-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Confavreux, Sandra Vukusic, Natural history of multiple sclerosis: a unifying concept, Brain, Volume 129, Issue 3, March 2006, Pages 606–616, https://doi.org/10.1093/brain/awl007
Close - Share Icon Share
Abstract
Multiple sclerosis can follow very different patterns of evolution and variable rates of disability accumulation. This raises the issue whether it represents one or several distinct diseases. We assessed demographic and clinical characteristics in 1844 patients with multiple sclerosis that we categorized according to the classification of Lublin and Reingold (1996) into 1066 (58%) relapsing–remitting, 496 (27%) secondary progressive, 109 (6%) progressive relapsing and 173 (9%) primary progressive cases of multiple sclerosis. Relapsing–remitting and secondary progressive cases shared similar age at disease onset (median = 28.7 versus 29.5 years; P = 0.21), initial symptoms of the relapsing–remitting phase, degree of recovery from the first neurological episode, and time from the first to the second episode. By contrast, disease duration was twice as long in secondary progressive than in relapsing–remitting cases (mean ± SD = 17.6 ± 9.6 versus 8.7 ± 8.6 years; P < 0.001). Progressive relapsing and primary progressive cases were essentially similar in their clinical characteristics. In patients experiencing a progressive course, median age at onset of progressive phase was similar in secondary progressive cases and in cases who were progressive from onset (39.1 versus 40.1 years; P = 0.47). The proportion of cases with superimposed relapses during progression was ∼40% in both categories. Finally, the 1562 patients with an exacerbating–remitting initial course and the 282 patients with a progressive initial course of the disease were essentially similar with respect to the time course of disability accumulation from assignment to a given disability score, and the age at assignment of disability landmarks. These observational data suggest that the clinical phenotype and course of multiple sclerosis are age dependent. Relapsing–remitting disease can be regarded as multiple sclerosis in which insufficient time has elapsed for the conversion to secondary progression; secondary progressive forms as relapsing–remitting multiple sclerosis that has ‘grown older’; and progressive from onset cases as multiple sclerosis ‘amputated’ from the usual preceding relapsing–remitting phase. Times to reach disability milestones, and ages at which these landmarks are reached, follow a predefined schedule not obviously influenced by relapses, whenever they may occur, or by the initial course of the disease, whatever its phenotype. This leads to a unifying concept of the disease in which primary and secondary progression might be regarded as essentially similar. From the clinical and statistical positions, multiple sclerosis might be considered as one disease with different clinical phenotypes rather than an entity encompassing several distinct diseases—the position of complexity rather than true heterogeneity.
Introduction
Multiple sclerosis can follow very different patterns of evolution and variable rates of disability accumulation. This raises the issue whether it represents one or several distinct diseases. There may be much to be learned on this topic from detailed scrutiny of the natural history of the disease. The course of multiple sclerosis may be considered as the expression of two clinical phenomena, relapses of acute neurological symptoms, which end with a partial or complete remission, and progression, which refers to the steady and irreversible worsening of symptoms and signs over ≥6 months. In turn, this analysis brings into the equation the interplay between two biological activities: inflammation and degeneration. There is strong evidence that relapses are mainly the expression of acute, focal, disseminated and recurrent inflammation occurring within the central nervous system (Youl et al., 1991). For each clinical episode, there is an average of 10 new MRI lesions (McDonald, 1994). One could say that ‘multiple sclerosis never sleeps’ and that relapses are therefore a direct but also a ‘filtered’ clinical expression of inflammation. There is also increasing evidence from pathology (Evangelou et al., 2000) and MR techniques (Losseff et al., 1996a, b; Fu et al., 1998; Arnold, 1999; Rudick et al., 1999; Brex et al., 2000; Fox et al., 2000; Tortorella et al., 2000; Ciccarelli et al., 2001; Traboulsee et al., 2002; Filippi et al., 2003) that progression and accumulation of disability correlate with the early, diffuse, chronic and progressive axonal loss, which is the hallmark of the neurodegenerative process in multiple sclerosis.
For 85% of the patients, relapses are the exclusive clinical expression of multiple sclerosis during the early years of the disease and this defines the relapsing–remitting phase of the disease. In a proportion of these patients which increases with disease duration, the course of multiple sclerosis converts to a secondary progressive phase. For 15% of the patients, the progressive phase is free of a preceding relapsing–remitting phase. Relapses are present during the primary or the secondary progressive phase of the disease in ∼40% of the patients (Confavreux et al., 2000).
Although these data are well acknowledged, the actual spectrum of the clinical course of multiple sclerosis is still much debated. Following an international survey of clinicians involved with multiple sclerosis, the current consensus is to consider four distinct categories (Lublin and Reingold, 1996). The overall course of multiple sclerosis is thus classified as ‘relapsing–remitting’ when the disease exhibits only relapses and remissions; ‘secondary progressive’ when an initial relapsing–remitting phase is followed by a progressive phase, whether superimposed with relapses or not; ‘primaryprogressive’ when the disease starts with a progressive phase and no relapse supervenes upon progression; ‘progressive relapsing’ when the progressive phase is present since the onset of the disease and superimposed with relapses. Some authors also consider a category of ‘transitional multiple sclerosis’ for depicting the cases with an isolated relapse occurring at some point before or after the onset of disease progression (Filippi et al., 1995; Gayou et al., 1997; Stevenson et al., 1999, 2000). Therefore, a variety of types of evolution does exist for multiple sclerosis, not to mention the vast range of rates of accumulation of irreversible neurological disability during the disease from one patient to another. For many clinicians, describing the course and the prognosis is therefore a kind of ‘mission impossible’ or, at least, a puzzling task. For some of them, the term of multiple sclerosis could even encompass separate disease entities (Lucchinetti et al., 2000; Weinshenker, 2000).
We have hypothesized that under this apparent disorder and complexity, some rules could be drawn from the statistical analysis of the natural history of multiple sclerosis. This could help go deeper in the discussion over a splitting or a unifying concept of the disease and lead to a reappraisal of the Lublin and Reingold's (1996) classification. The Lyon Multiple Sclerosis Cohort is a unique natural history database both in terms of size and quantity of data gathered since 1957. It has been used to address the present issues.
