-
PDF
- Split View
-
Views
-
Cite
Cite
Birgit Abler, Roman Hahlbrock, Alexander Unrath, Georg Grön, Jan Kassubek, At-risk for pathological gambling: imaging neural reward processing under chronic dopamine agonists, Brain, Volume 132, Issue 9, September 2009, Pages 2396–2402, https://doi.org/10.1093/brain/awp170
Close - Share Icon Share
Abstract
Treatment with dopamine receptor agonists has been associated with impulse control disorders and pathological gambling (PG) secondary to medication in previously unaffected patients with Parkinson's disease or restless legs syndrome (RLS). In a within-subjects design, we investigated the underlying neurobiology in RLS patients using functional magnetic resonance imaging. We scanned 12 female RLS patients without a history of PG. All patients were scanned twice: once whilst taking their regular medication with low dose dopamine receptor agonists and once after a washout phase interval. They performed an established gambling game task involving expectation and receipt or omission of monetary rewards at different levels of probabilities. Upon expectation of rewards, reliable ventral striatal activation was detected only when patients were on, but not when patients were off medication. Upon receipt or omission of rewards, the observed ventral striatal signal under medication differed markedly from its predicted pattern which by contrast was apparent when patients were off medication. Orbitofrontal activation was not affected by medication. Chronic dopamine receptor agonist medication changed the neural signalling of reward expectation predisposing the dopaminergic reward system to mediate an increased appetitive drive. Even without manifest PG, chronic medication with dopamine receptor agonists led to markedly changed neural processing of negative consequences probably mediating dysfunctional learning of contingencies. Intact orbitofrontal functioning, potentially moderating impulse control, may explain why none of the patients actually developed PG. Our results support the notion of a general medication effect in patients under dopamine receptor agonists in terms of a sensitization towards impulse control disorders.
Introduction
Pathological gambling (PG) has been reported as an expensive side-effect of treatment with dopamine agonists (DA). Accounts of previously unaffected patients with neurological illnesses that lost tens of thousands of dollars because of newly adopted gambling behaviour were issued and even received public recognition in the past years. While the first reports were on patients suffering from Parkinson's disease under high doses of DA medication (Dodd et al., 2005; Gallagher et al., 2007; Voon et al., 2007), recently patients with restless legs syndrome (RLS) attracted attention because they acquired dysfunctional habits of gambling even under low doses of DA (Driver-Dunckley et al., 2007; Tippmann-Peickert et al., 2007) within several weeks after a new treatment regimen was initiated or doses were increased. According to the reports, PG resolved in all patients after discontinuation of the treatment. DA are therefore assumed to cause a reversible increase of the predisposition towards PG (Voon et al., 2007).
PG, according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV, is classified as a compulsive disorder with failure to resist gambling impulses despite negative personal, family or vocational consequences. While the prevalence for PG in the general population is estimated ∼0.5% in the USA (Petry et al., 2005) and 0.2% in Germany (Meyer, 2007), ∼4–8% of patients with Parkinson's disease taking DA are reported to show PG behaviour (Gallagher et al., 2007). As various DA have been implicated in precipitating compulsive disorders like PG (Dodd et al., 2005; Gallagher et al., 2007; Voon et al., 2007), a class effect can be assumed, but the underlying neurobiology is still unclear. A breakdown of reward-hierarchies due to alteration of incentive processing has been suggested as a potential basis (Gallagher et al., 2007; Riba et al., 2008). Indeed, agonists preferring the D2/D3 receptor like pramipexole, ropinirole or cabergoline, frequently used in the treatment of Parkinson's disease or RLS, act predominantly on the mesolimbic dopamine system (Sokoloff et al., 2006) that mediates reward functions. Conclusively, studies of low single doses of DA in healthy subjects reported impairment of reward-related learning (Pizzagalli et al., 2008) and altered functional magnetic resonance imaging (fMRI) activation of the dopaminergic reward system (Riba et al., 2008). Stimulation of presynaptic D3 autoreceptors reducing phasic firing of dopamine neurons via inhibitory feedback (Pizzagalli et al., 2008) has been suggested as the underlying mechanism. However, while short-term administration of DA has been demonstrated to decrease firing of dopamine neurons, animal research has shown that chronic treatment is associated with recovery of the firing rates to normal levels, desentization of D2/D3 autoreceptors (Chernoloz et al., 2008) and downregulation of the dopamine transporter (Joyce et al., 2004). Furthermore, while the acute administration of DA in healthy subjects commonly relates to nausea, lowered alertness, reaction time and motor speed slowing and negative affect (Pizzagalli et al., 2008), chronic treatment in patients not only leads to higher motor functioning but is commonly associated with better well-being and positive affect (Sokoloff et al., 2006). Therefore, effects from single dose studies in healthy subjects are far from being capable to fully explain the predisposition towards pathologic gambling in patients on a regular DA medication.
