‘autos’ and literally translates to ‘self’. Early clinical accounts (Kanner, 1943; Asperger, 1944) anecdotally suggested that individuals with autism spectrum conditions are completely self-focused or ‘egocentric in the extreme’. Later work demonstrated that this egocentrism (Frith and de Vignemont, 2005) may be manifest in the lack of viewing oneself as embedded within social contexts (Lee and Hobson, 1998) and via the lack of distinguishing self from other (Loveland and Landry, 1986; Jordan, 1989; Lee et al., 1994; Lee and Hobson, 2006; Mitchell and O'K;eefe, 2008). In addition to this lack of distinguishing self from other, individuals with autism also have marked difficulties in self-referential cognitive processing. These difficulties extend to reflecting on one's; own false beliefs (Baron-Cohen, 1989; Perner et al., 1989; Leslie and Thaiss, 1992; Williams and Happe, 2009), or intentions (Phillips et al., 1998; Williams and Happe, in press), self-conscious emotion recognition and experience (Kasari et al., 1993; Heerey et al., 2003; Hobson et al., 2006), self-referential understanding of emotion (Hill et al., 2004; Lombardo et al., 2007; Silani et al., 2008), autobiographical/episodic memory (Klein et al., 1999; Crane and Goddard, 2008), and marked deficits in the facilitative effect that the self has on memory encoding and retrieval processes (Toichi et al., 2002; Lombardo et al., 2007; Henderson et al., 2009).
The co-occurrence of both egocentrism and impairments in self-referential cognitive processing in autism has led to several ideas that can broadly be characterized under the ‘absent-self’ hypothesis (Hurlburt et al., 1994; Frith and Happe, 1999; Frith, 2003; Happe, 2003; Baron-Cohen, 2005; Frith and de Vignemont, 2005; Hobson et al., 2006). Rather than implying a complete lack of self in autism (as the word ‘absent’ might suggest), the absent-self hypothesis proposes that a specific kind of higher order self-awareness, possibly administering top-down control, may be missing in autism. As observations from patients with focal lesions in the ventromedial prefrontal cortex may suggest, deficits in this type of higher order self-awareness are likely to have detrimental consequences on social behaviour (Beer et al., 2006). One such consequence in autism may be in appreciating the dual nature of oneself in the social world, as an agent who is both similar to, yet different from others (Frith, 2003; Frith and de Vignemont, 2005; Hobson and Meyer, 2005; Hobson et al., 2006). In other words, an intrapersonal deficit in high level self-awareness may be tightly linked to the interpersonal deficits in autism. Thus, the absent-self hypothesis makes two key predictions about the nature of the autistic self and its relation to social impairment. First, neural self-representation may be atypical in autism. Secondly, the atypical organization of neural self-representations may be intrinsically tied to the social impairments in autism.
To test these predictions, we designed a functional magnetic resonance imaging (fMRI) study where participants were scanned while reflecting on the self or a familiar non-close other (the British Queen) in either a mentalistic or a physical way. Quantitative meta-analyses across all normative neuroimaging studies to date that contrast self from other (e.g. Self > Other; see online supplementary material for the meta-analysis) demonstrate that the two most consistent and robust of these Self > Other effects are in the ventromedial prefrontal cortex [peak Montreal Neurological Institute (MNI) coordinate x = −2, y = 42, z = −8] and middle cingulate cortex (peak MNI coordinate x = 0, y = 0, z = 38). Based on this and other studies which find abnormalities in both ventromedial prefrontal cortex (Kennedy et al., 2006; Di Martino et al., 2009) and the middle cingulate cortex (Chiu et al., 2008; Di Martino et al., 2009) in autism, we predicted that these critical areas involved in preferentially coding for self-information are disrupted in autism and that such disruptions are related to the social impairments in autism.
Finally, a growing literature supports the idea that the neural mechanisms underlying autism are due to atypical neural connectivity (Belmonte et al., 2004; Just et al., 2004; Minshew and Williams, 2007). Previous theoretical work on embodied cognition (Barsalou, 1999; Aziz-Zadeh and Damasio, 2008) suggests that the neural mechanisms underlying high level conceptual representations are likely to be tightly integrated (i.e. functionally connected) with lower level ‘embodied’ sensorimotor representations that deal with mirroring of actions, emotions and sensations (Wicker et al., 2003; Keysers et al., 2004; Singer et al., 2004; Blakemore et al., 2005; Gazzola and Keysers, 2009). These theories predict that conceptual representations interact with the same sensorimotor circuitry engaged during the enactment or experience of such concepts (Aziz-Zadeh et al., 2006). Evidence has emerged demonstrating that high level social cognitive processes such as accurate empathic processing engages both medial prefrontal cortex and circuitry involved in low level embodied sensorimotor representations (Zaki et al., 2009). Thus, we predicted that in the typical brain, high level conceptual self-representation within the ventromedial prefrontal cortex should be tightly connected to lower level embodied sensorimotor areas such as somatosensory cortex and frontal operculum/ventral premotor cortex (FO/PMv) (Avenanti et al., 2005, 2007; Iacoboni and Dapretto, 2006; Gazzola and Keysers, 2009). Individuals with autism spectrum conditions have deficits in embodied sensorimotor representations (Dapretto et al., 2006; Cattaneo et al., 2007; Haswell et al., 2009; Minio-Paluello et al., 2009) and it has been speculated that in autism, interactions between embodied neural circuits and high level conceptual representation may be atypical (Iacoboni, 2006; Uddin et al., 2007; Williams, 2008). Therefore, we predicted such connectivity patterns would be reduced in autism spectrum conditions.
Methods
Participants
Thirty-three typically developing male adult participants (mean age 27.97 years ± 6.10 SD, range 18–42) and 33 male adults with autism spectrum conditions (mean age 26.59 years ± 7.04 SD, range 18–41) participated in this study. Both groups were matched on age and all subscales of the Wechsler Abbreviated Scales of Intelligence (Weschler, 1999) (Table 1). Autism spectrum condition participants were all diagnosed by ICD-10 criteria for Asperger Syndrome (ICD-10, 1994). The Toronto Alexithymia Scale (Bagby et al., 1994), Autism Spectrum Quotient (Baron-Cohen et al., 2001), Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994), and module 4 of the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) were administered to participants before the fMRI session. Diagnosis was confirmed for 30/33 participants on the ADI-R. The remaining three participants, who were subthreshold on the ADI-R, were 1 point below the cut-off on the Repetitive Behaviour domain. However, these participants were included since they met ADOS criteria, scored above the cut-off of 26 on the Autism Spectrum Quotient (Woodbury-Smith et al., 2005), and were diagnosed by experienced clinicians. Due to movement artifact (three autism spectrum condition participants) and stimulus delivery equipment malfunction (one autism spectrum condition participant), data for four autism spectrum condition participants were excluded, and the remaining 29 participants are reported in all subsequent analyses. Table 1 shows the participant characteristics. Informed consent was obtained for all participants in accord with procedures approved by the Suffolk Local Research Ethics Committee. All participants were native English speakers with normal or corrected vision and were right-handed.
