-
PDF
- Split View
-
Views
-
Cite
Cite
Myriam W. G. Vandenbroucke, H. Steven Scholte, Herman van Engeland, Victor A. F. Lamme, Chantal Kemner, A neural substrate for atypical low-level visual processing in autism spectrum disorder, Brain, Volume 131, Issue 4, April 2008, Pages 1013–1024, https://doi.org/10.1093/brain/awm321
Close - Share Icon Share
Abstract
An important characteristic of autism spectrum disorder (ASD) is increased visual detail perception. Yet, there is no standing neurobiological explanation for this aspect of the disorder. We show evidence from EEG data, from 31 control subjects (three females) and 13 subjects (two females) aged 16–28 years, for a specific impairment in object boundary detection in ASD, which is present as early as 120 ms after stimulus presentation. In line with a neural network model explicating the role of feedforward, horizontal and recurrent processing in visual perception, we can attribute this deficit to a dysfunction of horizontal connections within early visual areas. Interestingly, ASD subjects showed an increase in subsequent activity at lateral occipital sites (225 ms), which might reflect a compensational mechanism. In contrast, recurrent processing between higher and lower visual areas (around 260 ms), associated with the segregation between figure and background, was normal. Our results show specific neural abnormalities in ASD related to low-level visual processing. In addition, given the reconciliation between our findings and previous neuropathology and neurochemistry research, we suggest that atypical horizontal interactions might reflect a more general neural abnormality in this disorder.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social interaction and communication, and restricted and repetitive behaviours and interests. Another important characteristic of the disorder is a strong tendency for visual detail processing. In laboratory settings, this is reflected in superior performance in several tasks, such as the embedded figures test, the block design test and visual search tasks (for a review and discussion see Dakin and Frith, 2005). However, as yet, there is no standing neurobiological explanation for this aspect of ASD.
A model on visual perception, called reverse hierarchy theory (Hochstein and Ahissar, 2002), relates detail perception to feedback or recurrent activity in the visual cortex, while the processing of global stimulus aspects is associated with feedforward processing. When a visual stimulus enters the occipital cortex, initially a general, categorical interpretation is provided, called ‘vision at a glance’ by these authors. Thereafter, through feedback from higher visual areas, details are incorporated, called ‘vision with scrutiny’. Since people with ASD show enhanced detail perception, the balance between visual feedforward and feedback processing in these patients is of specific interest.
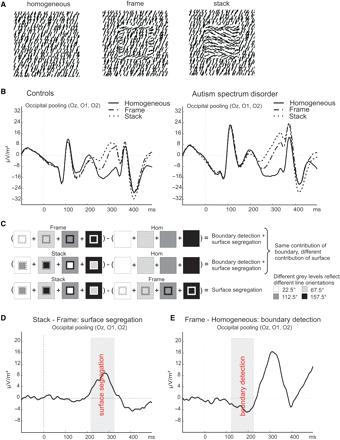
Findings from monkey research have provided direct evidence for the selective contributions of feedforward, feedback and also horizontal interactions in visual perception, especially in relation to figure-ground segregation, i.e. the segmentation of a scene into figure and background (Lamme, 1995; Lamme et al., 1998a; Lamme et al., 1999). In short, there are three essential neural processing steps in segregating a figure from its background: (i) texture elements (such as the lines in Fig. 1A) are detected by neurons that are selectively tuned to features such as orientation (Hubel and Wiesel, 1959). Information on the texture elements is mediated by feedforward processing; (ii) the detection of orientation boundaries (where two different orientations meet, see Fig. 1A) is mediated by lateral inhibition between neurons with similar orientation preference (Knierim and Vanessen, 1992). Such inhibition will lead to an enhanced response at locations where different orientations meet. Horizontal connections between cells with similar orientation tuning play an important role in such effects (Gilbert and Wiesel, 1989; Malach et al., 1993; Stettler et al., 2002); and (iii) the segregation of a surface from its background has been related to feedback processing (Lamme, 1995; Zipser et al., 1996; Lamme et al., 1998a; Angelucci and Bullier, 2003), i.e. recurrent interactions between higher and lower visual areas. Feedback loops lead to enhanced activity of neurons responding to the information inside the boundaries compared to neurons responding to the background (Lamme et al., 1999). This leads to so-called ‘filling-in’ of the surface region. The role of feedback in surface segregation has been directly demonstrated with lesion studies (Hupe et al., 1998; Lamme et al., 1998b): when extra striate areas in the monkey brain were eliminated, the signal related to surface filling-in was no longer apparent. The specific roles of feedforward, horizontal and feedback processing in texture segregation are thus supported by neurophysiological studies and a recent model of Roelfsema et al. (2002) makes their relative contributions explicit.
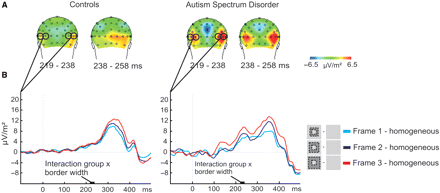
(A) The stimuli used in the discrimination task while EEG activity was measured. (B) The EEG responses (converted to spline laplacian's) to the stimuli for controls and subjects with ASD, before any subtraction. (C) The subtractions that were made to isolate activity related to boundary detection and surface segregation. Different grey levels represent different line orientations. (D) The stack–frame subtraction wave (for control subjects) to determine the time window, indicated by the grey panel, related to surface segregation. (E) The frame–homogeneous subtraction wave (for control subjects) to determine the time window, indicated by the grey panel, related to boundary detection.