Hence, we studied the effects of long-term DA medication on fMRI brain activation during a gambling task in a sample of RLS patients using a within-subject design with patients on and off medication. The clinical presentation of RLS commonly involves a wide spectrum of sensory discomfort during rest, accompanied by motor restlessness and an irresistible urge to move. As inactivity increases symptoms, sleep is often disturbed. Moving alters the sensations, providing temporary alleviation, but DA greatly relieves the symptoms (Allen et al., 2003; Trenkwalder et al., 2005). Severe impairment of motor coordination, cognitive deficits or neuropsychiatric problems, including impulse control disorders as described for Parkinson's disease irrespective of medication are not typically observed in untreated RLS, thus providing a basis to study side-effects of DA medication in combination with a markedly reduced risk of biasing co-morbidities.
Among the specific reward mechanisms potentially involved in the development of PG under DA, altered coding of errors in the prediction of rewards during gambles has been suggested (Pizzagalli et al., 2008; Riba et al., 2008). Prediction errors are coded in the mesolimbic dopaminergic system as demonstrated in studies of dopaminergic firing rates in monkeys (Fiorillo et al., 2003; Schultz, 2007) and of fMRI activation of dopaminergic brain regions, especially the ventral striatum in humans (Knutson et al., 2001; Abler et al., 2005, 2006). Receipt of unpredicted rewards (positive prediction error) is related to increases in signal and learning of the behaviour associated with the occurrence of rewards (Schultz, 2007). In contrast, the omission of predicted rewards (negative prediction error) relates to decreases in signal (Fiorillo et al., 2003; Abler et al., 2006) and extinction of the behaviour associated with losing the incentive. Altered fMRI activation of the ventral striatum upon winning and losing money, potentially representing dysfunctional prediction error processing, was accordingly observed in a sample of pathological gamblers (Reuter et al., 2005). Together with hypoactivation of the orbitofrontal cortex (Potenza et al., 2003a, b; Reuter et al., 2005), ventral striatal dysfunction seems to characterize pathological gamblers.
Besides changes in prediction error processing, alterations of appetitive motivation in the presence of prospective rewards and elevated novelty seeking have been identified as potential risk factors for the development of PG, at least in Parkinson's disease (Voon et al., 2007).
Using an established paradigm (Abler et al., 2006), well suited to investigate variations of reward expectation and prediction error as a discrete function of reward probabilities, we studied patients with RLS syndrome ‘on’ and ‘off’ their regular long-term DA medication in a within-subjects design. We assumed that DA medication is related to an increased predisposition towards PG in patients treated with DA, although only some develop manifest clinical symptoms. We therefore expected to find congruent neurobiological correlates.
We hypothesized that there would be an alteration in mesolimbic fMRI brain activation upon expectation of rewards when comparing patients on and off medication, and dysfunctional prediction error signalling upon receipt and omission of rewards when patients are under chronic DA medication. Thus, we expected changes related to medication in ventral striatal or orbitofrontal regions, in particular decreased activation upon receipt of rewards, as demonstrated for manifest PG (Potenza et al., 2003a, b; Reuter et al., 2005).
Methods
Patients
Twelve female outpatients of the Department of Neurology at the University Clinic of Ulm (one left-handed, aged 43–66 years, all non-smokers) with RLS treated with DA were included in and completed the study. The diagnosis was based on a detailed history and a standardized general and neurological examination. All patients fulfilled the revised essential criteria for the diagnosis of idiopathic RLS defined by the International RLS Study Group (IRLSSG) (Allen et al., 2003). On average, patients had been diagnosed with RLS 4 years before the study (Table 1) and had been suffering from RLS symptoms for several years more. Stable treatment with DA was initiated at least 1 month and usually >1 year before scanning. Psychiatric, especially substance-related problems were excluded in a psychiatric interview on the basis of the Structured Clinical Interview for DSM-IV (SCID) (APA, 1994). None of the patients ever engaged in regular gambling or had any evidence pointing to PG in the past. Severe acute or chronic current medical conditions were excluded.