Participant characteristics
| Variable . | Controls (entire sample) . | ASC (entire sample) . | P-value . | Controls (behaviour matched) . | ASC (behaviour matched) . | P-value . |
|---|---|---|---|---|---|---|
| n | 33 | 29 | 23 | 23 | ||
| Age, years | ||||||
| Range | 18–42 | 18–41 | 18–42 | 18–41 | ||
| Mean (SD) | 27.97 (6.10) | 26.59 (7.04) | 0.42 | 27.91 (6.56) | 25.87 (6.85) | 0.31 |
| IQ | ||||||
| VIQ | 110.79 (12.03) | 112.93 (15.56) | 0.54 | 111.91 (8.24) | 114.87 (14.37) | 0.40 |
| PIQ | 118.52 (11.37) | 112.31 (16.90) | 0.09 | 118.78 (10.64) | 113 (16.68) | 0.17 |
| FIQ | 116.27 (11.63) | 114.14 (16.43) | 0.55 | 117 (8.77) | 115.61 (15.41) | 0.71 |
| ADI-R | ||||||
| Social | N/A | 18.07 (5.07) | N/A | 17.87 (4.73) | ||
| Communication | N/A | 15.17 (4.24) | N/A | 14.83 (3.58) | ||
| Repetitive | N/A | 5.97 (2.76) | N/A | 5.78 (2.71) | ||
| AQ | 15.24 (6.89) | 32.59 (8.20) | 8.19 × 10−13 | 15.30 (7.74) | 31.26 (7.63) | 9.88 × 10−9 |
| TAS | 42.88 (10.66) | 59.28 (9.84) | 4.44 × 10−8 | 42.57 (10.53) | 59.65 (10.72) | 2.12 × 10−6 |
| Variable . | Controls (entire sample) . | ASC (entire sample) . | P-value . | Controls (behaviour matched) . | ASC (behaviour matched) . | P-value . |
|---|---|---|---|---|---|---|
| n | 33 | 29 | 23 | 23 | ||
| Age, years | ||||||
| Range | 18–42 | 18–41 | 18–42 | 18–41 | ||
| Mean (SD) | 27.97 (6.10) | 26.59 (7.04) | 0.42 | 27.91 (6.56) | 25.87 (6.85) | 0.31 |
| IQ | ||||||
| VIQ | 110.79 (12.03) | 112.93 (15.56) | 0.54 | 111.91 (8.24) | 114.87 (14.37) | 0.40 |
| PIQ | 118.52 (11.37) | 112.31 (16.90) | 0.09 | 118.78 (10.64) | 113 (16.68) | 0.17 |
| FIQ | 116.27 (11.63) | 114.14 (16.43) | 0.55 | 117 (8.77) | 115.61 (15.41) | 0.71 |
| ADI-R | ||||||
| Social | N/A | 18.07 (5.07) | N/A | 17.87 (4.73) | ||
| Communication | N/A | 15.17 (4.24) | N/A | 14.83 (3.58) | ||
| Repetitive | N/A | 5.97 (2.76) | N/A | 5.78 (2.71) | ||
| AQ | 15.24 (6.89) | 32.59 (8.20) | 8.19 × 10−13 | 15.30 (7.74) | 31.26 (7.63) | 9.88 × 10−9 |
| TAS | 42.88 (10.66) | 59.28 (9.84) | 4.44 × 10−8 | 42.57 (10.53) | 59.65 (10.72) | 2.12 × 10−6 |
Abbreviations: ASC = Autism Spectrum Condition; VIQ = Verbal IQ; PIQ = Performance IQ; FIQ = Full-Scale IQ; ADI-R = Autism Diagnostic Interview-Revised; AQ = Autism Spectrum Quotient; TAS = Toronto Alexithymia Scale; SD = standard deviation; N/A = not applicable. Data are presented as the mean and standard deviation (in parentheses).
Participant characteristics
| Variable . | Controls (entire sample) . | ASC (entire sample) . | P-value . | Controls (behaviour matched) . | ASC (behaviour matched) . | P-value . |
|---|---|---|---|---|---|---|
| n | 33 | 29 | 23 | 23 | ||
| Age, years | ||||||
| Range | 18–42 | 18–41 | 18–42 | 18–41 | ||
| Mean (SD) | 27.97 (6.10) | 26.59 (7.04) | 0.42 | 27.91 (6.56) | 25.87 (6.85) | 0.31 |
| IQ | ||||||
| VIQ | 110.79 (12.03) | 112.93 (15.56) | 0.54 | 111.91 (8.24) | 114.87 (14.37) | 0.40 |
| PIQ | 118.52 (11.37) | 112.31 (16.90) | 0.09 | 118.78 (10.64) | 113 (16.68) | 0.17 |
| FIQ | 116.27 (11.63) | 114.14 (16.43) | 0.55 | 117 (8.77) | 115.61 (15.41) | 0.71 |
| ADI-R | ||||||
| Social | N/A | 18.07 (5.07) | N/A | 17.87 (4.73) | ||
| Communication | N/A | 15.17 (4.24) | N/A | 14.83 (3.58) | ||
| Repetitive | N/A | 5.97 (2.76) | N/A | 5.78 (2.71) | ||
| AQ | 15.24 (6.89) | 32.59 (8.20) | 8.19 × 10−13 | 15.30 (7.74) | 31.26 (7.63) | 9.88 × 10−9 |
| TAS | 42.88 (10.66) | 59.28 (9.84) | 4.44 × 10−8 | 42.57 (10.53) | 59.65 (10.72) | 2.12 × 10−6 |
| Variable . | Controls (entire sample) . | ASC (entire sample) . | P-value . | Controls (behaviour matched) . | ASC (behaviour matched) . | P-value . |
|---|---|---|---|---|---|---|
| n | 33 | 29 | 23 | 23 | ||
| Age, years | ||||||
| Range | 18–42 | 18–41 | 18–42 | 18–41 | ||
| Mean (SD) | 27.97 (6.10) | 26.59 (7.04) | 0.42 | 27.91 (6.56) | 25.87 (6.85) | 0.31 |
| IQ | ||||||
| VIQ | 110.79 (12.03) | 112.93 (15.56) | 0.54 | 111.91 (8.24) | 114.87 (14.37) | 0.40 |
| PIQ | 118.52 (11.37) | 112.31 (16.90) | 0.09 | 118.78 (10.64) | 113 (16.68) | 0.17 |
| FIQ | 116.27 (11.63) | 114.14 (16.43) | 0.55 | 117 (8.77) | 115.61 (15.41) | 0.71 |
| ADI-R | ||||||
| Social | N/A | 18.07 (5.07) | N/A | 17.87 (4.73) | ||
| Communication | N/A | 15.17 (4.24) | N/A | 14.83 (3.58) | ||
| Repetitive | N/A | 5.97 (2.76) | N/A | 5.78 (2.71) | ||
| AQ | 15.24 (6.89) | 32.59 (8.20) | 8.19 × 10−13 | 15.30 (7.74) | 31.26 (7.63) | 9.88 × 10−9 |
| TAS | 42.88 (10.66) | 59.28 (9.84) | 4.44 × 10−8 | 42.57 (10.53) | 59.65 (10.72) | 2.12 × 10−6 |
Abbreviations: ASC = Autism Spectrum Condition; VIQ = Verbal IQ; PIQ = Performance IQ; FIQ = Full-Scale IQ; ADI-R = Autism Diagnostic Interview-Revised; AQ = Autism Spectrum Quotient; TAS = Toronto Alexithymia Scale; SD = standard deviation; N/A = not applicable. Data are presented as the mean and standard deviation (in parentheses).
Task design
The study design was a 2 × 2 within-subjects factorial block design where participants were asked to make either reflective ‘Mentalizing’ or ‘Physical’ judgements about two target individuals; the ‘Self’ or a familiar non-close ‘Other’ (the British Queen). For self-mentalizing (SM) blocks, participants judged on a scale from 1 to 4 (where 1 = not at all likely and 4 = very likely) how likely they themselves would agree with opinion questions that focused on mental characteristics (e.g. ‘How likely are you to think that keeping a diary is important’). On other-mentalizing (OM) blocks, the same mentalizing judgements were made, except this time it was in reference to how likely the British Queen would agree with the opinion questions (e.g. ‘How likely is the Queen to think that keeping a diary is important’). During self-physical (SP) blocks, participants judged how likely they would agree to opinion questions about their own physical characteristics (e.g. ‘How likely are you to have bony elbows?’). Conversely, the same physical judgements were made during other-physical (OP) blocks, except that participants rated these questions with the Queen as the target person (e.g. ‘How likely is the Queen to have bony elbows’). All opinion questions were acquired from Jason Mitchell's; lab and have been used in previous studies on reflective mentalizing judgements of the self and others that reliably elicit robust and consistent activity in mentalizing and self-referential neural circuits such as ventromedial prefrontal cortex (Mitchell et al., 2006; Jenkins et al., 2008). All stimuli did not differ per condition in the number of characters, syllables, frequency, or valence.
All participants completed one scanning session with one functional imaging run. Within this run there were 20 trials within each condition and five blocks per condition. Each trial type was presented in blocks of four trials and the trial-duration was 4 s each (16 s per block). After each block there was a rest period of 16 s where participants fixated on a cross in the middle of the screen and were instructed to relax. All trials within blocks and all blocks throughout the functional run were presented in pseudorandom order. Stimulus presentation was implemented with DMDX software and the stimulus presentation computer was synchronized with the onset of the functional run to ensure accuracy of event timing.
fMRI acquisition
Imaging was performed on a 3T GE Signa Scanner (General Electric Medical Systems, Milwaukee, WI) at the Cambridge Magnetic Resonance Imaging and Spectroscopy Unit (MRIS Unit). Our functional imaging run consisted of 325 whole-brain functional T2*-weighted echoplanar images (slice thickness, 3 mm; 0.8 mm skip; 33 axial slices; repetition time, 2000 ms; echo time, 30 ms; flip angle 90°; matrix, 64 × 64; field of view, 240 mm, sequential slice acquisition). The first five timepoints of the run were discarded to allow for T2 stabilization effects. In addition, a high-resolution 3D spoiled gradient anatomical image was acquired for each subject for registration purposes.