Indeed, the involvement of abnormal feedforward or recurrent mechanisms in ASD has been suggested. For instance, Gustafsson (1997) proposed that excessive inhibitory lateral feedback is a prominent feature of the disorder, causing high sensory discrimination. Also, it has been put forward that a failure of neuronal pruning (which is possibly the underlying cause of abnormally large brain size often found in subjects with ASD, see Frith, 2004; Happé and Frith, 2006), results in aberrancies in feedback processing, while feedforward systems are intact. Additionally, Happé and Frith suggest that a lack of the modulatory influence of feedback might result in perceptual abnormalities as found in ASD. Bertone and colleagues (2005) argue in a recent study, based on the finding of impaired orientation detection for second order, texture defined stimuli, that both lateral and feedback activity might be atypical in ASD. In addition, lateral or horizontal connections not only play an important role in processing texture stimuli as used by Bertone et al., but also in grouping or binding (Polat, 1999; Roelfsema, 2006). Specifically, monkey research has shown the selectivity of certain horizontal connections for neurons with similar orientation tuning (Malach et al., 1993) and these connections most likely play a role in perception by Gestalt laws, such as grouping by similarity (Roelfsema, 2006). A recent behavioural study demonstrated that grouping by Gestalt principles, amongst others grouping by similarity, is less strong in people with ASD compared to controls (Brosnan et al., 2004). Also, using the embedded figures test, where the stimulus configuration has to be suppressed to find the embedded figure in a complex pattern of line elements, it has repeatedly been shown that people with ASD perform better than healthy control subjects, demonstrating that this kind of grouping is weaker in this disorder (Jolliffe and Baron-Cohen, 1997; Ropar and Mitchell, 2001; De Jonge et al., 2006). These theoretical accounts and experimental findings implicate the involvement of abnormal functioning of horizontal and feedback connections in visual perception in ASD.
Altogether, we hypothesize that an imbalance between feedforward, horizontal and feedback processing is a core feature of ASD, associated with aberrant detail perception. The functioning of these different types of connections has never been, to our knowledge, systematically or explicitly studied in this disease. Since the specific roles of feedforward, horizontal and recurrent or feedback processing have been demonstrated in figure-ground segregation of textured figures (see Fig. 1A), we considered the use of such stimuli appropriate for examining our hypothesis. With electrophysiological recordings (event related potentials, ERPs), we studied texture segregation in a group of ASD subjects and compared them to healthy controls. We used stimuli composed of oriented line segments, which contained different amounts of figure surfaces and boundaries (see Fig. 1A and the Results section for a thorough explanation of the stimuli). By employing specific stimulus contrasts, we could single out ERP activity related to boundary detection and surface segregation, while selectively discounting activity related to local feature detection. This enabled us to draw inferences about the balance between feedforward, horizontal and feedback processing in subjects with ASD and age and IQ-matched healthy controls.
Material and methods
Subjects
Thirty-one control subjects (three females) and 13 subjects (two females) with ASD participated in this study (five with a diagnosis of autistic syndrome, eight with a diagnosis of Asperger syndrome), aged between 16 years and 4 months and 28 years and 10 months. There were no significant age or IQ differences between the groups (Table 1) and all subjects had normal or corrected to normal vision.
Age and IQ
| . | Age in years . | TIQ (SD) . |
|---|---|---|
| Control (n = 31) | 21.6 (2.1) | 117.3 (7.9) |
| ASD (n = 13) | 20.8 (4.1) | 120.5 (11.1) |
| . | Age in years . | TIQ (SD) . |
|---|---|---|
| Control (n = 31) | 21.6 (2.1) | 117.3 (7.9) |
| ASD (n = 13) | 20.8 (4.1) | 120.5 (11.1) |
IQ was measured using the full WAIS-III for subjects with ASD. A short version of the WAIS-III was used to determine IQ for the control subjects.
Age and IQ
| . | Age in years . | TIQ (SD) . |
|---|---|---|
| Control (n = 31) | 21.6 (2.1) | 117.3 (7.9) |
| ASD (n = 13) | 20.8 (4.1) | 120.5 (11.1) |
| . | Age in years . | TIQ (SD) . |
|---|---|---|
| Control (n = 31) | 21.6 (2.1) | 117.3 (7.9) |
| ASD (n = 13) | 20.8 (4.1) | 120.5 (11.1) |
IQ was measured using the full WAIS-III for subjects with ASD. A short version of the WAIS-III was used to determine IQ for the control subjects.
The diagnostic evaluation included a psychiatric observation and a review of prior records (developmental history, child psychiatric and psychological observations and tests). ASD was diagnosed by a child psychiatrist, using the DSM-IV criteria. The parents of these subjects were administered the autism diagnostic interview—revised (ADI-R, Lord et al., 1994) and subjects with ASD were administered the autism diagnostic observation schedule—generic (ADOS-G, Lord et al., 1989), both by a trained rater. Twelve subjects met ADI-R criteria for autism or autism spectrum disorder; one subject did not meet criteria for stereotyped behaviour (this subject did meet ADOS-G criteria). All subjects, but one (who did meet ADI-R criteria), met the full ADOS-G criteria for autism or autism spectrum disorder. The subjects were medication free except for one (who used 20 mg seroxat and 3 mg risperdal per day) and had no significant neurological history. Both the subjects with ASD and the control subjects received a money reward for their participation. The study was approved by the medical ethics committee of the University Medical Centre Utrecht and subjects gave written informed consent prior to participation.