Methods
Patient population and data collection
Patients were identified through the Lyon Multiple Sclerosis Cohort that was established in the Lyon Clinique de Neurologie in 1957. The cohort includes all the patients with a diagnosis of multiple sclerosis examined at least once at the clinic. Data were computerized in 1976 and, since 1990, have been entered on the European Database for Multiple Sclerosis (EDMUS) software (Confavreux et al., 1992).
Individual case reports include identification and demographic data, medical history, key episodes in the multiple sclerosis course (relapses, onset of the progressive phase, dates of assignment of the successive scores of irreversible disability), biological, electrophysiological and neuro-imaging data, and treatment. Data are entered retrospectively when the patient is first seen at the clinic. A specific effort is always made to obtain data from the original medical files, especially for the first neurological episode, and on the clinical course and disability. This effort is facilitated by the existing regional network of neurologists in our area and allows to update the database with follow-up data regularly. Data are collected prospectively whenever the patient returns, usually on a yearly basis. New data are automatically checked by the system for consistency with older information. Confidentiality and safety of the data are ensured in keeping with the recommendations of the French Commission Nationale Informatique et Libertés, which also provides approval. All patients give informed consent for having their data saved in the database.
Definition of cases and assessment of patients
By April 1997, a cohort of 2021 patients had been included in the database. At that time, the database was locked for the purpose of epidemiological studies. Diagnosis of multiple sclerosis was established according to Poser's classification (Poser et al., 1983).
A relapse of multiple sclerosis was defined as the occurrence, the recurrence or the worsening of symptoms of neurological dysfunction lasting over 24 h and usually ending up in a partial or complete remission (Confavreux et al., 1992; Lublin and Reingold, 1996). Fatigue alone and transient fever-related worsening of symptoms were not considered as a relapse. Symptoms occurring within a month were considered as part of the same relapse. The progression/progressive phase of multiple sclerosis was defined as the steady worsening of symptoms and signs for at least six months, whether superimposed with relapses or not (Schumacher et al., 1965). Once started, it continues throughout the disease, though occasional plateaus and temporary minor improvements may be observed (Lublin and Reingold, 1996). Course of the disease was categorized according to acknowledged classifications (Lublin and Reingold, 1996). Initial course was considered as ‘exacerbating–remitting’ or ‘progressive’. Overall course was classified as ‘relapsing–remitting’, ‘secondary progressive’, ‘primary progressive’ and ‘progressive relapsing’ as defined above.
The Kurtzke Disability Status Scale (DSS) score was recorded at each visit to determine the extent of the neurological disability (Kurtzke, 1961, 1983). We focused on the scores that could be easily identified, even by interviewing the patient retrospectively. A score of 4 corresponds to limited walking ability but without aid or rest for more than 500 m; a score of 6 corresponds to ability to walk with unilateral support no more than 100 m without rest; and a score of 7 corresponds to ability to walk no more than 10 m without rest while leaning against a wall or holding onto furniture for support. Disability was defined as irreversible when a given score persisted at least 6 months, excluding transient worsening of disability related to relapses. By definition, when a given score of irreversible disability had been assigned to a given patient, all the scores of disability that could be subsequently assessed during the follow-up of the patient were either equal to or higher than that score. For each patient, the date of assignment to a given score of irreversible disability was assessed whenever appropriate.
A series of clinical variables were systematically assessed for each patient. They included gender, date of onset of multiple sclerosis and age at onset of multiple sclerosis. Initial symptoms were categorized into isolated optic neuritis, isolated brainstem dysfunction, isolated dysfunction of long tracts and combination of these symptoms. Recovery from the first neurological episode was classified as complete when the irreversible score after the episode was 2 or less on the Kurtzke DSS; incomplete, when this score was 3 or more (Confavreux et al., 1992). Date of onset of the second neurological episode of multiple sclerosis, which may be a relapse or the onset of the progressive phase, was also systematically assessed whenever appropriate. The same was true for the subsequent relapses and onset of the progressive phase of the disease.
Statistical analysis
Comparisons of categorical data were made according to the chi-squared test. The Student's t-test was used for the comparison of quantitative data. The Kaplan–Meier technique was used for estimating the time to the assignment of an irreversible score of DSS 4, DSS 6 and DSS 7. The same technique was used for estimating the age at the time of assigning irreversible disability landmarks, at onset of the relapsing–remitting phase, and at onset of the progressive phase of the disease. Age can indeed be considered as a survival data, that is time from birth to assignment of the chosen disability scores. Data have been censored at the time/age at the last visit whenever the end-points had not been reached. Survival curves were compared using the log-rank test. All computations were performed using SPSS for Windows, version 11.0.
Results
Demographic and clinical characteristics
Among the 2021 patients potentially eligible, 170 classified as possible multiple sclerosis only (Poser et al., 1983) and 7 whose initial symptoms were unknown were excluded. The baseline characteristics of the remaining 1844 patients with a diagnosis of definite or probable multiple sclerosis have already been described (Confavreux et al., 2000, 2003). Noticeably, the 1844 patients could be distributed into 1066 (58%) relapsing–remitting, 496 (27%) secondary progressive, 109 (6%) progressive relapsing, and 173 (9%) primary progressive cases of multiple sclerosis according to the classification of Lublin and Reingold (1996).
Relapsing–remitting multiple sclerosis and secondary progressive multiple sclerosis
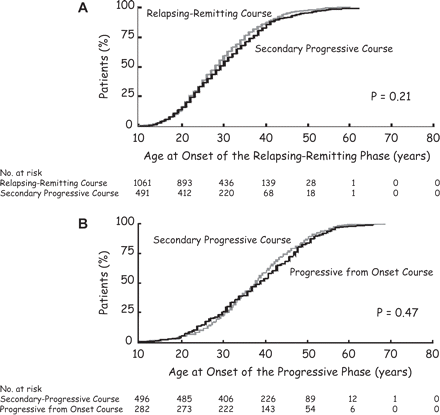
When comparing the patients with a relapsing–remitting overall course and the patients with a secondary progressive course, a difference could be noticed regarding gender distribution with a higher proportion of females in relapsing–remitting multiple sclerosis than in secondary progressive multiple sclerosis (68% versus 61%; P = 0.006) (Table 1). Conversely, the two populations were strikingly similar with respect to the age at onset of multiple sclerosis (Table 1 and Fig. 1A). They were also similar with respect to the initial symptoms of the relapsing–remitting phase of multiple sclerosis, the degree of recovery from the first neurological episode, and the time from onset of multiple sclerosis to the second neurological episode. By contrast, the two populations clearly differed in duration of the disease, which was twice as long in the secondary progressive than in the relapsing–remitting multiple sclerosis group (mean ± SD: 17.6 ± 9.6 versus 8.7 ± 8.6 years; P < 0.001) (Table 1).