Demographic and clinical characteristics of the sample
| Patients with RLS (n = 12) . | Mean (SD) . |
|---|---|
| Age | 55.8 (8.8) |
| Years since diagnosed with RLS | 3.9 (2.4) |
| Years on DA | 1.8 (1.6) |
| DA doses (in milligram pramipexole equivalent) | 0.47 (0.22) |
| Patients with RLS (n = 12) . | Mean (SD) . |
|---|---|
| Age | 55.8 (8.8) |
| Years since diagnosed with RLS | 3.9 (2.4) |
| Years on DA | 1.8 (1.6) |
| DA doses (in milligram pramipexole equivalent) | 0.47 (0.22) |
Pramipexole equivalent doses were calculated according to the suggestions. In: Riederer P, Laux G, Pöldinger W, editors. Neuropsychopharmaka. Vol. 5. Parkinsonmittel und Antidementiva, 1999; S.235.
Demographic and clinical characteristics of the sample
| Patients with RLS (n = 12) . | Mean (SD) . |
|---|---|
| Age | 55.8 (8.8) |
| Years since diagnosed with RLS | 3.9 (2.4) |
| Years on DA | 1.8 (1.6) |
| DA doses (in milligram pramipexole equivalent) | 0.47 (0.22) |
| Patients with RLS (n = 12) . | Mean (SD) . |
|---|---|
| Age | 55.8 (8.8) |
| Years since diagnosed with RLS | 3.9 (2.4) |
| Years on DA | 1.8 (1.6) |
| DA doses (in milligram pramipexole equivalent) | 0.47 (0.22) |
Pramipexole equivalent doses were calculated according to the suggestions. In: Riederer P, Laux G, Pöldinger W, editors. Neuropsychopharmaka. Vol. 5. Parkinsonmittel und Antidementiva, 1999; S.235.
Each patient was tested on two occasions, once whilst taking their regular DA and once after a washout phase without medication in a balanced, randomized design. Scans were conducted 2 weeks apart. Patients were treated either with pramipexole (five patients), cabergoline (four patients) or ropinirole (three patients) (Table 1) and were allowed an additional medication of l-dopa. For the scans without medication, patients were asked to discontinue cabergoline for at least 5 days, pramipexole and ropinirole for 48 h, and to refrain from taking l-dopa for 24 h before scanning, according to the half-lives of the drugs. The study was approved by the local ethics committee of the University of Ulm. Written informed consent was obtained after complete description of the study to the subjects and prior to inclusion.
Task
We used a well-established monetary incentive task described elsewhere in detail (Abler et al., 2006) with a parametric variation of probabilities (0, 25, 50, 75 and 100%) to win a fixed amount of money (1€). Each of the two sessions consisted of 60 trials (12 trials per each probability; 120 trials in total). The trials started with a cue (a coloured symbol) indicating the probability to win the money later on. After an expectation period, subjects had to correctly react with a button press to two different symbols. In reacting correctly, they retained the previously announced chance to win 1€. Feedback (outcome) followed the target's; disappearance and notified subjects of the amount of money (1€ or 0€) they won in the trial. Reaction times and errors were registered. Depending upon the reward probabilities, subjects were not rewarded despite pressing the correct button in a number of trials, i.e. a reward announced at a probability of 75% was actually distributed in 75% (receipt of reward) and held back in 25% (omission of reward) of the correct trials. Incorrect button presses resulted in a feedback of zero euros at any probability. Receipt and omission trials as well as the five trial types (0, 25, 50, 75 and 100% chance to win) appeared in a random order. Right before scanning, all subjects completed two sessions (60 trials/10 min each) of a practice version of the task.