Stimuli, conditions and procedure
Subjects performed a discrimination task, during which EEG activity was measured, with randomly presented stack, frame and homogenous stimuli (see Fig. 1A). The stimuli were made of black line segments (0.9 cd/m2), with a length of 0.36°, a width of 0.02° and an average density of 4.2 line segments per degree, projected randomly on a white background (103 cd/m2). Four orientations (22.5°, 67.5°, 112.5°, 157.5°) of the line segments were used in a balanced way to create the stimuli. The line orientation at each edge of the texture border of frame and stack stimuli was always at 45° with that of the background and at 45° with that of the region enclosed by the border. In frame stimuli, the line orientation of the enclosed region was the same as that of the background, whereas in stack stimuli the line orientation of the enclosed region was at 90° with that of the background. The inner square size of the stack and frame stimuli was always 1.93°.
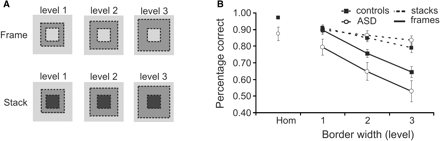
To establish a parametric design the difficulty level of the discrimination task was manipulated by varying the thickness of the borders of frame and stack stimuli (see Fig. 2A), i.e. 0.32°, 0.81° or 1.29°, resulting in a total stimulus size of 2.57°, 3.55° or 4.51°. We conjectured that thicker borders would make the distinction between stack and frame stimuli less visible (it would be more difficult to see the inside of the frame as continuous with the background). Concerning the ERP data, we hypothesized that the manipulation of border width would primarily affect surface segregation, reflected in the activity in the stack—frame contrast (see Results section for an explanation on the relation between the stack—frame contrast and surface segregation).
(A) The manipulation of the stack and frame stimuli, i.e. increasing the border width. (B) Performance data of the three-way alternative forced choice task between homogeneous (‘hom’), stack and frame stimuli. Subjects with ASD scored lower on frame and homogenous stimuli, whereas performance for stacks was the same compared with controls.
During the experiment, subjects fixated a red dot (24 cd/m2, 0.24°) in the centre of the computer screen which was present during the whole trial. A trial started with the presentation of a stack, frame or homogeneous stimulus for 267 ms at an unpredictable location in one of the quadrants of the screen (eccentricity between the fixation dot and the closest stimulus corner was always 1.7°). The stimulus was immediately followed by a mask (jittered presentation duration of 1817 to 2017 ms resulting in a total trial duration of 2084 to 2284 ms), consisting of the same line elements, but now in random orientations. When subjects pressed a response button, the fixation dot changed from red to green. To announce the next trial the colour changed back to red 267 ms before the start of the trial. Responses had to be as fast as possible and before the colour of the fixation dot changed back to red. Buttons for stack and homogeneous stimuli were always pressed by the index and middle finger of one hand (left or right, counterbalanced over subjects) and the button for frame stimuli was pressed by the index finger of the other hand. Since stack and homogeneous stimuli resemble each other least, this pattern of button responses would make confusion between button responses least likely.
Three experimental settings made sure subjects had to rely on their initial percept and a direct ‘cognitive’ comparison of the inner square with the background was impossible: (i) the short presentation duration of the stimuli (267 ms); (ii) the unpredictable appearance of the stimuli in one of the quadrants of the screen; and (iii) the appearance of the mask.
Subjects practised a discrimination task beforehand with three different practice blocks. Then, six experimental blocks of trials with 32 stimuli per level of border width of each frame and stack stimulus, and 32 homogeneous stimuli were presented to the subjects.
Psychophysics—data analysis
Percentage correct and reaction times were separately analysed for stack and frame stimuli using a repeated measures ANOVA with Border Width (three levels) as within subject factor and Group (patient/control) as a between-subjects factor. Since the homogenous stimuli could not be parametrically manipulated, percentage correct and reaction time data for this stimulus were compared between groups using a one-way ANOVA.
EEG—recording and data analysis
Electroencephalographic activity was recorded by means of a Biosemi 48-channel Active Two EEG system (Biosemi Instrumentation BV, Amsterdam, the Netherlands), and data were sampled at a rate of 256 Hz. Two electrodes in the cap, the CMS (common mode sense) and DRL (driven right leg), provided an ‘active ground’ in this system. To monitor eye movements, vertical and horizontal EOG was recorded with electrodes attached above, below and next to each eye.
Data were referenced to “frontal central electrode” and filtered offline with a high-pass filter at 0.5 Hz, a low-pass filter at 20 Hz and a 60 Hz notch filter. In order to compute event-related potentials, segments from 250 ms pre-stimulus until 700 ms post-stimulus were extracted offline. Before ocular correction, automatic raw artefact rejection was applied by removing segments containing voltage steps of more than 50 μV, removing any segments outside the –200 to 200 μV range as well as segments containing larger than 200 μV differences. EOG artefacts were removed from the EEG using an algorithm where correction factors are calculated on the basis of linear regression (Gratton et al., 1983). After ocular correction, artefact rejection was applied again by removing all segments outside the −75 to 75 μV. Artefact rejection resulted in 9% rejected segments in the control group and 14% rejected segments in the ASD group. Linear local DC de-trending was applied to remove current drift (by subtracting a linear function from each segment). Segments were corrected for baseline, using the data from the 150 ms prior to stimulus onset. All pre-processing steps were done using Brain Vision Analyzer (Brain Products GmbH, Munich, Germany).