Kaplan–Meier estimates for the age at onset of the relapsing–remitting phase (A) and of the progressive phase of multiple sclerosis (B) among 1844 patients with multiple sclerosis, according to the overall course of the disease. (A) Among the 1066 patients with a relapsing–remitting course of multiple sclerosis, five started the relapsing–remitting phase before the age of 10 years. Similarly, among the 496 patients with a secondary-progressive course of multiple sclerosis, five started the relapsing–remitting phase before the age of 10 years.
Comparative demographic and disease-related characteristics of relapsing–remitting cases and secondary progressive cases, among 1562 patients with an exacerbating–remitting onset of multiple sclerosis
. | Relapsing remitting multiple sclerosis†n = 1066 . | Secondary progressive multiple sclerosis†n = 496 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 342 (32) | 194 (39) | 0.006* | |||
| Females | 724 (68) | 302 (61) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 29.4 ± 9.3 | 29.8 ± 9.9 | 0.39*** | |||
| Median | 28.7 | 29.5 | ||||
| Range | 6–61 | 5–62 | ||||
| Initial symptoms of the relapsing–remitting phase: no. (%) | ||||||
| Isolated optic neuritis | 222 (21) | 108 (22) | 0.13* | |||
| Isolated brainstem dysfunction | 100 (9) | 58 (12) | ||||
| Isolated dysfunction of long tracts | 491 (46) | 236 (47) | ||||
| Combination of symptoms | 253 (24) | 94 (19) | ||||
| Recovery from the first episode: no. (%)# | ||||||
| Complete | 887 (83) | 401 (81) | 0.25* | |||
| Incomplete | 179 (17) | 95 (19) | ||||
| Kaplan–Meier estimate of the time from onset of multiple sclerosis to the second episode: (years) | ||||||
| Median | 1.7 | 2.3 | 0.07** | |||
| 95% CI | [1.5–1.9] | [2.0–2.7] | ||||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 8.7 ± 8.6 | 17.6 ± 9.6 | <0.001*** | |||
| Median | 6.0 | 16.0 | ||||
| Range | 0–52 | 1–47 | ||||
. | Relapsing remitting multiple sclerosis†n = 1066 . | Secondary progressive multiple sclerosis†n = 496 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 342 (32) | 194 (39) | 0.006* | |||
| Females | 724 (68) | 302 (61) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 29.4 ± 9.3 | 29.8 ± 9.9 | 0.39*** | |||
| Median | 28.7 | 29.5 | ||||
| Range | 6–61 | 5–62 | ||||
| Initial symptoms of the relapsing–remitting phase: no. (%) | ||||||
| Isolated optic neuritis | 222 (21) | 108 (22) | 0.13* | |||
| Isolated brainstem dysfunction | 100 (9) | 58 (12) | ||||
| Isolated dysfunction of long tracts | 491 (46) | 236 (47) | ||||
| Combination of symptoms | 253 (24) | 94 (19) | ||||
| Recovery from the first episode: no. (%)# | ||||||
| Complete | 887 (83) | 401 (81) | 0.25* | |||
| Incomplete | 179 (17) | 95 (19) | ||||
| Kaplan–Meier estimate of the time from onset of multiple sclerosis to the second episode: (years) | ||||||
| Median | 1.7 | 2.3 | 0.07** | |||
| 95% CI | [1.5–1.9] | [2.0–2.7] | ||||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 8.7 ± 8.6 | 17.6 ± 9.6 | <0.001*** | |||
| Median | 6.0 | 16.0 | ||||
| Range | 0–52 | 1–47 | ||||
Defined according to the Lublin and Reingold (1996) classification.
The recovery was considered as complete when the irreversible score after the first relapse was 2 or less on the Kurtzke DSS; incomplete, when this score was 3 or more.
SD denotes standard deviation and CI confidence intervals.
P-values are calculated with use of the *chi-squared test,
the log rank test or
the Student's t-test.
Comparative demographic and disease-related characteristics of relapsing–remitting cases and secondary progressive cases, among 1562 patients with an exacerbating–remitting onset of multiple sclerosis
. | Relapsing remitting multiple sclerosis†n = 1066 . | Secondary progressive multiple sclerosis†n = 496 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 342 (32) | 194 (39) | 0.006* | |||
| Females | 724 (68) | 302 (61) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 29.4 ± 9.3 | 29.8 ± 9.9 | 0.39*** | |||
| Median | 28.7 | 29.5 | ||||
| Range | 6–61 | 5–62 | ||||
| Initial symptoms of the relapsing–remitting phase: no. (%) | ||||||
| Isolated optic neuritis | 222 (21) | 108 (22) | 0.13* | |||
| Isolated brainstem dysfunction | 100 (9) | 58 (12) | ||||
| Isolated dysfunction of long tracts | 491 (46) | 236 (47) | ||||
| Combination of symptoms | 253 (24) | 94 (19) | ||||
| Recovery from the first episode: no. (%)# | ||||||
| Complete | 887 (83) | 401 (81) | 0.25* | |||
| Incomplete | 179 (17) | 95 (19) | ||||
| Kaplan–Meier estimate of the time from onset of multiple sclerosis to the second episode: (years) | ||||||
| Median | 1.7 | 2.3 | 0.07** | |||
| 95% CI | [1.5–1.9] | [2.0–2.7] | ||||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 8.7 ± 8.6 | 17.6 ± 9.6 | <0.001*** | |||
| Median | 6.0 | 16.0 | ||||
| Range | 0–52 | 1–47 | ||||
. | Relapsing remitting multiple sclerosis†n = 1066 . | Secondary progressive multiple sclerosis†n = 496 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 342 (32) | 194 (39) | 0.006* | |||
| Females | 724 (68) | 302 (61) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 29.4 ± 9.3 | 29.8 ± 9.9 | 0.39*** | |||
| Median | 28.7 | 29.5 | ||||
| Range | 6–61 | 5–62 | ||||
| Initial symptoms of the relapsing–remitting phase: no. (%) | ||||||
| Isolated optic neuritis | 222 (21) | 108 (22) | 0.13* | |||
| Isolated brainstem dysfunction | 100 (9) | 58 (12) | ||||
| Isolated dysfunction of long tracts | 491 (46) | 236 (47) | ||||
| Combination of symptoms | 253 (24) | 94 (19) | ||||
| Recovery from the first episode: no. (%)# | ||||||
| Complete | 887 (83) | 401 (81) | 0.25* | |||
| Incomplete | 179 (17) | 95 (19) | ||||
| Kaplan–Meier estimate of the time from onset of multiple sclerosis to the second episode: (years) | ||||||
| Median | 1.7 | 2.3 | 0.07** | |||
| 95% CI | [1.5–1.9] | [2.0–2.7] | ||||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 8.7 ± 8.6 | 17.6 ± 9.6 | <0.001*** | |||
| Median | 6.0 | 16.0 | ||||
| Range | 0–52 | 1–47 | ||||
Defined according to the Lublin and Reingold (1996) classification.