fMRI acquisition
A 3.0 T Siemens ALLEGRA Scanner (Siemens, Erlangen, Germany) equipped with a head coil was used to acquire T1-anatomical volume images (1 × 1 × 1 mm voxels) and functional magnetic resonance images. Twenty-three sagittal slices were acquired with an image size of 64 × 64 pixels and a field of view of 192 mm. Slice thickness was 3 mm with 0.75 mm gap resulting in a voxel size of 3 × 3 × 3.75 mm. Images were centred on basal structures of the brain including subcortical regions of interest (basal ganglia and prefrontal regions). Functional images were recorded using a T2*-sensitive gradient echo sequence measuring changes in BOLD-contrast. 401 volumes were obtained during each session at a TR of 1500 ms (TE 35 ms, flip angle 90°).
fMRI analysis
Image processing and statistical analysis were carried out using Statistical Parametric Mapping (SPM5, Wellcome Trust Centre for Neuroimaging, London, UK) with a random effects model for group analyses. Pre-processing of the individual functional scans included realignment to correct for motion artefacts, slice timing, spatial normalization to a standard template (Montreal Neurological Institute, MNI) and smoothing with an 8 mm full width at half maximum (FWHM) Gaussian kernel. Intrinsic autocorrelations were accounted for by AR (1) and low-frequency drifts were removed via high pass filtering.
After pre-processing, first level analysis was performed on each subject estimating the variance of voxels for each trial according to a general linear model. As in our previous study on healthy subjects, we defined regressors to analyse each of the five types of expectation phases sorted by reward probabilities of 0, 25, 50, 75 and 100%. Regressors modelled reward expectation including presentation of the cue, the button press and the eight different types of outcome (Fig. 2). Depending on the preceding reward expectation (0–100%) and actual outcome (receipt of reward: R, omission of reward: O) the eight outcome events were: (i) 0%; (ii) 25% R; (iii) 25% O; (iv) 50% R; (v) 50% O; (vi) 75% R; (vii) 75% O; and (viii) 100%. According to their actual durations, trials were modelled as timely extended events and convolved with the hemodynamic response function. The six re-alignment parameters modelling residual motion were also included in the individual models.
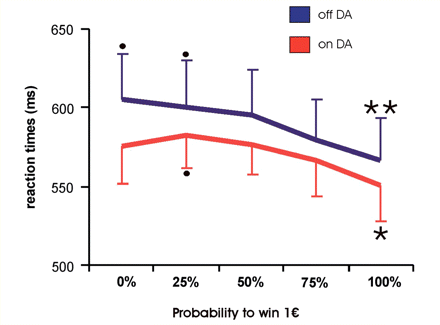
Reaction times: mean reaction times and their standard error during scanning of the reward task when off and on DA with a trend towards higher reaction times ‘on DA’ and significant (paired t-test, P < 0.05) acceleration of reaction times when rewards were expected at 100% probability as compared to rewards expected at 0% (only ‘on DA’) or 25% (on and off DA). Asterisks denote significant differences (P < 0.05) between mean reaction times on trials with reward probabilities of either 0 or 25% against trials of 100% probability on medication and between 25% and 100% off medication.
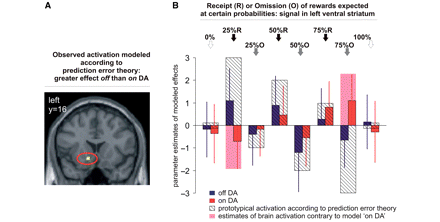
Reward outcome/prediction error. (A) Left ventral striatal activity as found for the repeated measures analysis of outcome regressors modelling reward prediction error. (B) Parameter estimates of modelled effects with standard errors in left ventral striatum and model of putative signal according to prediction error theory with highest signal upon most unexpected receipt of rewards and lowest signal upon mot unexpected omission of rewards. (0/25/50/75/100%: probability at which reward of 1€ was expected). At 0 and 100% probabilities predictions are definite and no errors occur. fMRI activation represented by parameter estimates followed the prediction error model when patients were not taking DA medication. On DA, fMRI activation in the ventral striatum showed a pattern contrary to the model, particularly when a rather unlikely reward (expected at only 25% probability) was received (25% R) and when a very likely reward (75% O) was omitted. Activities are shown at P < 0.001.