To establish if the scalp distributions of the subtraction ERPs were at occipital sites, comparable to previous electrophysiology studies on figure-ground segregation (Knierim and Vanessen, 1992; Lamme, 1995; Caputo and Casco, 1999), spline Laplacian distribution maps were calculated. This was done by interpolating ERP waves using spherical splines and approximating current source densities (Perrin et al., 1989). The resulting maps are spatial second-order derivatives of the scalp potentials lending greater weight to local contributions of cortical generators, filtering out deep sources, as well as being reference free (Nunez and Srinivasan, 2006).
For each subject, the data were averaged for each stimulus and for each quadrant separately. We first averaged the data within quadrants across subjects since stimulation of the different spatial locations led to a retinotopic organization of the ERP signals, which was similar in both groups. Then, individual subject data were averaged for each group, followed by averaging the quadrant data, leaving group averages for homogeneous stimuli, and stack and frame stimuli with different border widths.
Before doing the analyses, we pooled some electrodes to increase the signal-to-noise ratio. Stack and frame stimuli were averaged over border width and, as well as homogeneous stimuli, averaged over groups. The subtraction ‘(stack + frame)–homogeneous’ was used to determine pooling of electrodes by visually inspecting the data. Consistent with earlier electrophysiology findings (Knierim and Vanessen, 1992; Lamme, 1995; Caputo and Casco, 1999), this figure-ground contrast elicited the largest differential activity at occipital sites, i.e. at Oz, O1 and O2; this pool of electrodes was used for further analyses.
Next, we wanted to outline in the control group the time windows related to boundary detection and surface segregation, to subsequently compare these between the groups. Therefore, subtraction potentials were calculated for frame and stack stimuli with thin borders: ‘frame–homogeneous’ and ‘stack–frame’ (see Results section for an explanation and see Fig. 1C). To validly define the time windows in the control group, the first moment of significant deflection was determined with a random effects analysis, performed on the difference waves by employing a one-sample t-test at each time point. The average of each subject at that time point was treated as an observation. Correction for multiple comparisons with respect to the number of time points being tested was done using the False Discovery Rate (FDR, P < 0.01, Benjamini and Hochberg, 1995) in MATLAB (The MathWorks, Inc., Natick, MA, USA). Then, from the first moment of significant deflection, one-sample t-tests were done in SPSS (SPSS Inc., Chicago, IL, USA) on both difference waves over pooled time segments of 20 ms, to determine the total time window of the deflection (P < 0.005).
For the resulting time windows of the subtraction potentials ‘frame–homogeneous’ and ‘stack–frame’ a comparison between groups was done on segments of 20 ms, using a repeated measures ANOVA with Border Width (three levels) as within-subjects factor and Group (patient/control) as between-subjects factor. P-values of <0.005 were considered significant. The occipito-parietal effect studied post hoc (see Results section) was found by continuing the analysis of the 20 ms segments after the time window related to boundary detection.
Results
Contrasting stack, frame and homogeneous stimuli to separate surface segregation from boundary detection
Figure 1A shows the stimuli used in our experiment. In a homogeneous stimulus, no figure is present. A frame stimulus consists of an ‘empty’ frame (border) on a homogeneous background whereas in case of the stack stimulus the inside of the border is filled with lines of a third orientation. These three stimuli contain the same elementary features, i.e. local line elements with specific orientations (see Material and methods section). By using, in different exemplars of each stimulus, all orientations for background, frame or the region within the frame, these low-level features can be fully balanced over trials. In this way all three stimuli will, on average, elicit identical responses from orientation-selective mechanisms, i.e. neurons in early visual areas in the feedforward sweep (Hubel and Wiesel, 1959). In addition, the experimental set-up allows for selectively discounting activity that is caused by the orientation discontinuity, arising from horizontal interactions (Gilbert and Wiesel, 1989; Malach et al., 1993; Stettler et al., 2002): stacks and frames contain the same amount and strength of orientation boundaries. The only difference between these stimuli is that stacks contain an extra texture-defined surface, which results in the percept of the stacking of two squares. This extra surface will elicit a relatively enhanced texture segregation signal in lower occipital areas, arising from feedback from higher cortical areas (Lamme, 1995; Zipser et al., 1996; Lamme et al., 1998b; Angelucci and Bullier, 2003).
In Fig. 1B, we show ERP responses to stack, frame and homogeneous texture stimuli, obtained from both control and ASD subjects. After the subtraction procedure, outlined in detail in Fig. 1C, we obtain more selective responses. As explained above, the remaining signal from the subtraction ‘stack – frame’ is due to the difference in surface organization between stack and frame stimuli. This signal (from control subjects), is shown in Fig. 1D. A significant positive deflection related to surface segregation is found between 215 and 320 ms after stimulus presentation.
The contrasts ‘frame – homogeneous’ and ‘stack – homogeneous’ isolate activity due to both surface segregation and boundary detection. The contribution of surface-segregation mechanisms will be lowest in the ‘frame – homogeneous’ contrast, as the frame has only a minimal amount of surface. In addition, we know from earlier work in both human subjects and monkeys that boundary detection precedes surface segregation in time (Caputo and Casco, 1999; Lamme et al., 1999; Roelfsema et al., 2002). In the control subjects, our stack–frame contrast reveals surface segregation mechanisms to emanate from 215 ms onwards (see above). Therefore, it is logically warranted to attribute any activity found in the frame-homogeneous contrast prior to 215 ms to boundary detection mechanisms. This activity is indeed present, from 121 ms onwards, as shown in Fig. 1E.