The recovery was considered as complete when the irreversible score after the first relapse was 2 or less on the Kurtzke DSS; incomplete, when this score was 3 or more.
SD denotes standard deviation and CI confidence intervals.
P-values are calculated with use of the *chi-squared test,
the log rank test or
the Student's t-test.
Progressive relapsing multiple sclerosis and primary progressive multiple sclerosis
The median age at onset of the disease was earlier in progressive relapsing than in primary progressive cases (38 versus 41 years; P = 0.005) (Table 2). The median age at the time of assigning a score of DSS 4 was also earlier in progressive relapsing than in primary progressive cases (40 versus 43 years; P = 0.003). These were the only differences observed when comparing these two forms of multiple sclerosis according to demographic and disease-related clinical characteristics such as age at the time of assigning a score of DSS 6 or DSS 7, gender, initial symptoms of the disease and disease duration (Table 2). Moreover, the rates at which irreversible disability progresses, calculated from the onset of multiple sclerosis or from assignment of a given disability score, were essentially similar (Table 2).
Comparative demographic and disease-related characteristics of progressive relapsing cases and primary progressive cases, among 282 patients with a progressive onset of multiple sclerosis
. | Progressive relapsing multiple sclerosis†n = 109 . | Primary progressive multiple sclerosis†n = 173 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 41 (38) | 80 (46) | 0.15* | |||
| Females | 68 (62) | 93 (54) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 37.3 ± 11.5 | 40.6 ± 10.7 | 0,02*** | |||
| Median | 38.1 | 41.3 | ||||
| Range | 11–58 | 13–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (1) | 4 (2) | 0.14* | |||
| Isolated brainstem dysfunction | 0 (0) | 1 (1) | ||||
| Isolated dysfunction of long tracts | 87 (80) | 150 (87) | ||||
| Combination of symptoms | 21 (19) | 18 (10) | ||||
| Kaplan–Meier estimates of the time [median (95% CI)]: (years) | ||||||
| From onset of multiple sclerosis to assignment of a disability status score of | ||||||
| DSS 4 | 0.0 | 0.0 | 0.50** | |||
| DSS 6 | 7.5 [5.8–9.2] | 6.8 [6.1–7.6] | 0.37** | |||
| DSS 7 | 13.7 [10.1–17.2] | 12.8 [9.9–15.7] | 0.92** | |||
| From assignment of DSS score of 4 to assignment of a score of | ||||||
| DSS 6 | 5.4 [3.3–7.5] | 5.5 [4.5–6.5] | 0.71** | |||
| DSS 7 | 11.3 [7.8–14.7] | 12.4 [10.2–14.7] | 0.65** | |||
| From assignment of DSS score of 6 to assignment of a score of | ||||||
| DSS 7 | 3.6 [2.2–5.0] | 4.0 [2.8–5.2] | 0.68** | |||
| Kaplan–Meier estimates of the age [median (95% CI)] at the time of assigning DSS: (years) | ||||||
| DSS 4 | 40.0 [36.7–43.3] | 43.3 [40.4–46.1] | 0.003** | |||
| DSS 6 | 52.2 [47.8–56.6] | 54.7 [50.7–58.6] | 0.09** | |||
| DSS 7 | 58.7 [53.6–63.7] | 64.4 [61.9–66.9] | 0.11** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 10.9 ± 7.4 | 9.6 ± 8.4 | 0.38*** | |||
| Median | 10.0 | 7.0 | ||||
| Range | 1–40 | 0–62 | ||||
. | Progressive relapsing multiple sclerosis†n = 109 . | Primary progressive multiple sclerosis†n = 173 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 41 (38) | 80 (46) | 0.15* | |||
| Females | 68 (62) | 93 (54) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 37.3 ± 11.5 | 40.6 ± 10.7 | 0,02*** | |||
| Median | 38.1 | 41.3 | ||||
| Range | 11–58 | 13–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (1) | 4 (2) | 0.14* | |||
| Isolated brainstem dysfunction | 0 (0) | 1 (1) | ||||
| Isolated dysfunction of long tracts | 87 (80) | 150 (87) | ||||
| Combination of symptoms | 21 (19) | 18 (10) | ||||
| Kaplan–Meier estimates of the time [median (95% CI)]: (years) | ||||||
| From onset of multiple sclerosis to assignment of a disability status score of | ||||||
| DSS 4 | 0.0 | 0.0 | 0.50** | |||
| DSS 6 | 7.5 [5.8–9.2] | 6.8 [6.1–7.6] | 0.37** | |||
| DSS 7 | 13.7 [10.1–17.2] | 12.8 [9.9–15.7] | 0.92** | |||
| From assignment of DSS score of 4 to assignment of a score of | ||||||
| DSS 6 | 5.4 [3.3–7.5] | 5.5 [4.5–6.5] | 0.71** | |||
| DSS 7 | 11.3 [7.8–14.7] | 12.4 [10.2–14.7] | 0.65** | |||
| From assignment of DSS score of 6 to assignment of a score of | ||||||
| DSS 7 | 3.6 [2.2–5.0] | 4.0 [2.8–5.2] | 0.68** | |||
| Kaplan–Meier estimates of the age [median (95% CI)] at the time of assigning DSS: (years) | ||||||
| DSS 4 | 40.0 [36.7–43.3] | 43.3 [40.4–46.1] | 0.003** | |||
| DSS 6 | 52.2 [47.8–56.6] | 54.7 [50.7–58.6] | 0.09** | |||
| DSS 7 | 58.7 [53.6–63.7] | 64.4 [61.9–66.9] | 0.11** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 10.9 ± 7.4 | 9.6 ± 8.4 | 0.38*** | |||
| Median | 10.0 | 7.0 | ||||
| Range | 1–40 | 0–62 | ||||
Defined according to the Lublin and Reingold (1996) classification. SD denotes standard deviation; CI, confidence intervals; DSS, Kurtzke Disability Status Scale.