The contrast images of parameter estimates for each level of expectation and for each combination of probabilities and outcome were then included in a second level group analysis. Replicating our previous experiment (Abler et al., 2006), we computed analyses separately for expectation and outcome trials: one on the five different expectations (Analysis 1a) and one on the eight outcome events (Analysis 2). Conditions were weighted with a linear contrast to model neural activations related to increasing reward expectation with increasing probabilities (Analysis 1a) and a linear contrast to model activation following prediction error theory (Analysis 2), formally modelling the size of the prediction error (Fig. 2B). A third analysis was calculated including only the two extremes of expectation regressors (Analysis 1b: 100 > 0%). Treatment effects (on, off medication) were tested by interaction analyses of medication with either expectation or outcome. Statistical maps were thresholded at P < 0.05 with false discovery rate (FDR) corrections for multiple comparisons. If effects failed significance at that threshold, statistical maps were thresholded at P < 0.001 uncorrected at the voxel level, however, with a threshold of P < 0.05 at the cluster level to avoid false positive results due to multiple comparisons.
Psychological testing
After each scan, subjects performed three reaction time tasks (see online Supplementary material: self-paced and externally triggered reaction upon appearance of a visual stimulus, and a divided attention task, forcing subjects to respond on visual and auditory stimuli simultaneously). Also, subjective RLS symptoms at each of the two occasions were assessed in a brief interview with open questions, and by using a rating scale from 1 (no RLS symptoms) to 10 (strongest RLS symptoms ever experienced). Paired t-tests were applied to compare reaction time and rating scale results on and off medication.
Results
Behavioural responding
Subjects pressed the correct button within the required time interval in 98% of the trials, irrespective of dopaminergic medication. An analysis of variance for repeated measures on mean reaction times revealed a significant main effect for levels of probability [F(4,44) = 4.66, P = 0.003], but not medication [F(1,11) = 1.11, P = 0.315], although patients appeared to respond slightly faster when taking DA (Fig. 1). The interaction of both factors was not significant [F(4,44) = 0.52, P = 0.724]. Post hoc tests (Bonferroni) yielded significant acceleration of mean reaction times when comparing trials with 0 and 25% against trials with 100% probability (0% versus 100%: P = 0.003; 25% versus 100%: P = 0.018) for runs without dopaminergic medication and a significant acceleration comparing trials with 25% against trials with 100% probability (P = 0.03) when patients were on medication.
Mean reaction times in the neuropsychological tests after scanning were significantly faster in three of four tests when subjects took their regular medication than without DA (see Supplementary material). Patients rated their symptoms significantly worse when being off medication [average 6.0 on a visual analog scale from 1 to 10; t(11) = 5.9, P = 0.0001] against being on medication (average 2.0 on scale from 1 to 10). Without medication, they commonly reported re-occurrence or deterioration of their common symptoms like prickling, fidgeting, urge to move their legs and troubles with sleeping.
fMRI results
We directed our analyses to the question whether medication with DA alters fMRI activation in the dopaminergic reward system and particularly in those regions where altered activation was found in patients with manifest PG, i.e. the ventral striatum and orbitofrontal cortex. fMRI activation of these areas can be described as a function of reward probability and prediction error as shown for firing rates of dopamine neurons in animal experiments (Fiorillo et al., 2003; Schultz, 2007) and for fMRI signals in human subjects (Abler et al., 2006).
Reward expectation
The linear contrast predicting increasing neural activations with increasing probabilities (analysis 1a) revealed a significant effect (P < 0.001) in right lateral orbitofrontal cortex (Table 2), when patients were on DA medication, but not when patients were off medication. Significant linear trends were additionally observed in occipital and parietal regions and in the cerebellum, predominantly when patients were ‘off DA’, but also ‘on DA’ (for details, see online Supplementary material). Comparing linear trends between on and off medication, no significant differences were to observe in the reward system even at lowered thresholds of P < 0.05.