Data analysis
Behavioural and region of interest data were analysed in SPSS 16 (http://www.spss.com). Functional MRI data preprocessing and statistics were implemented using SPM5 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm). The preprocessing steps were conducted in the following manner: slice timing correction, realignment to the mean functional image, co-registration of the functional data with a high-resolution structural image, segmentation of the structural image, normalization into standard anatomical space (MNI) by applying the transformations estimated from the segmentation step, and spatial smoothing with an 8 mm full width half maximum (FWHM) Gaussian kernel.
Whole-brain statistical analysis was performed using the general linear model in SPM5. Each trial was convolved with the canonical haemodynamic response function. High-pass temporal filtering with a cut-off of 128 s was applied to remove low frequency drift in the time series and global changes were removed by proportional linear scaling. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model of order 1. Factorial contrasts images were outputted automatically in the first level single-subject analysis. To test for a group difference in the interaction effect [(SM > SP) > (OM > OP)] we computed two-sample t-tests on each participant's; interaction effect contrast image from the single-subject analysis. Group differences in main effects were also tested with two-sample t-tests on participant's; main effects contrasts.
All results from whole-brain analyses were thresholded at P < 0.05, false-discovery rate (FDR) corrected, extent 25 voxels. Regions of interest in the middle cingulate cortex and the ventromedial prefrontal cortex were independently selected on the basis of suprathreshold voxels identified from the quantitative meta-analyses (see online Supplementary material for details). Follow up statistical analyses to verify the direction of any voxel-based Group × Condition interaction effects were done by examining on a within-group basis, the percent signal change at the peak voxel from the contrast of interest. This was specifically done because of the inherent limitations in alternatively interpreting between-group differences on any one condition alone. Given that the groups are known to differ on physiological measures (Rumsey et al., 1985; Horwitz et al., 1988; George et al., 1992; Zilbovicius et al., 1995; Haznedar et al., 2000; Ohnishi et al., 2000; Hazlett et al., 2004; Kennedy et al., 2006; Kennedy and Courchesne, 2008a) one cannot assume that groups are equivalent in terms of underlying physiology and blood oxygenated level dependent (BOLD) signal baseline. Thus, follow-up analyses on any interaction effects are done on a within-group basis.
To explore individual differences in social symptom severity and activity we ran correlational analyses of ADI-R and ADOS social subscale scores on self-mentalizing (SM) > other-mentalizing (OM) and self-physical (SP) > other-physical (OP) contrasts within ventromedial prefrontal cortex and middle cingulate cortex regions of interest. Comparison of the difference between correlations from these two contrasts was tested after converting correlation coefficients to z scores with Fisher's; r to z transform (Steiger, 1980).
Functional connectivity analyses were implemented with ‘psychophysiological interaction’ analyses within SPM5 (Friston et al., 1997). The seed region was the ventromedial prefrontal cortex meta-analytic region of interest. Time courses from the seed region were extracted and multiplied by a condition vector of 0, 1 or −1, where self-trials were coded as 1, other-trials were coded as −1, and all other events were 0. The product vector of (time courses × condition vector) was our psychophysiological interaction vector. The seed time course, condition vector, and psychophysiological interaction vector were entered as regressors into individual subject analyses and contrast maps were computed for the psychophysiological interaction regressor. Psychophysiological interaction contrast maps for each participant were entered into a second level random effects group analysis thresholded at P < 0.05 (FDR corrected, extent 25 voxels) across the whole brain. For a priori hypotheses in regards to the somatosensory cortex and the frontal operculum/ventral premotor cortex, regions of interest of Brodmann area 1/2 for somatosensory cortex, and Brodmann area 44 for frontal operculum/ventral premotor cortex, were defined from the SPM Anatomy toolbox (Eickhoff et al., 2005) (see online Supplementary material for details).
Results
Behavioural data
Participants in both groups were matched on age and IQ (Table 1). Replicating prior work (Hill et al., 2004; Lombardo et al., 2007; Silani et al., 2008), we also observed a group difference in alexithymia. Autism spectrum condition participants report significantly more alexithymic traits than controls [controls, 42.88; ASC, 59.28; t(60) = 6.26, P = 4.44 × 10−8].
We conducted separate group (control, ASC) × target (self, other) × judgement (mentalizing, physical) repeated measures ANOVAs for relevance ratings and reaction-times during the fMRI task. The analysis of relevance ratings revealed non-significant 3-way and 2-way interactions (all P > 0.40), indicating that the groups rated the judgements similarly. While the main effect of target was non-significant (P = 0.51), the main effect for Judgement was highly significant [F(1,60) = 171.931, P < 0.0001], such that mentalizing trials were judged to be more relevant than physical trials (Table 2).
fMRI rating and reaction-time data
| Condition . | Controls rating, n = 33 . | ASC rating, n = 29 . | Controls RTa, n = 33 . | ASC RTa, n = 29 . | Controls RTa (behaviour matched), n = 23 . | ASC RTa (behaviour matched), n = 23 . |
|---|---|---|---|---|---|---|
| SM | 2.75 (0.28) | 2.70 (0.30) | 2413.65 (325.83) | 2603.76 (389.61) | 2454.28 (274.34) | 2475.21 (289.80) |
| SP | 2.27 (0.25) | 2.34 (0.31) | 2523.14 (300.49) | 2724.70 (392.14) | 2545.30 (242.47) | 2578.53 (278.09) |
| OM | 2.65 (0.24) | 2.73 (0.29) | 2564.49 (338.17) | 2671.83 (366.28) | 2579.54 (280.93) | 2561.39 (243.02) |
| OP | 2.27 (0.18) | 2.32 (0.25) | 2565.45 (331.05) | 2665.93 (388.93) | 2573.29 (247.54) | 2564.47 (299.74) |
| Condition . | Controls rating, n = 33 . | ASC rating, n = 29 . | Controls RTa, n = 33 . | ASC RTa, n = 29 . | Controls RTa (behaviour matched), n = 23 . | ASC RTa (behaviour matched), n = 23 . |
|---|---|---|---|---|---|---|
| SM | 2.75 (0.28) | 2.70 (0.30) | 2413.65 (325.83) | 2603.76 (389.61) | 2454.28 (274.34) | 2475.21 (289.80) |
| SP | 2.27 (0.25) | 2.34 (0.31) | 2523.14 (300.49) | 2724.70 (392.14) | 2545.30 (242.47) | 2578.53 (278.09) |
| OM | 2.65 (0.24) | 2.73 (0.29) | 2564.49 (338.17) | 2671.83 (366.28) | 2579.54 (280.93) | 2561.39 (243.02) |
| OP | 2.27 (0.18) | 2.32 (0.25) | 2565.45 (331.05) | 2665.93 (388.93) | 2573.29 (247.54) | 2564.47 (299.74) |
Abbreviations: SM = self-mentalizing; OM = other-mentalizing; SP = self-physical; OP = other-physical; ASC = autism spectrum condition. Data are given as the mean and standard deviation (in parentheses).
a Reaction-time data is in milliseconds.
fMRI rating and reaction-time data
| Condition . | Controls rating, n = 33 . | ASC rating, n = 29 . | Controls RTa, n = 33 . | ASC RTa, n = 29 . | Controls RTa (behaviour matched), n = 23 . | ASC RTa (behaviour matched), n = 23 . |
|---|---|---|---|---|---|---|
| SM | 2.75 (0.28) | 2.70 (0.30) | 2413.65 (325.83) | 2603.76 (389.61) | 2454.28 (274.34) | 2475.21 (289.80) |
| SP | 2.27 (0.25) | 2.34 (0.31) | 2523.14 (300.49) | 2724.70 (392.14) | 2545.30 (242.47) | 2578.53 (278.09) |
| OM | 2.65 (0.24) | 2.73 (0.29) | 2564.49 (338.17) | 2671.83 (366.28) | 2579.54 (280.93) | 2561.39 (243.02) |
| OP | 2.27 (0.18) | 2.32 (0.25) | 2565.45 (331.05) | 2665.93 (388.93) | 2573.29 (247.54) | 2564.47 (299.74) |
| Condition . | Controls rating, n = 33 . | ASC rating, n = 29 . | Controls RTa, n = 33 . | ASC RTa, n = 29 . | Controls RTa (behaviour matched), n = 23 . | ASC RTa (behaviour matched), n = 23 . |
|---|---|---|---|---|---|---|
| SM | 2.75 (0.28) | 2.70 (0.30) | 2413.65 (325.83) | 2603.76 (389.61) | 2454.28 (274.34) | 2475.21 (289.80) |
| SP | 2.27 (0.25) | 2.34 (0.31) | 2523.14 (300.49) | 2724.70 (392.14) | 2545.30 (242.47) | 2578.53 (278.09) |
| OM | 2.65 (0.24) | 2.73 (0.29) | 2564.49 (338.17) | 2671.83 (366.28) | 2579.54 (280.93) | 2561.39 (243.02) |
| OP | 2.27 (0.18) | 2.32 (0.25) | 2565.45 (331.05) | 2665.93 (388.93) | 2573.29 (247.54) | 2564.47 (299.74) |
Abbreviations: SM = self-mentalizing; OM = other-mentalizing; SP = self-physical; OP = other-physical; ASC = autism spectrum condition. Data are given as the mean and standard deviation (in parentheses).
a Reaction-time data is in milliseconds.