P-values are calculated with use of the *chi-squared test,
the log rank test,
the Student's t-test.
Comparative demographic and disease-related characteristics of progressive relapsing cases and primary progressive cases, among 282 patients with a progressive onset of multiple sclerosis
. | Progressive relapsing multiple sclerosis†n = 109 . | Primary progressive multiple sclerosis†n = 173 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 41 (38) | 80 (46) | 0.15* | |||
| Females | 68 (62) | 93 (54) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 37.3 ± 11.5 | 40.6 ± 10.7 | 0,02*** | |||
| Median | 38.1 | 41.3 | ||||
| Range | 11–58 | 13–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (1) | 4 (2) | 0.14* | |||
| Isolated brainstem dysfunction | 0 (0) | 1 (1) | ||||
| Isolated dysfunction of long tracts | 87 (80) | 150 (87) | ||||
| Combination of symptoms | 21 (19) | 18 (10) | ||||
| Kaplan–Meier estimates of the time [median (95% CI)]: (years) | ||||||
| From onset of multiple sclerosis to assignment of a disability status score of | ||||||
| DSS 4 | 0.0 | 0.0 | 0.50** | |||
| DSS 6 | 7.5 [5.8–9.2] | 6.8 [6.1–7.6] | 0.37** | |||
| DSS 7 | 13.7 [10.1–17.2] | 12.8 [9.9–15.7] | 0.92** | |||
| From assignment of DSS score of 4 to assignment of a score of | ||||||
| DSS 6 | 5.4 [3.3–7.5] | 5.5 [4.5–6.5] | 0.71** | |||
| DSS 7 | 11.3 [7.8–14.7] | 12.4 [10.2–14.7] | 0.65** | |||
| From assignment of DSS score of 6 to assignment of a score of | ||||||
| DSS 7 | 3.6 [2.2–5.0] | 4.0 [2.8–5.2] | 0.68** | |||
| Kaplan–Meier estimates of the age [median (95% CI)] at the time of assigning DSS: (years) | ||||||
| DSS 4 | 40.0 [36.7–43.3] | 43.3 [40.4–46.1] | 0.003** | |||
| DSS 6 | 52.2 [47.8–56.6] | 54.7 [50.7–58.6] | 0.09** | |||
| DSS 7 | 58.7 [53.6–63.7] | 64.4 [61.9–66.9] | 0.11** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 10.9 ± 7.4 | 9.6 ± 8.4 | 0.38*** | |||
| Median | 10.0 | 7.0 | ||||
| Range | 1–40 | 0–62 | ||||
. | Progressive relapsing multiple sclerosis†n = 109 . | Primary progressive multiple sclerosis†n = 173 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 41 (38) | 80 (46) | 0.15* | |||
| Females | 68 (62) | 93 (54) | ||||
| Age at onset of multiple sclerosis: (years) | ||||||
| Mean ± SD | 37.3 ± 11.5 | 40.6 ± 10.7 | 0,02*** | |||
| Median | 38.1 | 41.3 | ||||
| Range | 11–58 | 13–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (1) | 4 (2) | 0.14* | |||
| Isolated brainstem dysfunction | 0 (0) | 1 (1) | ||||
| Isolated dysfunction of long tracts | 87 (80) | 150 (87) | ||||
| Combination of symptoms | 21 (19) | 18 (10) | ||||
| Kaplan–Meier estimates of the time [median (95% CI)]: (years) | ||||||
| From onset of multiple sclerosis to assignment of a disability status score of | ||||||
| DSS 4 | 0.0 | 0.0 | 0.50** | |||
| DSS 6 | 7.5 [5.8–9.2] | 6.8 [6.1–7.6] | 0.37** | |||
| DSS 7 | 13.7 [10.1–17.2] | 12.8 [9.9–15.7] | 0.92** | |||
| From assignment of DSS score of 4 to assignment of a score of | ||||||
| DSS 6 | 5.4 [3.3–7.5] | 5.5 [4.5–6.5] | 0.71** | |||
| DSS 7 | 11.3 [7.8–14.7] | 12.4 [10.2–14.7] | 0.65** | |||
| From assignment of DSS score of 6 to assignment of a score of | ||||||
| DSS 7 | 3.6 [2.2–5.0] | 4.0 [2.8–5.2] | 0.68** | |||
| Kaplan–Meier estimates of the age [median (95% CI)] at the time of assigning DSS: (years) | ||||||
| DSS 4 | 40.0 [36.7–43.3] | 43.3 [40.4–46.1] | 0.003** | |||
| DSS 6 | 52.2 [47.8–56.6] | 54.7 [50.7–58.6] | 0.09** | |||
| DSS 7 | 58.7 [53.6–63.7] | 64.4 [61.9–66.9] | 0.11** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 10.9 ± 7.4 | 9.6 ± 8.4 | 0.38*** | |||
| Median | 10.0 | 7.0 | ||||
| Range | 1–40 | 0–62 | ||||
Defined according to the Lublin and Reingold (1996) classification. SD denotes standard deviation; CI, confidence intervals; DSS, Kurtzke Disability Status Scale.
P-values are calculated with use of the *chi-squared test,
the log rank test,
the Student's t-test.