Maximum z-values and MNI-coordinates of contrasts of interest derived by whole-brain second-level group analyses
| Contrast/Region . | . | Off DA . | On DA . | Off versus on DA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Z . | NV . | Peak coordinates . | Z . | NV . | Peak coordiates . | Z . | NV . | Peak coordinates . |
| . | . | . | . | x/y/z . | . | . | x/y/z . | . | . | x/y/z . |
| Expectation: analysis 1a —increasing reward probability | ||||||||||
| Lateral orbitofrontal cortex | R | NS | 4.71 | 29 | 44/26/–12 | NS | ||||
| Expectation: analysis 1b —100% > 0% reward probability | ||||||||||
| Ventral striatum | L | NS | 3.52 | 20 | –18/20/–8 | Trend: P = 0.006 (on > off DA) | ||||
| Lateral orbitofrontal cortex | R | NS | 3.87 | 50 | –40/20/–2 | NS | ||||
| L | NS | 3.85 | 42 | 44/26/–32 | NS | |||||
| Outcome: analysis 2 —reward prediction error | ||||||||||
| Ventral striatum | L | 3.6* | 9 | −10/14/–10 | NS | 3.7* | 19 | −10/16/−8 | ||
| (off > on DA) | ||||||||||
| Medial orbitofrontal cortex | 4.3* | 61 | 0/44/–20 | 5.5* | 296 | –2/44/–18 | NS | |||
| Contrast/Region . | . | Off DA . | On DA . | Off versus on DA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Z . | NV . | Peak coordinates . | Z . | NV . | Peak coordiates . | Z . | NV . | Peak coordinates . |
| . | . | . | . | x/y/z . | . | . | x/y/z . | . | . | x/y/z . |
| Expectation: analysis 1a —increasing reward probability | ||||||||||
| Lateral orbitofrontal cortex | R | NS | 4.71 | 29 | 44/26/–12 | NS | ||||
| Expectation: analysis 1b —100% > 0% reward probability | ||||||||||
| Ventral striatum | L | NS | 3.52 | 20 | –18/20/–8 | Trend: P = 0.006 (on > off DA) | ||||
| Lateral orbitofrontal cortex | R | NS | 3.87 | 50 | –40/20/–2 | NS | ||||
| L | NS | 3.85 | 42 | 44/26/–32 | NS | |||||
| Outcome: analysis 2 —reward prediction error | ||||||||||
| Ventral striatum | L | 3.6* | 9 | −10/14/–10 | NS | 3.7* | 19 | −10/16/−8 | ||
| (off > on DA) | ||||||||||
| Medial orbitofrontal cortex | 4.3* | 61 | 0/44/–20 | 5.5* | 296 | –2/44/–18 | NS | |||
Only effects within the reward system are listed (for a full table, please see online Supplementary material) Reward expectation Analyses (1a and 1b) modelling effects of increasing reward probability (five levels) and comparing neural activations upon reward expectation at 100% versus 0% probability. Reward outcome Analyses of outcome regressors (eight levels) linearly related to prediction error. Results for whole-brain second-level comparisons were significant at P < 0.001 uncorrected for multiple comparisons at the voxel level, resulting in cluster extents significant at a level of P < 0.05. Effects surviving a FDR correction for multiple comparisons at the voxel level (P < 0.05 FDR corrected) are indicated by an asterisk. For the interaction with medication (direction of contrast: ‘off > on DA’), ‘off DA’ results at P < 0.005 at the voxel level and P < 0.05 at the cluster level were used as inclusive mask. For the inverted contrast (direction: ‘on > off DA’), ‘on DA’ results were used as an inclusive mask (same levels of significance as above). Only activations in brain regions of interest (ventral tegmental area, ventral striatum, orbitofrontal cortex) are listed. Coordinates are SPM/MNI-coordinates. L/R/M = left/right/midline; Z = Z-level at peak coordinate; NV = Number of voxels in cluster; NS = no significant effect.