Analysis of reaction-times revealed non-significant 3-way group × target × judgement (P = 0.77) and group × judgement interactions (P = 0.95). There was however, a significant group × target interaction [F(1,60) = 6.088, P = 0.016] and main effects of target [F(1,60) = 7.383, P = 0.009] and judgement [F(1,60) = 11.379, P = 0.001]. The group × target interaction was due to the control group responding faster for self-judgements than the autism spectrum condition group, while the main effect of Judgement was due to faster responses to mentalizing judgements (Table 2). However, given the unconstrained nature of the task (i.e. no explicit instruction to respond as quickly as possible), reaction-time cannot necessarily be assumed to be a measure of ‘efficient performance’ on the task and is unlikely to affect the fMRI data comparisons. To check this, we pair-wise matched participants in each group based on reaction-time during the task. This procedure resulted in 23 participants in each group (still matched on age and IQ, Table 1) and eliminated the group × target interaction in reaction-time. This subset of reaction-time-matched participants produced identical fMRI results to those of the entire sample. Thus, similar to other studies that find no effects of reaction-time on activity elicited during similar tasks (Mitchell et al., 2006; Pfeifer et al., 2007), we can rule out any interpretation of the fMRI group differences as simply a function of reaction-time group differences (see online Supplementary material).
fMRI data: Atypical neural self-representation
Our first analysis evaluated group differences in the interaction effect among all four conditions [e.g. (SM > SP) > (OM > OP)]. Given the a priori hypothesis for the middle cingulate cortex and the ventromedial prefrontal cortex, we ran region of interest analyses using the meta-analytic regions of interest as the search space and employing false-discovery rate (FDR) small volume correction (SVC) within each region of interest. This analysis revealed a significant group difference (controls > ASC) in the interaction effect contrast images within the middle cingulate cortex (Brodmann area 24, MNI x = 2, y = 4, z = 42, t = 3.27, P = 0.016 FDR, small volume corrected) but not the ventromedial prefrontal cortex. The middle cingulate cortex peak was six voxels away in Euclidean distance from the meta-analysis peak of x = 0, y = 0, z = 38.
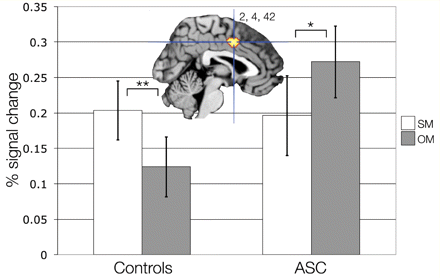
Based on a previous study (Chiu et al., 2008) that observed marked reductions in middle cingulate cortex activity during self-decisions in a social context, we hypothesized that this effect was due to atypical recruitment of the middle cingulate cortex specifically during self-mentalizing. To examine this, signal change was extracted from the peak voxel in the middle cingulate cortex and planned comparisons between self-mentalizing and other-mentalizing were made separately for each group with paired samples t-tests. In the autism spectrum condition group there was reduced activation during self-mentalizing compared with other-mentalizing [t(28) = 2.18, P = 0.04] while controls showed increased activation during self-mentalizing compared with other-mentalizing [t(32) = 3.118, P = 0.004] (Fig. 1 and Table 3, panel a). A whole-brain analysis corrected for multiple comparisons across the whole brain (P < 0.05, FDR corrected) revealed no other significant group differences.
Activation in middle cingulate cortex for self-mentalizing (SM) compared with other-mentalizing (OM) (SM > OM). This figure shows group differences in the middle cingulate cortex response (MNI x = 2, y = 4, z = 42; thresholded at P < 0.005, uncorrected for display purposes) to SM compared with OM (SM > OM) for controls (left) and autism spectrum conditions (ASC) (right). Error bars indicate ±1 SEM. **P < 0.005; *P < 0.05. Note that percent signal change values cannot be assumed to represent equivalent values between-groups, because groups may differ in their underlying physiological baseline level of activity.
fMRI activation results
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls > ASC (SM > SP) > (OM > OP) interaction effecta | ||||||
| Middle cingulate cortex | B | 24 | 2, 4, 42 | 3.27 | 0.016 | |
| B | 24 | −4, 2, 36 | 3.20 | 0.016 | ||
| B | 24 | 6, −10, 46 | 2.53 | 0.023 | ||
| B | 24 | 2, −22, 42 | 2.26 | 0.029 | ||
| Panel b: Controls Self > Other | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −6, 50, –6 | 7.06 | 0.001 | 287 |
| 10 | −4, 62, 2 | 5.38 | 0.006 | |||
| Cerebellum lobe crus I | R | 22, −82, −24 | 5.43 | 0.005 | 42 | |
| Panel c: Controls > ASC Self > Othera | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −4, 46, –10 | 4.12 | 0.016 |
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls > ASC (SM > SP) > (OM > OP) interaction effecta | ||||||
| Middle cingulate cortex | B | 24 | 2, 4, 42 | 3.27 | 0.016 | |
| B | 24 | −4, 2, 36 | 3.20 | 0.016 | ||
| B | 24 | 6, −10, 46 | 2.53 | 0.023 | ||
| B | 24 | 2, −22, 42 | 2.26 | 0.029 | ||
| Panel b: Controls Self > Other | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −6, 50, –6 | 7.06 | 0.001 | 287 |
| 10 | −4, 62, 2 | 5.38 | 0.006 | |||
| Cerebellum lobe crus I | R | 22, −82, −24 | 5.43 | 0.005 | 42 | |
| Panel c: Controls > ASC Self > Othera | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −4, 46, –10 | 4.12 | 0.016 |
Abbreviations: ASC = autism spectrum conditions; Hemi = hemisphere; L = left; R = right; B = bilateral; BA = Brodmann area; MNI = Montreal Neurological Institute; FDR = false-discovery rate; SM = self-mentalizing; SP = self-physical; OM = other-mentalizing; OP = other-physical.
a Results are from a priori region of interest analyses using FDR small volume correction at P < 0.05.
fMRI activation results
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls > ASC (SM > SP) > (OM > OP) interaction effecta | ||||||
| Middle cingulate cortex | B | 24 | 2, 4, 42 | 3.27 | 0.016 | |
| B | 24 | −4, 2, 36 | 3.20 | 0.016 | ||
| B | 24 | 6, −10, 46 | 2.53 | 0.023 | ||
| B | 24 | 2, −22, 42 | 2.26 | 0.029 | ||
| Panel b: Controls Self > Other | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −6, 50, –6 | 7.06 | 0.001 | 287 |
| 10 | −4, 62, 2 | 5.38 | 0.006 | |||
| Cerebellum lobe crus I | R | 22, −82, −24 | 5.43 | 0.005 | 42 | |
| Panel c: Controls > ASC Self > Othera | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −4, 46, –10 | 4.12 | 0.016 |
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls > ASC (SM > SP) > (OM > OP) interaction effecta | ||||||
| Middle cingulate cortex | B | 24 | 2, 4, 42 | 3.27 | 0.016 | |
| B | 24 | −4, 2, 36 | 3.20 | 0.016 | ||
| B | 24 | 6, −10, 46 | 2.53 | 0.023 | ||
| B | 24 | 2, −22, 42 | 2.26 | 0.029 | ||
| Panel b: Controls Self > Other | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −6, 50, –6 | 7.06 | 0.001 | 287 |
| 10 | −4, 62, 2 | 5.38 | 0.006 | |||
| Cerebellum lobe crus I | R | 22, −82, −24 | 5.43 | 0.005 | 42 | |
| Panel c: Controls > ASC Self > Othera | ||||||
| ventromedial prefrontal cortex | L | 10/11 | −4, 46, –10 | 4.12 | 0.016 |
Abbreviations: ASC = autism spectrum conditions; Hemi = hemisphere; L = left; R = right; B = bilateral; BA = Brodmann area; MNI = Montreal Neurological Institute; FDR = false-discovery rate; SM = self-mentalizing; SP = self-physical; OM = other-mentalizing; OP = other-physical.
a Results are from a priori region of interest analyses using FDR small volume correction at P < 0.05.