Having identified, in control subjects, the ERP signals that relate to either boundary detection or surface segregation, we can now proceed towards investigating whether there are differences between the control (n = 31) and ASD (n = 13) subjects with respect to these two elementary visual processes. First, however, we show the psychophysical data of the behavioural task.
Stack, frame and homogeneous stimuli: performance data from a parametric design
During the EEG measurement behavioural data were obtained, of which group averages are shown in Fig. 2B. Performance decreased with increasing border width (see Fig. 2A) both for frames [F(84,2) = 62.616, P = 0.000] and for stacks [F(84,2) = 15.729, P = 0.000]. In addition, subjects with ASD scored lower than controls on homogeneous stimuli [F(42,1) = 11.578, P = 0.001] and on frame stimuli [F(42,1) = 5.363, P = 0.026), but performance was the same in both groups for stack stimuli [F(42,1) = 0.137, P = 0.713]. There were no interactions between group and border width for either stacks or frames. These data suggest that subjects with ASD are able to detect a stimulus that has a highly salient surface (stacks), whereas detection of a stimulus on the basis of mostly boundaries (frames) is impaired. This impairment could also have led to confusion between homogeneous and frame stimuli, explaining lower performance scores on homogeneous stimuli in the ASD subjects. Finally, it should be noted that there were no differences between the groups on RT for either frame [F(42,1) = 0.552, P = 0.462], stack [F(42,1) = 1.156, P = 0.288] or homogeneous stimuli [F(42,1) = 0.872, P = 0.356].
Impaired boundary detection in subjects with ASD
As explained before, we investigated the specific stimulus subtractions and time windows related to boundary detection and surface segregation to reveal differences between subjects with ASD and controls in the underlying visual processing mechanisms. We should note that when looking at the ERP data, prior to subtraction, in Fig. 1B, one will see additional differences between the groups, at various latencies, both before and after the intervals discussed here. These differences could very well reflect other fundamental distinctions between controls and ASD in the processing of visual stimuli. However, the current stimuli and paradigm only allow stimulus subtractions that reveal the underlying neural mechanisms specifically related to boundary detection and surface segregation. (For example, the detection of line elements was subtracted out and could not be compared between the groups). We do, therefore, not feel free to speculate about the origin of other differences between the groups.
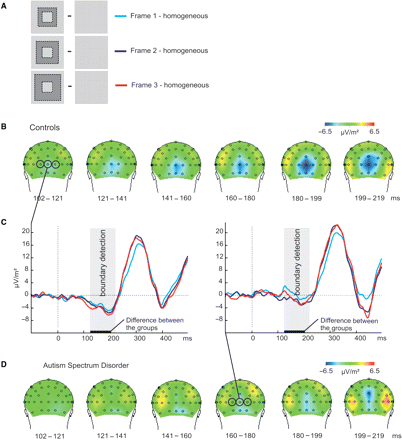
We first compared the ASD subjects with controls on the early part of the frame–homogeneous contrast, which is specific for boundary detection. This contrast was made for all three border widths of the frame stimuli (Fig. 3A). Figure 3C shows the subtraction waves for the three levels of border width. Figure 3B and D depict the activation maps of the control and ASD subjects respectively, during the temporal interval that is specific for boundary detection (roughly 100–200 ms). In these maps, activity related to the frame–homogeneous contrast is pooled for all three frame stimuli, for reasons outlined subsequently.
(A) The subtractions for the three different border widths of frame stimuli that were made to isolate activity related to boundary detection. Different grey levels represent different line orientations (not all line orientations are shown). (B) Activation maps of the frame–homogeneous contrast for the control group, pooled over the three levels of border width. (C) The graphs represent the subtraction waves frame–homogeneous for the three levels of border width at central occipital sites (O1, O2, Oz; controls left; ASD right). During the time window related to boundary detection, indicated by the grey panels, activity was strongly diminished for subjects with ASD compared to controls. (D) Activation maps of the frame–homogeneous contrast for the ASD group, pooled over the three levels of border width.
As already revealed, the control group showed a negative deflection related to boundary detection from 121 ms after stimulus presentation at central occipital electrodes (Fig. 3B). Remarkably, this negativity was strongly diminished in the ASD group (compare the maps of Fig. 3D with those of Fig. 3B, and the ERP traces in Fig. 3C). A repeated measures ANOVA confirmed this: from 121 to 203 ms the subtraction waves (Fig. 3C) differed significantly between the groups [F(42,1) > 9, P < 0.005] over all three levels of border width. Note that the ERP data include trials for both correct and incorrect responses. The accuracy of the behavioural response might have confounded the ERP difference in boundary detection between the groups. However, excluding the incorrect trials (about 35%) would strongly reduce the statistical power and it would undo counterbalancing of the line orientations that we carefully applied when designing the stimuli (see Material and methods section).
In addition, we found that activity increased with thicker borders on the boundary-related signal from 121 to 141 ms [F(42,1) > 5, P < 0.005]. This is probably due to the fact that when the ‘width’ of the border increases, the ‘length’ of the orientation boundary also enlarges, resulting in enhanced boundary-related signal. However, the effect of border width was very small and not distinguishable at the level of the activation maps. Therefore, we pooled the activity of all three border widths before computing the maps of Fig. 3B and D.