Maximum z-values and MNI-coordinates of contrasts of interest derived by whole-brain second-level group analyses
| Contrast/Region . | . | Off DA . | On DA . | Off versus on DA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Z . | NV . | Peak coordinates . | Z . | NV . | Peak coordiates . | Z . | NV . | Peak coordinates . |
| . | . | . | . | x/y/z . | . | . | x/y/z . | . | . | x/y/z . |
| Expectation: analysis 1a —increasing reward probability | ||||||||||
| Lateral orbitofrontal cortex | R | NS | 4.71 | 29 | 44/26/–12 | NS | ||||
| Expectation: analysis 1b —100% > 0% reward probability | ||||||||||
| Ventral striatum | L | NS | 3.52 | 20 | –18/20/–8 | Trend: P = 0.006 (on > off DA) | ||||
| Lateral orbitofrontal cortex | R | NS | 3.87 | 50 | –40/20/–2 | NS | ||||
| L | NS | 3.85 | 42 | 44/26/–32 | NS | |||||
| Outcome: analysis 2 —reward prediction error | ||||||||||
| Ventral striatum | L | 3.6* | 9 | −10/14/–10 | NS | 3.7* | 19 | −10/16/−8 | ||
| (off > on DA) | ||||||||||
| Medial orbitofrontal cortex | 4.3* | 61 | 0/44/–20 | 5.5* | 296 | –2/44/–18 | NS | |||
| Contrast/Region . | . | Off DA . | On DA . | Off versus on DA . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Z . | NV . | Peak coordinates . | Z . | NV . | Peak coordiates . | Z . | NV . | Peak coordinates . |
| . | . | . | . | x/y/z . | . | . | x/y/z . | . | . | x/y/z . |
| Expectation: analysis 1a —increasing reward probability | ||||||||||
| Lateral orbitofrontal cortex | R | NS | 4.71 | 29 | 44/26/–12 | NS | ||||
| Expectation: analysis 1b —100% > 0% reward probability | ||||||||||
| Ventral striatum | L | NS | 3.52 | 20 | –18/20/–8 | Trend: P = 0.006 (on > off DA) | ||||
| Lateral orbitofrontal cortex | R | NS | 3.87 | 50 | –40/20/–2 | NS | ||||
| L | NS | 3.85 | 42 | 44/26/–32 | NS | |||||
| Outcome: analysis 2 —reward prediction error | ||||||||||
| Ventral striatum | L | 3.6* | 9 | −10/14/–10 | NS | 3.7* | 19 | −10/16/−8 | ||
| (off > on DA) | ||||||||||
| Medial orbitofrontal cortex | 4.3* | 61 | 0/44/–20 | 5.5* | 296 | –2/44/–18 | NS | |||
Only effects within the reward system are listed (for a full table, please see online Supplementary material) Reward expectation Analyses (1a and 1b) modelling effects of increasing reward probability (five levels) and comparing neural activations upon reward expectation at 100% versus 0% probability. Reward outcome Analyses of outcome regressors (eight levels) linearly related to prediction error. Results for whole-brain second-level comparisons were significant at P < 0.001 uncorrected for multiple comparisons at the voxel level, resulting in cluster extents significant at a level of P < 0.05. Effects surviving a FDR correction for multiple comparisons at the voxel level (P < 0.05 FDR corrected) are indicated by an asterisk. For the interaction with medication (direction of contrast: ‘off > on DA’), ‘off DA’ results at P < 0.005 at the voxel level and P < 0.05 at the cluster level were used as inclusive mask. For the inverted contrast (direction: ‘on > off DA’), ‘on DA’ results were used as an inclusive mask (same levels of significance as above). Only activations in brain regions of interest (ventral tegmental area, ventral striatum, orbitofrontal cortex) are listed. Coordinates are SPM/MNI-coordinates. L/R/M = left/right/midline; Z = Z-level at peak coordinate; NV = Number of voxels in cluster; NS = no significant effect.
Contrasting effects of expectation probabilities at their extremes (100% minus 0%, analysis 1b) revealed a statistically reliable effect in the left parahippocampus, the left ventral striatum and lateral orbitofrontal cortex bilaterally only when patients were on medication (Table 2). An interaction of this contrast with medication marginally failed pre-defined significance (P = 0.006) in the left ventral striatum (–18/10/–4) demonstrating higher differential activation when patients were ‘on DA’. Irrespective of medication status, additional locations bearing a significant contrast (100–0%) were found in occipital regions, and the cerebellum (for details, see online Supplementary material).
Receipt or omission of reward
To contrast differential effects of outcomes depending on preceding reward expectations we tested linear effects of a positive prediction error versus linear effects of a negative prediction error (brain regions beyond the reward system are detailed in the Supplementary material. Briefly, the contrast revealed a significant effect in bilateral occipital regions, both when on and off DA, in the posterior cingulate, and the right insula only when patients were on DA, and in medial prefrontal cortex when patients were off medication). With patients off DA, this analysis revealed significant effects (P < 0.05, FDR corrected) in the left ventral striatum and medial orbitofrontal cortex. On DA, effects were significant only in the medial orbitofrontal cortex (P < 0.05 FDR corrected). Accordingly, a significant (P < 0.05 FDR corrected) interaction of this contrast with medication was observed in left ventral striatum (Table 2). As illustrated in Fig. 2B, these differential effects were mainly due to a decrease of differential activation when patients ‘on DA’ medication received rewards expected at a rather low probability of 25% (25% R), and due to an increased differential activation when they did not receive a reward announced at the high probability of 75% (75% O).