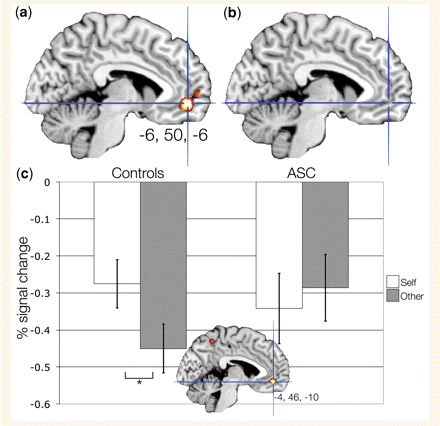
Next, we examined the main effect of increased activation during self-judgements compared with other-judgements (Self > Other) collapsing across mentalizing and physical judgements. Whole-brain analyses within the control group revealed that the ventromedial prefrontal cortex (Brodmann area 10/11) and cerebellum was activated more for self than other (Fig. 2a and Table 3, panel b). In contrast, within the autism spectrum condition group there was no significant difference in activation for self compared with other (Fig. 2b). Given the a priori hypotheses regarding group differences in the ventromedial prefrontal cortex and the middle cingulate cortex, we next ran a region of interest analysis constrained to a search space within the meta-analytic regions of interest. Hypoactivation in the autism spectrum condition group (e.g. controls > ASC) for this contrast (Self > Other) was found in the ventromedial prefrontal cortex (Brodmann area 10/11, MNI x = −4, y = 46, z = −10, t = 4.12, P = 0.016 FDR, small volume corrected) but not the middle cingulate cortex. This ventromedial prefrontal cortex peak was 6.63 voxels away in Euclidean distance from the meta-analysis peak of x = −2, y = 42, z = −8.
Activation in ventromedial prefrontal cortex (vMPFC) for self-compared with other-judgements (Self > Other). This figure displays activation for the Self > Other contrast within (a) controls, (b) ASC (both displayed at P < 0.05, FDR corrected). Panel (c) shows the group difference in activation for Controls > ASC during Self > Other (MNI x = −4, y = 46, z = −10; thresholded at P < 0.005, uncorrected for display purposes). The bar graph depicts the group difference in activation (controls, left; ASC, right). Error bars indicate ±1 SEM. *P < 0.0005. Note that percent signal change values cannot be assumed to represent equivalent values between-groups, because groups may differ in their underlying physiological baseline level of activity.
To further qualify the nature of this group × target effect in ventromedial prefrontal cortex we extracted the signal change from the peak voxel and specifically compared self with other in each group separately. The ventromedial prefrontal cortex was more active in controls for self compared with other-judgements [F(1,32) = 17.43, P = 2.13 × 10−4], while within the autism spectrum condition group, ventromedial prefrontal cortex activation was equivalent across self- or other-judgements [F(1,28) = 0.808, P = 0.376] (Fig. 2c and Table 3, panel c). This effect remained in the subset of behaviour-matched participants (see online Supplementary material). Whole-brain analyses corrected for multiple comparisons across the whole brain (P < 0.05, FDR corrected) revealed no other significant group differences.
Following these analyses, we flipped the main effect of target in order to examine group differences in other-referential compared with self-referential processing (e.g. Other > Self). Controls recruit virtually identical regions to those of the autism spectrum group (Supplementary Table S2, panels a and b) and the analysis of group differences on this contrast revealed no significant differences across the whole brain. This analysis demonstrates that the groups do not differ in the neural systems recruited preferentially for thinking about others compared with oneself.
Altered functional connectivity during self-referential cognition
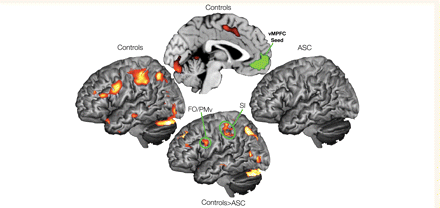
In our functional connectivity analyses, we employed psychophysiological interaction analyses to determine which areas show greater changes in functional connectivity during self-judgements compared with other-judgements. Specifically, we looked at connectivity from the ventromedial prefrontal cortex using the Self > Other meta-analysis region of interest as the seed region. Controls exhibited greater ventromedial prefrontal cortex connectivity during self-judgements compared with other-judgements within a wide distribution of regions, comprising frontal operculum/ventral premotor cortex, somatosensory cortex and middle cingulate cortex extending into caudal anterior cingulate cortex, intraparietal sulcus, visual cortex/cerebellum, temporal pole, anterior temporal lobe and middle temporal gyrus (Fig. 3 and Table 4, panel a). In the autism spectrum condition group, no significant results were found at the whole-brain corrected P < 0.05 FDR threshold. Given the specific hypotheses with regards to group differences in ventromedial prefrontal cortex connectivity to somatosensory cortex and frontal operculum/ventral premotor cortex, we ran region of interest analyses within anatomical regions of somatosensory cortex and frontal operculum/ventral premotor cortex. These analyses revealed that the control participants had greater functional connectivity compared with autism spectrum conditions during self-judgements compared with connectivity during other-judgements (P < 0.05 FDR, small volume corrected) (Fig. 3 and Table 4, panel b). No other group differences in connectivity were observed after whole-brain correction for multiple comparisons (P < 0.05 FDR corrected).
Functional connectivity in ventromedial prefrontal cortex during self-judgements compared with other-judgements (Self > Other). This figure displays increases in ventromedial prefrontal cortex (seed region labelled in green) connectivity for self-judgements compared with connectivity during other-judgements (Self > Other). Connectivity within the control group is displayed on the left and top middle brains, while connectivity within the ASC group is displayed on the right (both displayed at P < 0.05 FDR corrected). Group differences (Controls > ASC) in connectivity (bottom middle) are displayed at P < 0.005, uncorrected for display purposes.