Secondary progressive multiple sclerosis and multiple sclerosis with a progressive initial course
Patients with progressive relapsing multiple sclerosis and those with primary progressive multiple sclerosis were pooled in a group of 282 patients with a progressive initial course of multiple sclerosis and compared with the group of 496 patients with secondary progressive multiple sclerosis (Table 3). The two populations were similar with respect to distribution by gender and symptoms at onset of the progressive phase. They were also similar with respect to age at onset of progression (Fig. 1B). The proportion of cases with superimposed relapses during progression was ∼40% in both categories. Times from onset of multiple sclerosis to assignment of irreversible disability scores were much longer in secondary progressive multiple sclerosis than in individuals with a progressive onset (P < 0.001 for all comparisons). However, the time course of disability accumulation as assessed from assignment of a score of 4 or 6 was more rapid and occurred earlier in secondary progressive multiple sclerosis than in individuals with a progressive onset. For instance, median survival from DSS 4 to DSS 6 was 4.0 years in secondary progressive multiple sclerosis and 5.4 years in multiple sclerosis with progression from onset (P = 0.001). Similarly, median age at reaching DSS 4 was 37.6 and 42.1 years in these two groups, respectively (P < 0.001). Finally, duration of the disease was significantly longer in secondary progressive multiple sclerosis (17.6 ± 9.6 years) than in cases with a progressive initial course (10.1 ± 8.0 years; P < 0.001).
Comparative demographic and disease-related characteristics of secondary progressive multiple sclerosis and cases with a progressive initial course, among 1844 patients with multiple sclerosis
. | Secondary progressive multiple sclerosis†n = 496 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 194 (39) | 121 (43) | 0.32* | |||
| Females | 302 (61) | 161 (57) | ||||
| Age at onset of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 39.5 ± 10.3 | 39.3 ± 11.3 | 0.84*** | |||
| Median | 39.1 | 40.1 | ||||
| Range | 14–72 | 11–67 | ||||
| Initial symptoms of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (0) | 5 (2) | 0.11* | |||
| Isolated brainstem dysfunction | 1 (0) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 418 (85) | 236 (84) | ||||
| Combination of symptoms | 76 (15) | 40 (14) | ||||
| Superimposed relapses during the progressive phase: no. (%) | ||||||
| Yes | 196 (40) | 109 (39) | 0.81* | |||
| No | 300 (60) | 173 (61) | ||||
| Kaplan-Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 6.1 [5.1–7.0] | 0.0 | <0.001** | |||
| DSS 6 | 12.5 [11.6–13.4] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 19.1 [16.9–21.3] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 4.0 [3.5–4.5] | 5.4 [4.3–6.6] | 0.001** | |||
| DSS 7 | 9.0 [7.9–10.1] | 12.0 [10.1–13.9] | <0.001** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.0 [2.5–3.5] | 4.0 [2.9–5.1] | 0.09** | |||
| Kaplan-Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 37.6 [36.1–39.1] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 45.5 [43.6–47.4] | 53.0 [51.1–54.9] | <0.001** | |||
| DSS 7 | 53.3 [51.0–55.7] | 63.1 [60.0–66.2] | <0.001** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 17.6 ± 9.6 | 10.1 ± 8.0 | <0.001*** | |||
| Median | 16.0 | 9.0 | ||||
| Range | 1–47 | 0–62 | ||||
. | Secondary progressive multiple sclerosis†n = 496 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 194 (39) | 121 (43) | 0.32* | |||
| Females | 302 (61) | 161 (57) | ||||
| Age at onset of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 39.5 ± 10.3 | 39.3 ± 11.3 | 0.84*** | |||
| Median | 39.1 | 40.1 | ||||
| Range | 14–72 | 11–67 | ||||
| Initial symptoms of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (0) | 5 (2) | 0.11* | |||
| Isolated brainstem dysfunction | 1 (0) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 418 (85) | 236 (84) | ||||
| Combination of symptoms | 76 (15) | 40 (14) | ||||
| Superimposed relapses during the progressive phase: no. (%) | ||||||
| Yes | 196 (40) | 109 (39) | 0.81* | |||
| No | 300 (60) | 173 (61) | ||||
| Kaplan-Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 6.1 [5.1–7.0] | 0.0 | <0.001** | |||
| DSS 6 | 12.5 [11.6–13.4] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 19.1 [16.9–21.3] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 4.0 [3.5–4.5] | 5.4 [4.3–6.6] | 0.001** | |||
| DSS 7 | 9.0 [7.9–10.1] | 12.0 [10.1–13.9] | <0.001** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.0 [2.5–3.5] | 4.0 [2.9–5.1] | 0.09** | |||
| Kaplan-Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 37.6 [36.1–39.1] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 45.5 [43.6–47.4] | 53.0 [51.1–54.9] | <0.001** | |||
| DSS 7 | 53.3 [51.0–55.7] | 63.1 [60.0–66.2] | <0.001** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 17.6 ± 9.6 | 10.1 ± 8.0 | <0.001*** | |||
| Median | 16.0 | 9.0 | ||||
| Range | 1–47 | 0–62 | ||||
Defined according to the Lublin and Reingold (1996) classification.
Denotes the pooling of cases with ‘progressive relapsing multiple sclerosis’ and of cases with ‘primary progressive multiple sclerosis’ (Lublin and Reingold, 1996). SD denotes standard deviation; CI, confidence intervals; DSS, Kurtzke Disability Status Scale.
P-values are calculated with use of the *chi-squared test,
the log rank test,
the Student's t-test.