We should also note that the boundary-related signal was weaker for a stimulus presented in the upper visual field compared to a stimulus in the lower visual field, which was evident in both groups. Therefore, we re-analysed the data for the upper and the lower visual fields separately. For stimulation of the lower visual field, the difference between the groups for the boundary-related ERP was of the same magnitude and strength as displayed in Fig. 3, while stimulation of the upper visual field led to a similar, but less significant trend. There were no differences between stimulation of the left or right hemifield.
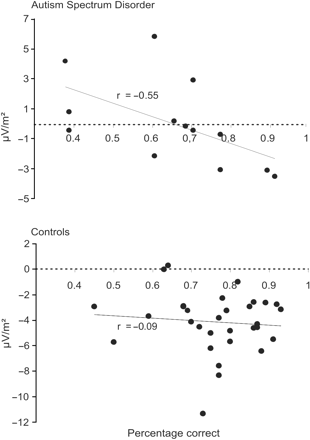
Our finding that ASD subjects show a diminished boundary-related negativity is consistent with the psychophysical results as the subjects with ASD scored lower on frame stimuli than controls. Correct detection of frames relies more heavily on processing of orientation boundaries, since there is little signal related to surface segregation. A deficit in the identification of frames could well be related to the diminished boundary-related negativity in the patient group. To test this, we correlated the performance on frame stimuli, averaged over border width, to the boundary-related ERP signal, also averaged over border width, separately for each subject group. Interestingly, we found that there was a negative correlation between the performance data and the subtraction ERP for the ASD subjects on the first 20 ms time bin (i.e. 121–141 ms, Pearson's r = −0.55, P = 0.05, see Fig. 4). This means that ASD subjects who scored higher on frame stimuli showed a more negative ERP deflection. A correlation between performance on frames and the boundary-related negativity was not evident for the control subjects (Pearson's r = −0.09, P = 0.65). This finding confirms that a deficit in the detection of frames in ASD subjects is probably related to the diminished boundary-related ERP signal, whereas when subjects with ASD performed similar to controls, their ERP signals also showed a comparable boundary-related negative deflection.
Correlation graph of the performance scores on frame stimuli and the boundary-related ERP (i.e. frame–homogeneous, averaged over border width) for the time window 121–141 ms, separately for each subject group (top: ASD, bottom: controls). The negative correlation for the ASD subjects (P = 0.05) indicates that with increasing performance the boundary-related ERP was more negative, i.e. more comparable to controls. There was no significant correlation in the control group between performance and the boundary related ERP (P = 0.65)
Normal surface segregation in subjects with ASD
Surface segregation was singled out by the stack–frame contrast (see Fig. 5). This contrast was made for all three levels of border width of the frame and stack stimuli (Fig. 5A). Figure 5C shows the subtraction waves for the three levels of border width. Figure 5B and D depict the activation maps of the control and ASD subjects, respectively, during the temporal interval that is specific for surface segregation (roughly 200–300 ms), and for stacks and frames with the thinnest borders (stack 1–frame 1, see Fig. 5A). This contrast was selected for presentation because it yielded the highest signal-to-noise ratio of the stack–frame activity.
(A) The subtractions for the three different border widths of stack and frame stimuli that were made to isolate activity related to surface segregation. Different grey levels represent different line orientations (not all line orientations are shown). (B) Activation maps of the stack–frame contrast for the control group, only for the stack and frame stimuli with thin borders [level 1; see A)]. (C)The graphs represent the subtraction waves stack–frame for the three conditions at central occipital sites (O1, O2, Oz; controls left; ASD right). During the time window related to surface segregation, indicated by the grey panels, activity was the same for subjects with ASD and controls. (D) Activation maps of the frame–homogeneous contrast for the ASD group, only for the stack and frame stimuli with thin borders (level 1; see A).
As already revealed, the control group showed a positive deflection related to surface segregation from 215 to 320 ms after stimulus presentation at central occipital electrodes, indicated in red at the maps in Fig. 5B. Figure 5D shows the activation maps for the ASD group. The subtraction waves (Fig. 5C) and the maps show that there were no differences between the groups during this time window. Although it appears as if the positive deflection continued in the ASD group after this time interval, i.e. after 320 ms, group effects were not significant. These data are again consistent with our psychophysical findings: the subjects with ASD had the same performance scores compared to controls on stack stimuli, for which the detection relies heavily on surface segregation.
On the basis of our behavioural data showing that the discrimination between stacks and frames decreased with increasing border width (see Fig. 2B), we expected that the stack–frame difference wave would also decrease (see also Material and methods section). The graphs in Fig. 5C show that this was indeed the case in both groups from 242 to 320 ms [F(84,2) > 7, P < 0.005]. It should be noted that the decrease in the performance data and the decrease in the ERP activity for the stack–frame contrast were both linear [performance: F(42,1) > 18, P < 0.005; ERP: F(42,1) > 10, P < 0.005]. Since the ERP activity at central occipital sites probably reflects processing in striate and extra-striate areas, the results provide additional evidence for the role of these visual areas in the perceptual interpretation of a visual scene, as has been suggested previously (Lee et al., 1998; Super et al., 2001; Guo et al., 2004).
Compensation in higher cortical areas?