fMRI functional connectivity (PPI) results
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls, vMPFC PPI, Self > Other | ||||||
| Cerebellum lobe crus I | L | −36, −78, –26 | 5.21 | 0.017 | 3662 | |
| Lingual gyrus | R | 18 | 16, −82, −10 | 4.78 | 0.017 | |
| Cerebellum lobe crus I | L | −40, −82, –20 | 4.76 | 0.017 | ||
| Intraparietal sulcus (IPS) | L | 7 | −30, −62, 52 | 4.87 | 0.017 | 3321 |
| Primary somatosensory cortex (SI) | L | 1/2 | −48, −34, 54 | 4.82 | 0.017 | |
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −48, 4, 32 | 4.76 | 0.017 | |
| Temporal pole | L | 38 | −34, 20, –24 | 4.70 | 0.017 | 108 |
| Middle temporal gyrus | L | 20 | −54, −22, –14 | 4.22 | 0.017 | 143 |
| L | 20 | −56, −36, –12 | 3.42 | 0.031 | ||
| Anterior inferior temporal gyrus | L | 20 | −44, 2, –40 | 4.16 | 0.017 | 27 |
| L | 20 | −36, 8, –38 | 3.13 | 0.044 | ||
| Intraparietal sulcus (IPS) | R | 7 | 36, −74, 38 | 4.05 | 0.018 | 133 |
| R | 7 | 18, −72, 46 | 3.37 | 0.033 | ||
| Middle cingulate cortex | B | 23/24 | −4, −4, 50 | 3.88 | 0.019 | 239 |
| Caudal anterior cingulate cortex | B | 24 | 0, 16, 40 | 3.55 | 0.027 | |
| Cerebellum lobe IV | L | −6, −50, 0 | 3.78 | 0.021 | 79 | |
| Middle occipital gyrus | L | 37 | −46, −72, 6 | 3.58 | 0.026 | 31 |
| Middle temporal gyrus | L | 21 | −52, −46, 2 | 3.50 | 0.029 | 46 |
| Posterior middle temporal gyrus | L | 37 | −56, −58, 2 | 3.43 | 0.031 | 30 |
| Panel b: Controls > ASC, vMPFC PPI, Self > Othera | ||||||
| Primary somatosensory cortex (SI) | L | 1/2 | −38, −32, 44 | 3.14 | 0.041 | |
| −40, −30, 60 | 3.02 | 0.041 | ||||
| −40, −26, 52 | 2.94 | 0.041 | ||||
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −46, 6, 28 | 3.02 | 0.053 | |
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls, vMPFC PPI, Self > Other | ||||||
| Cerebellum lobe crus I | L | −36, −78, –26 | 5.21 | 0.017 | 3662 | |
| Lingual gyrus | R | 18 | 16, −82, −10 | 4.78 | 0.017 | |
| Cerebellum lobe crus I | L | −40, −82, –20 | 4.76 | 0.017 | ||
| Intraparietal sulcus (IPS) | L | 7 | −30, −62, 52 | 4.87 | 0.017 | 3321 |
| Primary somatosensory cortex (SI) | L | 1/2 | −48, −34, 54 | 4.82 | 0.017 | |
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −48, 4, 32 | 4.76 | 0.017 | |
| Temporal pole | L | 38 | −34, 20, –24 | 4.70 | 0.017 | 108 |
| Middle temporal gyrus | L | 20 | −54, −22, –14 | 4.22 | 0.017 | 143 |
| L | 20 | −56, −36, –12 | 3.42 | 0.031 | ||
| Anterior inferior temporal gyrus | L | 20 | −44, 2, –40 | 4.16 | 0.017 | 27 |
| L | 20 | −36, 8, –38 | 3.13 | 0.044 | ||
| Intraparietal sulcus (IPS) | R | 7 | 36, −74, 38 | 4.05 | 0.018 | 133 |
| R | 7 | 18, −72, 46 | 3.37 | 0.033 | ||
| Middle cingulate cortex | B | 23/24 | −4, −4, 50 | 3.88 | 0.019 | 239 |
| Caudal anterior cingulate cortex | B | 24 | 0, 16, 40 | 3.55 | 0.027 | |
| Cerebellum lobe IV | L | −6, −50, 0 | 3.78 | 0.021 | 79 | |
| Middle occipital gyrus | L | 37 | −46, −72, 6 | 3.58 | 0.026 | 31 |
| Middle temporal gyrus | L | 21 | −52, −46, 2 | 3.50 | 0.029 | 46 |
| Posterior middle temporal gyrus | L | 37 | −56, −58, 2 | 3.43 | 0.031 | 30 |
| Panel b: Controls > ASC, vMPFC PPI, Self > Othera | ||||||
| Primary somatosensory cortex (SI) | L | 1/2 | −38, −32, 44 | 3.14 | 0.041 | |
| −40, −30, 60 | 3.02 | 0.041 | ||||
| −40, −26, 52 | 2.94 | 0.041 | ||||
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −46, 6, 28 | 3.02 | 0.053 | |
Abbreviations: ASC = autism spectrum conditions; PPI = psychophysiological interaction; Hemi = Hemisphere; L = Left; R = Right; B = Bilateral; BA = Brodmann area; MNI = Montreal Neurological Institute; FDR = false discovery rate; vMPFC = ventromedial prefrontal cortex.
Results are from a priori region of interest analyses using FDR SVC at P < 0.05.
fMRI functional connectivity (PPI) results
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls, vMPFC PPI, Self > Other | ||||||
| Cerebellum lobe crus I | L | −36, −78, –26 | 5.21 | 0.017 | 3662 | |
| Lingual gyrus | R | 18 | 16, −82, −10 | 4.78 | 0.017 | |
| Cerebellum lobe crus I | L | −40, −82, –20 | 4.76 | 0.017 | ||
| Intraparietal sulcus (IPS) | L | 7 | −30, −62, 52 | 4.87 | 0.017 | 3321 |
| Primary somatosensory cortex (SI) | L | 1/2 | −48, −34, 54 | 4.82 | 0.017 | |
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −48, 4, 32 | 4.76 | 0.017 | |
| Temporal pole | L | 38 | −34, 20, –24 | 4.70 | 0.017 | 108 |
| Middle temporal gyrus | L | 20 | −54, −22, –14 | 4.22 | 0.017 | 143 |
| L | 20 | −56, −36, –12 | 3.42 | 0.031 | ||
| Anterior inferior temporal gyrus | L | 20 | −44, 2, –40 | 4.16 | 0.017 | 27 |
| L | 20 | −36, 8, –38 | 3.13 | 0.044 | ||
| Intraparietal sulcus (IPS) | R | 7 | 36, −74, 38 | 4.05 | 0.018 | 133 |
| R | 7 | 18, −72, 46 | 3.37 | 0.033 | ||
| Middle cingulate cortex | B | 23/24 | −4, −4, 50 | 3.88 | 0.019 | 239 |
| Caudal anterior cingulate cortex | B | 24 | 0, 16, 40 | 3.55 | 0.027 | |
| Cerebellum lobe IV | L | −6, −50, 0 | 3.78 | 0.021 | 79 | |
| Middle occipital gyrus | L | 37 | −46, −72, 6 | 3.58 | 0.026 | 31 |
| Middle temporal gyrus | L | 21 | −52, −46, 2 | 3.50 | 0.029 | 46 |
| Posterior middle temporal gyrus | L | 37 | −56, −58, 2 | 3.43 | 0.031 | 30 |
| Panel b: Controls > ASC, vMPFC PPI, Self > Othera | ||||||
| Primary somatosensory cortex (SI) | L | 1/2 | −38, −32, 44 | 3.14 | 0.041 | |
| −40, −30, 60 | 3.02 | 0.041 | ||||
| −40, −26, 52 | 2.94 | 0.041 | ||||
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −46, 6, 28 | 3.02 | 0.053 | |
| Anatomical label . | Hemi . | BA . | MNI (x, y, z) . | t-value . | P(FDR) . | Cluster size . |
|---|---|---|---|---|---|---|
| Panel a: Controls, vMPFC PPI, Self > Other | ||||||
| Cerebellum lobe crus I | L | −36, −78, –26 | 5.21 | 0.017 | 3662 | |
| Lingual gyrus | R | 18 | 16, −82, −10 | 4.78 | 0.017 | |
| Cerebellum lobe crus I | L | −40, −82, –20 | 4.76 | 0.017 | ||
| Intraparietal sulcus (IPS) | L | 7 | −30, −62, 52 | 4.87 | 0.017 | 3321 |
| Primary somatosensory cortex (SI) | L | 1/2 | −48, −34, 54 | 4.82 | 0.017 | |
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −48, 4, 32 | 4.76 | 0.017 | |
| Temporal pole | L | 38 | −34, 20, –24 | 4.70 | 0.017 | 108 |
| Middle temporal gyrus | L | 20 | −54, −22, –14 | 4.22 | 0.017 | 143 |
| L | 20 | −56, −36, –12 | 3.42 | 0.031 | ||
| Anterior inferior temporal gyrus | L | 20 | −44, 2, –40 | 4.16 | 0.017 | 27 |
| L | 20 | −36, 8, –38 | 3.13 | 0.044 | ||
| Intraparietal sulcus (IPS) | R | 7 | 36, −74, 38 | 4.05 | 0.018 | 133 |
| R | 7 | 18, −72, 46 | 3.37 | 0.033 | ||
| Middle cingulate cortex | B | 23/24 | −4, −4, 50 | 3.88 | 0.019 | 239 |
| Caudal anterior cingulate cortex | B | 24 | 0, 16, 40 | 3.55 | 0.027 | |
| Cerebellum lobe IV | L | −6, −50, 0 | 3.78 | 0.021 | 79 | |
| Middle occipital gyrus | L | 37 | −46, −72, 6 | 3.58 | 0.026 | 31 |
| Middle temporal gyrus | L | 21 | −52, −46, 2 | 3.50 | 0.029 | 46 |
| Posterior middle temporal gyrus | L | 37 | −56, −58, 2 | 3.43 | 0.031 | 30 |
| Panel b: Controls > ASC, vMPFC PPI, Self > Othera | ||||||
| Primary somatosensory cortex (SI) | L | 1/2 | −38, −32, 44 | 3.14 | 0.041 | |
| −40, −30, 60 | 3.02 | 0.041 | ||||
| −40, −26, 52 | 2.94 | 0.041 | ||||
| Frontal operculum/ventral premotor cortex (FO/PMv) | L | 44 | −46, 6, 28 | 3.02 | 0.053 | |
Abbreviations: ASC = autism spectrum conditions; PPI = psychophysiological interaction; Hemi = Hemisphere; L = Left; R = Right; B = Bilateral; BA = Brodmann area; MNI = Montreal Neurological Institute; FDR = false discovery rate; vMPFC = ventromedial prefrontal cortex.
Results are from a priori region of interest analyses using FDR SVC at P < 0.05.