Comparative demographic and disease-related characteristics of secondary progressive multiple sclerosis and cases with a progressive initial course, among 1844 patients with multiple sclerosis
. | Secondary progressive multiple sclerosis†n = 496 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 194 (39) | 121 (43) | 0.32* | |||
| Females | 302 (61) | 161 (57) | ||||
| Age at onset of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 39.5 ± 10.3 | 39.3 ± 11.3 | 0.84*** | |||
| Median | 39.1 | 40.1 | ||||
| Range | 14–72 | 11–67 | ||||
| Initial symptoms of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (0) | 5 (2) | 0.11* | |||
| Isolated brainstem dysfunction | 1 (0) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 418 (85) | 236 (84) | ||||
| Combination of symptoms | 76 (15) | 40 (14) | ||||
| Superimposed relapses during the progressive phase: no. (%) | ||||||
| Yes | 196 (40) | 109 (39) | 0.81* | |||
| No | 300 (60) | 173 (61) | ||||
| Kaplan-Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 6.1 [5.1–7.0] | 0.0 | <0.001** | |||
| DSS 6 | 12.5 [11.6–13.4] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 19.1 [16.9–21.3] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 4.0 [3.5–4.5] | 5.4 [4.3–6.6] | 0.001** | |||
| DSS 7 | 9.0 [7.9–10.1] | 12.0 [10.1–13.9] | <0.001** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.0 [2.5–3.5] | 4.0 [2.9–5.1] | 0.09** | |||
| Kaplan-Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 37.6 [36.1–39.1] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 45.5 [43.6–47.4] | 53.0 [51.1–54.9] | <0.001** | |||
| DSS 7 | 53.3 [51.0–55.7] | 63.1 [60.0–66.2] | <0.001** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 17.6 ± 9.6 | 10.1 ± 8.0 | <0.001*** | |||
| Median | 16.0 | 9.0 | ||||
| Range | 1–47 | 0–62 | ||||
. | Secondary progressive multiple sclerosis†n = 496 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 194 (39) | 121 (43) | 0.32* | |||
| Females | 302 (61) | 161 (57) | ||||
| Age at onset of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 39.5 ± 10.3 | 39.3 ± 11.3 | 0.84*** | |||
| Median | 39.1 | 40.1 | ||||
| Range | 14–72 | 11–67 | ||||
| Initial symptoms of the progressive phase of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 1 (0) | 5 (2) | 0.11* | |||
| Isolated brainstem dysfunction | 1 (0) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 418 (85) | 236 (84) | ||||
| Combination of symptoms | 76 (15) | 40 (14) | ||||
| Superimposed relapses during the progressive phase: no. (%) | ||||||
| Yes | 196 (40) | 109 (39) | 0.81* | |||
| No | 300 (60) | 173 (61) | ||||
| Kaplan-Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 6.1 [5.1–7.0] | 0.0 | <0.001** | |||
| DSS 6 | 12.5 [11.6–13.4] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 19.1 [16.9–21.3] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 4.0 [3.5–4.5] | 5.4 [4.3–6.6] | 0.001** | |||
| DSS 7 | 9.0 [7.9–10.1] | 12.0 [10.1–13.9] | <0.001** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.0 [2.5–3.5] | 4.0 [2.9–5.1] | 0.09** | |||
| Kaplan-Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 37.6 [36.1–39.1] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 45.5 [43.6–47.4] | 53.0 [51.1–54.9] | <0.001** | |||
| DSS 7 | 53.3 [51.0–55.7] | 63.1 [60.0–66.2] | <0.001** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 17.6 ± 9.6 | 10.1 ± 8.0 | <0.001*** | |||
| Median | 16.0 | 9.0 | ||||
| Range | 1–47 | 0–62 | ||||
Defined according to the Lublin and Reingold (1996) classification.
Denotes the pooling of cases with ‘progressive relapsing multiple sclerosis’ and of cases with ‘primary progressive multiple sclerosis’ (Lublin and Reingold, 1996). SD denotes standard deviation; CI, confidence intervals; DSS, Kurtzke Disability Status Scale.
P-values are calculated with use of the *chi-squared test,
the log rank test,
the Student's t-test.
Multiple sclerosis with an exacerbating–remitting initial course and multiple sclerosis with a progressive initial course
The 1562 patients with an exacerbating–remitting initial course of multiple sclerosis were eventually compared with the 282 patients with a progressive initial course of the disease (Table 4). The two groups differed in several respects: the first group was characterized by a greater female preponderance (P = 0.006), an earlier onset of multiple sclerosis (P < 0.001), initial symptoms of the disease related more often to optic neuritis and brainstem dysfunction and less frequently to dysfunction of long tracts (P < 0.001), and much longer times from onset of multiple sclerosis to assignment of irreversible disability scores (P < 0.001 for all comparisons). By contrast, both populations were strikingly similar with respect to the time course of disability accumulation from assignment to a given disability score to a higher score. Finally, age at assignment of disability landmarks was older for a score of DSS 4 or DSS 6 in patients with an exacerbating–remitting onset compared with patients with a progressive onset (P < 0.001 and P = 0.002). It was similar for a score of DSS7 in both populations (P = 0.24).
Comparative demographic and disease-related characteristics of cases with an exacerbating–remitting initial course and cases with a progressive initial course of multiple sclerosis, among 1844 patients with multiple sclerosis
. | Multiple sclerosis with an exacerbating– remitting initial course†n = 1562 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 536 (34) | 121 (43) | 0.006* | |||
| Females | 1026 (66) | 161 (57) | ||||
| Age at onset of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 29.6 ± 9.5 | 39.3 ± 11.3 | <0.001*** | |||
| Median | 29.0 | 40.1 | ||||
| Range | 5–62 | 11–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 330 (21) | 5 (2) | <0.001* | |||
| Isolated brainstem dysfunction | 158 (10) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 727 (47) | 236 (84) | ||||
| Combination of symptoms | 347 (22) | 40 (14) | ||||
| Kaplan–Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 11.4 [10.5–12.3] | 0.0 | <0.001** | |||
| DSS 6 | 23.1 [20.1–26.1] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 33.1 [29.2–37.0] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 5.7 [4.9–6.4] | 5.4 [4.3–6.6] | 0.74** | |||
| DSS 7 | 12.1 [10.0–14.2] | 12.0 [10.1–13.9] | 0.70** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.3 [2.8–3.9] | 4.0 [2.9–5.1] | 0.48** | |||
| Kaplan–Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 44.8 [43.8–45.9] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 55.3 [54.2–56.7] | 53.0 [51.1–54.9] | 0.002** | |||
| DSS 7 | 62.8 [60.3–65.4] | 63.1 [60.0–66.2] | 0.24** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 11.5 ± 9.9 | 10.1 ± 8.0 | 0.02*** | |||
| Median | 10.0 | 9.0 | ||||
| Range | 0–52 | 0–62 | ||||
. | Multiple sclerosis with an exacerbating– remitting initial course†n = 1562 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 536 (34) | 121 (43) | 0.006* | |||
| Females | 1026 (66) | 161 (57) | ||||
| Age at onset of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 29.6 ± 9.5 | 39.3 ± 11.3 | <0.001*** | |||
| Median | 29.0 | 40.1 | ||||
| Range | 5–62 | 11–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 330 (21) | 5 (2) | <0.001* | |||
| Isolated brainstem dysfunction | 158 (10) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 727 (47) | 236 (84) | ||||
| Combination of symptoms | 347 (22) | 40 (14) | ||||
| Kaplan–Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 11.4 [10.5–12.3] | 0.0 | <0.001** | |||
| DSS 6 | 23.1 [20.1–26.1] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 33.1 [29.2–37.0] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 5.7 [4.9–6.4] | 5.4 [4.3–6.6] | 0.74** | |||
| DSS 7 | 12.1 [10.0–14.2] | 12.0 [10.1–13.9] | 0.70** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.3 [2.8–3.9] | 4.0 [2.9–5.1] | 0.48** | |||
| Kaplan–Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 44.8 [43.8–45.9] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 55.3 [54.2–56.7] | 53.0 [51.1–54.9] | 0.002** | |||
| DSS 7 | 62.8 [60.3–65.4] | 63.1 [60.0–66.2] | 0.24** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 11.5 ± 9.9 | 10.1 ± 8.0 | 0.02*** | |||
| Median | 10.0 | 9.0 | ||||
| Range | 0–52 | 0–62 | ||||
Denotes the pooling of cases with ‘relapsing–remitting multiple sclerosis’ and of cases with ‘secondary progressive multiple sclerosis’ (Lublin and Reingold, 1996).