Discussion
With the present study in DA-treated RLS patients on and off medication, we undertook the first experimental step to investigate the neural substrate of a pre-disposition that may mediate PG under chronic administration of DA. Neural signalling of reward expectation was increased by trend, thus potentially pre-disposing the dopaminergic reward system to mediate an increased appetitive drive under medication. We could further demonstrate a striking pattern of dysfunctional prediction error processing when patients received DA medication. Medial orbitofrontal activation, however, was not changed by medication.
Upon reward expectation, we found statistically reliable reward system activation only when the patients were taking their regular DA medication. The acceleration of overall reaction times under DA medication further supports this interpretation. Enhanced dopamine transmission caused by the medication has been related to this effect (Servan-Schreiber et al., 1998), which has been observed in our own previous study using the same task (Abler et al., 2006) and which is different from the motor speed slowing observed in single dose studies of DA (Pizzagalli et al., 2008).
Off the medication, patients displayed subthreshold activation. Therefore, the findings allow speculation about increased reward expectations and appetitive drive in the patients when ‘on DA’.
Upon receipt of omission of rewards, patients off DA medication displayed the expected signal distribution according to prediction error theory in the ventral striatum. The highest signal was observed when patients received a very unlikely but still possible reward expected at a probability of only 25%, i.e. upon a highly positive prediction error. The lowest signal occurred when patients did not receive a rather likely reward expected at a probability of 75%, i.e. upon a highly negative prediction error. Outcome events associated with lower prediction errors were accordingly lower in activation and followed the linear distribution along probabilities as expected from prior investigations (Abler et al., 2006). Strikingly, when patients were on steady state medication, the cardinal fMRI signals followed an opposite pattern, especially at high prediction errors: a highly positive signal was observed upon omission of an almost certain reward, the lowest signal was associated with the surprising receipt of the most unexpected reward. Given that highly positive prediction error signals relate to boost learning about the preceding behaviour and negative signals relate to its extinction, a pre-disposition to dysfunctional reward-related behaviour seems obvious. This observation contributes to an understanding of why pathological gamblers often do not learn from the negative consequences of losing even large amounts of money, and helps the understanding of their irrational beliefs in gambling strategies without comprehensible bases.
Given these marked changes in reward processing, an explanation is needed as to why none of the patients in our study actually reported problems with gambling or impulse control. That is, the dysfunction ventral striatal prediction error signalling obviously did not come with notable behavioural consequences. Studying actual pathological gamblers with reward tasks during fMRI, both mesolimbic and mesocortical dopaminergic pathways were involved. Besides changes of activation in the ventral striatum, decreases of activation were observed in the medial orbitofrontal cortex as part of the mesocortical system (Reuter et al., 2005). Also, pathological gamblers displayed decreased fMRI activation specifically in the medial orbital prefrontal cortex when tested with a stroop task (Potenza et al., 2003a) or during gambling cue presentation (Potenza et al., 2003b). This region has also been associated with impulse control disorders (Rogers et al., 1999). Thus, the preserved or even pronounced activation in the medial orbitofrontal cortex with patients on DA is presumably involved in an adaptive modulation of impulse control mediating the lack of behavioural consequences of altered ventral–striatal signalling under medication.
We conclude that the observed ventral striatal changes in fMRI activation on DA may lead to an increased predisposition towards PG under this medication. DA medication may stimulate an appetitive drive under which the subjective experience of possible rewards is stronger than without medication. This mesolimbic dopaminergic hyperactivation is paired with a dysfunctional processing of prediction errors leading to ignorance of negative consequences. Intact mesocortical functioning, however, may prevent symptom manifestation. Discontinuation of medication obviously leads to a rapid reversal of this effect as already observed in patients with manifest PG (1).
It is of note that the modest number of participants may limit generalization. In contrast, the on–off medication within-subject design in a naturalistic sample with standard clinical medication of different classes appears clinically relevant and suggests a general medication effect in patients under DA in terms of sensitization towards impulse control disorders.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Dr Abler, R. Hahlbrock, Dr Unrath, Prof. Grön and Prof. Kassubek report no competing interests and did not receive funding from any source other than their employer, the University of Ulm.