Relationships with social symptom severity
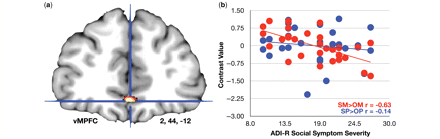
Next, we examined whether individual differences in social symptom severity in autism spectrum conditions (as measured on the ADI-R and ADOS) correlated with activity either during self-mentalizing (SM) > other-mentalizing (OM) or self-physical (SP) > other-physical (OP). The meta-analytic middle cingulate cortex and ventromedial prefrontal cortex regions of interest were used as the search space for this analysis and significant results passed the FDR small volume corrected threshold. ADOS and ADI-R social symptom severity did not correlate with middle cingulate cortex activity. Furthermore, ventromedial prefrontal cortex activity was not correlated with ADOS social symptom severity. However, ADI-R social symptom severity was negatively correlated with ventromedial prefrontal cortex activity during SM > OM (MNI x = 2, y = 44, z = −12, t = 4.21, r = −0.63, P = 1.27 × 10−4). This result indicates that those individuals with autism spectrum conditions whose ventromedial prefrontal cortex made the biggest distinction between self-mentalizing and other-mentalizing were the least socially impaired in early childhood, while those whose ventromedial prefrontal cortex made little to no distinction between self-mentalizing and other-mentalizing were the most socially impaired in early childhood. During SP > OP, no correlation was observed between ventromedial prefrontal cortex and ADI-R social symptom severity (MNI x = 2, y = 44, z = −12, t = 0.75, r = −0.14, P = 0.20). The difference between these two correlations (i.e. SM > OM versus SP > OP) was significant (z = −2.17, P = 0.03) (Fig. 4a and b).
Individual differences in ventromedial prefrontal cortex (vMPFC) self-other distinction and early childhood social symptom severity. This figure displays (a) the vMPFC region (MNI x = 2, y = 44, z = −12) that is correlated with early childhood social symptom severity (measured by the ADI-R). (b) The correlation for the contrasts of self-mentalizing compared with other-mentalizing (SM > OM; red dots) and self-physical compared with other-physical (SP > OP; blue dots).
Discussion
Theory and prior research suggest that the neural systems involved in self-representation are atypically organized in autism. In the current study, we directly tested this hypothesis in 33 neurotypical male adults and 29 age and IQ-matched individuals with autism spectrum conditions. We observed specific disruptions in the neural systems involved in preferentially coding for self-information. The first of these effects was in the middle cingulate cortex. Rather than preferentially responding to self-mentalizing, the middle cingulate cortex in autism responds more to other-mentalizing. These observations replicate and extend prior work showing reduced activity in the middle cingulate cortex when making self-decisions in a social context (Chiu et al., 2008). While the previous study could not discern whether such effects in the middle cingulate cortex were due to self-mentalizing, other-mentalizing, or both (Frith and Frith, 2008), the current study was able to separate both processes independently of each other. This separation highlights that the disruption of middle cingulate cortex function is due to reversal of the typical SM > OM effect in the middle cingulate cortex, such that individuals with autism spectrum conditions recruit this region more for other-mentalizing than self-mentalizing (OM > SM).
The reversal of the middle cingulate cortex's preferential response to self (e.g. OM > SM) is intriguing given that the middle cingulate cortex responds preferentially to self-relevant information (Moran et al., 2006) and shows Self > Other effects during trait reflection (Gutchess et al., 2007; Ersner-Hershfield et al., 2009), visual perspective taking (Vogeley et al., 2004; David et al., 2006), empathy for pain (Singer et al., 2004; Jackson et al., 2006; Lamm et al., 2007) and self-decisions in a social context (King-Casas et al., 2006; Tomlin et al., 2006; Chiu et al., 2008). Furthermore, studies on similar or dissimilar others observed that the middle cingulate cortex responds more to others who are similar to self than to others who are dissimilar (Mitchell et al., 2006; Lamm et al., in press). Middle cingulate cortex activity also tracks with learning parameters that indicate how one’s own actions influence others (Hampton et al., 2008).
It is also notable that the middle cingulate cortex has been found to be both structurally and functionally atypical in autism. In a post-mortem neuropathological study, Vargas et al. (2005) found increased neuroglial activation and presence of inflammatory cytokines in the middle cingulate cortex, suggesting the presence of neuroinflammation. In recent quantitative meta-analyses of fMRI studies in autism, the middle cingulate cortex is an area of consistent hypoactivation across the literature of studies tapping social processes, but is not hypo- or hyperactive during non-social processes (Di Martino et al., 2009). Given these considerations, the altered role of the middle cingulate cortex in autism is critical and future research investigating this region is likely to render new insights into the neural basis of autism.
The second observation of the current study is the complete lack of preferential responsiveness to self-information in the ventromedial prefrontal cortex of individuals with autism. While controls significantly recruited the ventromedial prefrontal cortex more for self than other, individuals with autism did not. Instead, the ventromedial prefrontal cortex treated self and other equivalently in autism. This equivalence for self and other is striking since previous behavioural studies have shown that individuals with autism do not benefit from processing information in self-relevant ways. Three studies have now shown a reduced or absent self-reference effect in memory in autism (Toichi et al., 2002; Lombardo et al., 2007; Henderson et al., 2009). The current study extends this observation by showing a lack of a ‘neural self-reference effect’ in the ventromedial prefrontal cortex.
The ventromedial prefrontal cortex result from the current study differs from a previous study where no group differences in Self > Other activity were found (Kennedy and Courchesne, 2008a). However, it is worth noting that the Kennedy and Courchesne study may not have been sensitive enough to detect a group difference primarily because the ‘other’ person was someone close to the participants (i.e. one's; mother). Prior research (Schmitz et al., 2004; Seger et al., 2004; Ochsner et al., 2005; Mitchell et al., 2006; Jenkins et al., 2008) demonstrates that thinking about others who share significant amounts of self-relevant information may reduce the power to elicit a neural distinction between self and other in the ventromedial prefrontal cortex. The current study uses a familiar but non-close other, and the inclusion of this type of person as the ‘other’ condition increases the reliability in consistently eliciting such an effect in the general population. Thus given the current study's; manipulation between self and other was designed to be an obviously large difference, it is all the more intriguing that such a manipulation does not elicit the Self > Other effect in the ventromedial prefrontal cortex of individuals with autism.
However, the lack of a neural self-other distinction in the ventromedial prefrontal cortex of autism does not mean that individuals with autism do not recruit areas that code for such a distinction. On the contrary, in areas of the brain where self and other are distinguished via its preferential response to other-information (i.e. Other > Self), both controls and autism spectrum conditions activated these regions similarly. Thus, there is specificity in the deficit for neurally distinguishing self from other. In autism, these deficits only occur in areas that preferentially respond to self-information. This specificity in the lack of such a mechanism like ventromedial prefrontal cortex for preferentially coding self-information confirms predictions made by the absent-self theory and sheds new insight into the nature of the autistic self. The neural deficit in self-representation may also be crucial for explaining the simultaneous presence of both impaired self-referential cognition and the self-other equivalence that appears on the surface to be egocentrism.
While the ventromedial prefrontal cortex is key for distinguishing self from other, it clearly does not work alone. We observed within our control sample that the neural circuit functionally connected with the ventromedial prefrontal cortex during high-level conceptual self-processing (compared with other-processing) was distributed across lower level sensorimotor regions (frontal operculum/ventral premotor cortex, somatosensory cortex) involved in embodied processes essential for sensation/perception and action. These findings lend support for the broader idea that building high-level conceptual self-representations relies on the coordination of information from lower level embodied sensorimotor systems (Barsalou, 1999; Aziz-Zadeh and Damasio, 2008). In contrast, individuals with autism spectrum conditions do not show any areas where ventromedial prefrontal cortex connectivity is stronger during self-judgements compared with other-judgements. Thus, in addition to not distinguishing self from other in the ventromedial prefrontal cortex, self-representation deficits in autism extend across a crucial neural circuit that coordinates conceptual self-processing with lower level embodied representations.
To add to the mounting evidence for the critical role of the ventromedial prefrontal cortex in neural self-representation in autism, the current study demonstrates a tight link between atypical neural self-representation and the social impairments in autism. The magnitude of distinguishing self from other in the ventromedial prefrontal cortex was related to the magnitude of early childhood social impairments. Individuals with the greatest social impairments in early childhood showed the least ventromedial prefrontal cortex self-other mentalizing distinction, while the least socially impaired individuals showed the largest ventromedial prefrontal cortex self-other mentalizing distinction. Thus, the marked deficits in neural self-representation are strongly linked to the early social impairments in autism.