Thus far, we found that boundary detection mechanisms are aberrant in ASD, while surface-segregation mechanisms are not. How can this be, given that boundary detection is considered to be an essential prerequisite for surface segregation? We further investigated the frame–homogeneous contrast; that is, we looked at the activity ‘after’ the time window that was specific to boundary detection. Surprisingly, it turned out that from 223 to 243 ms the signal at occipito-parietal electrodes (PO7, PO8, P7 and P8) was more positive in the ASD group compared to controls (see Fig. 6A). This enhanced positivity was dependent on the border width of the frame stimuli: the positive deflection increased with increasing border width in the ASD group but not in the control group [F(84,2) > 6, P < 0.005, see Fig. 6B]. This effect at lateral occipital sites in the ASD group could cautiously be interpreted as enhanced processing and may reflect a compensatory mechanism for the earlier atypical processing, around 120 ms, at lower level—central occipital—sites. See the Discussion section for a more extensive debate on this issue.
(A) Activation maps of the frame–homogeneous contrast for the control group and ASD group, pooled over the three levels of border width. (B) The graphs represent the subtraction waves frame–homogeneous for the three conditions at lateral occipital sites (PO7, PO8, P7, P8). Activity increased with increasing border width for subjects with ASD but not for control subjects from 223 to 243 ms.
Discussion
In the current research, the neural basis of atypical visual detail perception in ASD was investigated with ERPs. We conjectured that this aspect of ASD is due to an imbalance between feedforward, horizontal and feedback activity. By using a texture segregation task, where surface segregation could be varied independently of orientation-based boundary detection, feedforward, horizontal and recurrent processing could be explicitly tested. The results showed that from 121 ms after stimulus presentation, subjects with ASD had strongly diminished activity at central occipital electrodes compared to controls in response to orientation boundaries. Interestingly, when contrasting activity of stack and frame stimuli, we found activity associated with surface segregation from 215 to 320 ms at occipital electrodes, which did not differ between the groups. The ERP data parallel the results of the behavioural data since subjects with ASD scored lower on frame stimuli (for which correct identification relied mainly on boundary detection), but not on stack stimuli (for which the correct identification relied mainly on surface segregation). A significant correlation for the ASD subjects between performance on frame stimuli and the boundary-related negativity, i.e. a more negative deflection for subjects with higher performance scores, confirmed the association between the behavioural and electrophysiological results. Specifically, ASD subjects who were better in detecting frame stimuli, also showed a more ‘normal’ boundary-related ERP signal, i.e. more comparable to that of controls.
As explained in the Introduction section, there is evidence from neurophysiological, neuroanatomical and modelling studies that horizontal connections, mainly those between cells with similar orientation tuning (Malach et al., 1993), are vital to the process of boundary detection through lateral inhibition (Knierim and Vanessen, 1992; Lamme et al., 1999). Surface segregation, on the other hand, is associated with recurrent or feedback processing from higher to lower visual areas (Lamme et al., 1998a; Lamme and Roelfsema, 2000). Therefore, these data give strong evidence for aberrant inhibition through horizontal connections in low-level visual areas in ASD, whereas feedback mechanisms are intact in these patients. We would like to note that this inference is in agreement with the notion of Bertone and colleagues (Bertone et al., 2005) that lateral connectivity is atypical in ASD, which they based on the findings of impaired orientation discrimination of textured stimuli. It is the first time that direct evidence for a neural mechanism related to atypical visual perception has been found in ASD. Subsequently, we will indicate how the findings on lateral inhibition relate to neuropathology and neurochemistry research, and how malfunctioning of horizontal connections might be related to aberrant visual perception in ASD.
Horizontal connections in ASD
An impairment in lateral inhibition
Interestingly, an imbalance between excitation and inhibition in neural circuits in ASD has been suggested as a consequence of an imbalance between the amount of excitatory and inhibitory neurotransmitters. Hussman (2001) proposed that the imbalance could be due to either increased glutamergic signalling, leading to enhanced excitation, or to malfunctioning of the inhibitory neurotransmitter GABA, leading to a reduction in inhibition. There are two arguments for this idea. First, pathology relating to GABA receptors emerges as a common factor in several suspected aetiologies of autism. Second, both disinhibition of the GABAergic influence and excessive stimulation of non-NMDA glutamate receptors, generate pathology which mirrors that observed in autism (Hussman, 2001). This idea of an enhanced excitation to inhibition ratio is supported by others (Rubenstein and Merzenich, 2003; Cline, 2005) and Collins et al. (2006) showed, by studying GABA receptor subunit genes, that indeed the GABAergic system is involved in the aetiology of autism.
A second line of evidence for disturbed lateral inhibition through horizontal connections comes from studies on the neuropathology of ASD. Casanova et al. (2002, 2003, 2006a, b) have shown that the minicolumn organization in several areas of the cerebral cortex (including Brodmann areas 3, 4, 9, 17, 21 and 22) of patients with ASD is altered. Minicolumns are functional units of the brain that organize about 80–100 neurons with a common set of functional properties in cortical space. Several minicolumns (60 to 80) in turn combine into a hypercolumn, as defined by Hubel and Wiesel (1977). The minicolumnar aberrancies in ASD include that neurons inside a minicolumn are more widely spaced, minicolumns are smaller and, given that the cerebral cortex is not smaller in ASD patients, the authors inferred that there must be a larger total number of minicolumns filling the cortical space. In addition, in Brodmann areas 9, 17, 21 and 22, they showed that the minicolumns contained less neuropil space. Neuropil consists of unmyelinated fibres, in part from inhibitory projections of double-bouquet interneurons. Casanova et al. (2002, 2003) hypothesize that the altered structural organization of minicolumns will lead to the disturbance of the flow of information between minicolumns, diminished lateral inhibition, as well as an enhanced rate of epilepsy-seizures. Around puberty, one-third of the people with ASD will have suffered at least two unprovoked seizures (depending on the strictness of the diagnosis and on the level of mental retardation, Ballaban-Gil and Tuchman, 2000).