While specifying the directionality of such a relationship solely on the basis of the current data may be difficult (i.e. does a self-deficit lead to social deficits or vice versa?), it is worth stating developmental considerations that may shed light on such a relationship. First, Meltzoff has proposed that the starting state for social cognition is one where infants take the stance that others are ‘like me’ (Meltzoff, 2007; Meltzoff and Brooks, 2008). However, as development progresses, social cognitive ability invariably develops past the simple acknowledgement of self-other equivalence and into a simultaneous or ‘dual’ understanding that self can also be different from others. Much of what is known of later developing social cognition is predicated on this push and pull between similarities and differences between self and other (Brewer, 1991; Banaji and Prentice, 1994; Nickerson, 1999; Ames, 2004; Epley et al., 2004; Birch and Bloom, 2007; Pronin, 2008). To illustrate, in theory of mind development the ability to inhibit privileged self-knowledge facilitates success on standard false belief tasks (Birch and Bloom, 2003). Perhaps the most difficult developmental feat crucial for social cognitive development beyond the ‘like me’ stage is the development of understanding the ‘duality’ of self in mentalistic terms (i.e. by ‘duality of self’ we mean a simultaneous understanding that oneself is both similar to yet different from others). The beginning of this transition to developing this dual understanding of self starts around the end of the first year of life (9–14 months) (Amsterdam, 1972; Scaife and Bruner, 1975) and continues well on into the second year of life (Kagan, 1981). Strikingly, some of the earliest signs of autism are behaviours indicative of this dual understanding of self; namely, deficits in joint attention (Landa et al., 2007) and a lack of responding to one’s own name (Osterling and Dawson, 1994; Zwaigenbaum et al., 2005; Nadig et al., 2007). Both early risk signs of autism also emerge around the end of the first year (12–14 months).
Thus, while the data from the current study cannot by itself distinguish between whether self-deficits cause social deficits or vice versa, both the developmental time-course of self-other equivalence (i.e. ‘like me’ stage) followed by a dual understanding of self and evidence on the early development of autism suggests that the current findings may be a developmental ‘fingerprint’ of atypical neural organization laid down during a critical period of development where such processes are beginning to take shape. Such atypical early development of the ventromedial prefrontal cortex may be a driving factor underlying the observed relationship with early childhood social impairments in autism. Work remains to be done on the typical development of regions such as the ventromedial prefrontal cortex in self-referential and social cognition, but initial studies in adolescence appear promising (Pfeifer et al., 2007; Sebastian et al., 2008; Burnett et al., 2009).
Aside from discussing the cognitive and developmental significance of atypical ventromedial prefrontal cortex function, it is important to note the wealth of data supporting underlying structural, neurochemical and physiological anomalies in the ventromedial prefrontal cortex. Localized medial prefrontal grey matter enlargement in autism occurs in early childhood (Carper and Courchesne, 2005) and may persist into early adolescence (Waiter et al., 2004; Bonilha et al., 2008). Complementing this grey matter enlargement, adjacent ventromedial prefrontal cortex white matter density (Bonilha et al., 2008; McAlonan et al., 2009), fractional anisotropy, and tract number are reduced (Barnea-Goraly et al., 2004; Cheung et al., 2009; Pugliese et al., 2009; Pardini et al., in press), which may ultimately manifest as an information processing ‘bottleneck’. In terms of the physiological and neurochemical composition of the ventromedial prefrontal cortex, only one magnetic resonance spectroscopy study has specifically explored this region and found decreases in the concentration of metabolites (i.e. Cho) that may reflect altered membrane metabolism (Levitt et al., 2003). Serotonin (Murphy et al., 2006; Makkonen et al., 2008) and dopamine (Ernst et al., 1997) receptor binding are also reduced in the medial prefrontal cortex in autism. Positron emission tomography studies have documented abnormalities in glucose metabolism that is increased at rest (Rumsey et al., 1985; Horwitz et al., 1988) and decreased during task performance (Haznedar et al., 2000; Hazlett et al., 2004). Regional cerebral blood flow is also reduced (George et al., 1992; Zilbovicius et al., 1995) and correlates with social symptom severity (Ohnishi et al., 2000). Finally, recent fMRI evidence suggests that resting ventromedial prefrontal cortex blood oxygenated level dependent (BOLD) signal (Kennedy et al., 2006; Kennedy and Courchesne, 2008a) is also reduced in a task-independent manner (i.e. irrespective of the comparison cognitive task) and correlates with social symptom severity. Resting state functional connectivity from the ventromedial prefrontal cortex is also significantly reduced (Kennedy and Courchesne, 2008,b). Converging on these findings, recent quantitative meta-analyses of task-related functional neuroimaging studies finds consistent hypoactivation of the ventromedial prefrontal cortex in autism across the literature of social (but not non-social) tasks (Di Martino et al., 2009). Taken together, these observations highlight the paramount role of the ventromedial prefrontal cortex in the neurodevelopment of autism. We speculate that early atypical pathophysiological process(es)/mechanism(s) are at work in the ventromedial prefrontal cortex that derail the normative structural and functional development of this region, hindering critical developmental transitions in self-referential and social-cognitive development. For example, a recently discovered genetic variant associated with autism near the gene cadherin 10 (CDH10) is involved in neuronal cell adhesion molecules and is specifically expressed in ventromedial prefrontal cortex of the developing human foetal brain (Wang et al., 2009). Future work targeting this region and autism-associated genetic variants are likely to illuminate core neurodevelopmental insights into autism (Lombardo et al., 2010a).
In conclusion, we have observed disruptions in the neural systems critical for coding self-information in autism. The disruption of such systems is integrally related to the early social impairments in autism. The abundance of evidence highlighting atypical development, structure, function, and physiology of the ventromedial prefrontal cortex suggests that the current study highlights the end result of an early pathophysiological biological mechanism in this area. The expression of such a pathophysiological mechanism may derail the normative development of the ventromedial prefrontal cortex in a critical period where a dual understanding of self is beginning to emerge. Future work on the ventromedial prefrontal cortex will be crucial for elucidating core neural mechanisms in the neurodevelopment of autism.
Funding
This work was funded by doctoral studentship/bursaries from the Shirley Foundation (to M.V.L.); Cambridge Overseas Trust and Medical Research Council (MRC) [to the AIMS Consortium and to S.B.C.-(program grant)].
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Jason Mitchell and Adrianna Jenkins for generously letting us use their stimuli, and Mike Cohen, Matthew Belmonte, Caroline Robertson, Teresa Tavassoli, Meng-Chuan Lai and Ilaria Minio-Paluello for their valuable discussion and comments, as well as support from the MRC Autism Imaging Multi-Centre Study (AIMS) Consortium. This work was conducted in association with the NIHR CLAHRC for Cambridgeshire and Peterborough NHS Mental Health Trust. E.T.B. is employed half-time by the University of Cambridge and half-time by GlaxoSmithKline plc.
References
Abbreviations
- ADI-R
Autism Diagnostic Interview Revised
- ADOS
Autism Diagnostic Observational Schedule
- BA
Brodmann Area
- BOLD
blood oxygenated level dependent
- FDR
false-discovery rate
- fMRI
functional magnetic resonance imaging
- FO/PMv
frontal operculum/ventral premotor cortex
- MNI
Montreal Neurological Institute
- OM
other-mentalizing
- OP
other-physical
- PPI
psychophysiological interaction
- ROI
region of interest
- SI/SII
somatosensory cortex
- SM
self-mentalizing
- SP
self-physical
- SVC
small-volume correction
Appendix 1
The MRC AIMS Consortium is a UK collaboration between the Institute of Psychiatry at Kings College, London, the Autism Research Centre and Brain Mapping Unit at the University of Cambridge, and the Autism Research Group at the University of Oxford. It is funded by the Medical Research Council (MRC) UK and headed by the Section of Brain Maturation, Institute of Psychiatry. The Consortium members are in alphabetical order: A. J. Bailey, S. Baron-Cohen, P. F. Bolton, E. T. Bullmore, S. Carrington, B. Chakrabarti, E. M. Daly, S. C. Deoni, C. Ecker, F. Happé, J. Henty, P. Jezzard, P. Johnston, D. K. Jones, M. V. Lombardo, A. Madden, D. Mullins, C. Murphy, D. G. Murphy, G. Pasco, S. Sadek, D. Spain, R. Stewart, J. Suckling, S. Wheelwright and S. C. Williams.
Author notes
*The members of the MRC AIMS Consortium are placed in the Appendix 1.