Both lines of research indicate that inhibition through horizontal connections is impaired in ASD, in line with our own findings. While we imply that impaired lateral inhibition is related to atypical visual perception in ASD, altered organization of minicolumns has been found in several different brain areas, including the pre-frontal cortex, and GABAergic inhibition plays a role throughout the brain. Therefore, we suggest that aberrant lateral inhibition could be a more general neurobiological deficit in ASD (see also Hussman, 2001), not only specific to visual perception. It would be a promising direction for future research to study the functional integrity of these inhibitory interactions more thoroughly to see how they are related to other behavioural symptoms of ASD.
Malfunctioning of horizontal connections
As already indicated in the Introduction section, besides the role of horizontal connections in the detection of figure boundaries through lateral inhibition, horizontal interactions also play an important role in a different aspect of perception, namely grouping or binding (Polat, 1999; Roelfsema, 2006). Accordingly, malfunctioning of horizontal connections could also provide an explanation for other features of atypical visual perception in ASD, namely impaired Gestalt processing and grouping (Brosnan et al., 2004), as well as superior performance on the embedded figures test (see Introduction section, Jolliffe and Baron-Cohen, 1997; Ropar and Mitchell, 2001; De Jonge et al., 2006).
Enhanced processing at occipito-parietal sites: a compensation mechanism?
When we investigated the frame–homogeneous signal after the time window related to boundary detection only, we found an enhanced positivity in the patient group compared to controls, around 225 ms at occipito-parietal sides. This suggests that, probably due to diminished lateral inhibition through horizontal connections at early stages, the brain puts more effort in the correct identification of a stimulus at subsequent analysis stages, eventually resulting in normal recurrent activity back to early visual areas. At higher-level areas, the orientation discontinuity that is present in the scene is probably detected by neurons with larger receptive fields (Roelfsema et al., 2002). In the ASD subjects processing by these neurons apparently is enhanced compared to controls, which is supported by the observation that the difference between ASD and control subjects depends on border width: it is strongest for larger borders. We interpret this finding as a compensation mechanism. Interestingly, Belmonte and Yurgelun-Todd (2003) showed with fMRI compensatory processing in the posterior intraparietal sulcus in people with ASD during a visual selective attention task. The combination of disrupted early processing with compensation at higher levels may be a general feature of the physiology of perception in ASD.
Conclusion
We have shown a fundamental visual processing aberrancy in ASD, which is probably caused by impaired interactions through horizontal connections in lower visual areas. This aberrancy can be compensated later in time in higher cortical areas, but the exact mechanism or implication of this compensation is not entirely clear. Interestingly, a deficit in neuronal interactions within cortical areas in ASD has been suggested before, stemming from different lines of research such as neuropathology and neurochemistry research, and is apparent in different brain areas besides the occipital cortex. Possibly, malfunctioning of horizontal connections is a more general neurobiological deficit underlying several symptoms of ASD.
Acknowledgements
The work described was supported by an Innovational Research Incentives grant (VIDI-scheme, 402-01-094) of the Netherlands Organization for Scientific Research (NWO) to C.K.
References
Abbreviations:
- ADI-R
autism diagnostic interview—revised
- ADOS-G
autism diagnostic observation schedule—generic
- ASD
autism spectrum disorder
- CMS
common mode sense
- DRL
driven right leg
- ERP
electrophysiological recording
- FDR
false discovery rate.

![(A) The subtractions for the three different border widths of stack and frame stimuli that were made to isolate activity related to surface segregation. Different grey levels represent different line orientations (not all line orientations are shown). (B) Activation maps of the stack–frame contrast for the control group, only for the stack and frame stimuli with thin borders [level 1; see A)]. (C)The graphs represent the subtraction waves stack–frame for the three conditions at central occipital sites (O1, O2, Oz; controls left; ASD right). During the time window related to surface segregation, indicated by the grey panels, activity was the same for subjects with ASD and controls. (D) Activation maps of the frame–homogeneous contrast for the ASD group, only for the stack and frame stimuli with thin borders (level 1; see A).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/brain/131/4/10.1093/brain/awm321/2/m_awm321f5.gif?Expires=1716379870&Signature=lyzG8Uvd7u9O9Ub6IyW92UWXxv9zIUdVaeSVN~C0A0H2CB0rMHDa~i-dqV3k6COJUdeXjIuVwFz9Ls4zXuDMQfnu~XhrV8RSdvp85T21zSI5-547J4cXfWf8F7y5d4-CPVWl2v1xkb91XVz5qTj70Y6zJD7RCqkh2YW60wNYM8QKBmTW2A6ubTBGf7Y-he2Gr0tAINeG86WYRHabwzBJDv-ieJEDHooPMtGlu7nnMXEyx3oUrJQqX6CleHzXtGcLXsxv0ae1drp7wdKlL4-y5BoNFMn-iU-7LLly6y~FY0ClsYa6B5OTIWe425kajINLX8LUw32vl6mjB0HTYVEsEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)