Denotes the pooling of cases with ‘progressive relapsing multiple sclerosis’ and of cases with ‘primary progressive multiple sclerosis’ (Lublin and Reingold, 1996).
SD denotes standard deviation; CI, confidence intervals; DSS, Kurtzke Disability Status Scale.
P-values are calculated with use of the *chi-squared test,
the log rank test,
the Student's t-test.
Comparative demographic and disease-related characteristics of cases with an exacerbating–remitting initial course and cases with a progressive initial course of multiple sclerosis, among 1844 patients with multiple sclerosis
. | Multiple sclerosis with an exacerbating– remitting initial course†n = 1562 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 536 (34) | 121 (43) | 0.006* | |||
| Females | 1026 (66) | 161 (57) | ||||
| Age at onset of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 29.6 ± 9.5 | 39.3 ± 11.3 | <0.001*** | |||
| Median | 29.0 | 40.1 | ||||
| Range | 5–62 | 11–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 330 (21) | 5 (2) | <0.001* | |||
| Isolated brainstem dysfunction | 158 (10) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 727 (47) | 236 (84) | ||||
| Combination of symptoms | 347 (22) | 40 (14) | ||||
| Kaplan–Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 11.4 [10.5–12.3] | 0.0 | <0.001** | |||
| DSS 6 | 23.1 [20.1–26.1] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 33.1 [29.2–37.0] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 5.7 [4.9–6.4] | 5.4 [4.3–6.6] | 0.74** | |||
| DSS 7 | 12.1 [10.0–14.2] | 12.0 [10.1–13.9] | 0.70** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.3 [2.8–3.9] | 4.0 [2.9–5.1] | 0.48** | |||
| Kaplan–Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 44.8 [43.8–45.9] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 55.3 [54.2–56.7] | 53.0 [51.1–54.9] | 0.002** | |||
| DSS 7 | 62.8 [60.3–65.4] | 63.1 [60.0–66.2] | 0.24** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 11.5 ± 9.9 | 10.1 ± 8.0 | 0.02*** | |||
| Median | 10.0 | 9.0 | ||||
| Range | 0–52 | 0–62 | ||||
. | Multiple sclerosis with an exacerbating– remitting initial course†n = 1562 . | Multiple sclerosis with a progressive initial course#n = 282 . | P-value . | |||
|---|---|---|---|---|---|---|
| Gender: no. (%) | ||||||
| Males | 536 (34) | 121 (43) | 0.006* | |||
| Females | 1026 (66) | 161 (57) | ||||
| Age at onset of multiple sclerosis: no. (%) | ||||||
| Mean ± SD | 29.6 ± 9.5 | 39.3 ± 11.3 | <0.001*** | |||
| Median | 29.0 | 40.1 | ||||
| Range | 5–62 | 11–67 | ||||
| Initial symptoms of multiple sclerosis: no. (%) | ||||||
| Isolated optic neuritis | 330 (21) | 5 (2) | <0.001* | |||
| Isolated brainstem dysfunction | 158 (10) | 1 (0) | ||||
| Isolated dysfunction of long tracts | 727 (47) | 236 (84) | ||||
| Combination of symptoms | 347 (22) | 40 (14) | ||||
| Kaplan–Meier estimates of the time (median [95% CI]): (years) | ||||||
| From onset of multiple sclerosis to assignment of | ||||||
| DSS 4 | 11.4 [10.5–12.3] | 0.0 | <0.001** | |||
| DSS 6 | 23.1 [20.1–26.1] | 7.1 [6.3–7.9] | <0.001** | |||
| DSS 7 | 33.1 [29.2–37.0] | 13.4 [11.0–15.9] | <0.001** | |||
| From assignment of DSS 4 to assignment of | ||||||
| DSS 6 | 5.7 [4.9–6.4] | 5.4 [4.3–6.6] | 0.74** | |||
| DSS 7 | 12.1 [10.0–14.2] | 12.0 [10.1–13.9] | 0.70** | |||
| From assignment of DSS 6 to assignment of | ||||||
| DSS 7 | 3.3 [2.8–3.9] | 4.0 [2.9–5.1] | 0.48** | |||
| Kaplan–Meier estimates of the age (median [95% CI]) at the time of assigning DSS (years) | ||||||
| DSS 4 | 44.8 [43.8–45.9] | 42.1 [40.2–44.0] | <0.001** | |||
| DSS 6 | 55.3 [54.2–56.7] | 53.0 [51.1–54.9] | 0.002** | |||
| DSS 7 | 62.8 [60.3–65.4] | 63.1 [60.0–66.2] | 0.24** | |||
| Duration of multiple sclerosis: (years) | ||||||
| Mean ± SD | 11.5 ± 9.9 | 10.1 ± 8.0 | 0.02*** | |||
| Median | 10.0 | 9.0 | ||||
| Range | 0–52 | 0–62 | ||||
Denotes the pooling of cases with ‘relapsing–remitting multiple sclerosis’ and of cases with ‘secondary progressive multiple sclerosis’ (Lublin and Reingold, 1996).
Denotes the pooling of cases with ‘progressive relapsing multiple sclerosis’ and of cases with ‘primary progressive multiple sclerosis’ (Lublin and Reingold, 1996).
SD denotes standard deviation; CI, confidence intervals; DSS, Kurtzke Disability Status Scale.
P-values are calculated with use of the *chi-squared test,
the log rank test,
the Student's t-test.
Discussion
The reasons why progression may start de novo or after a period of episodes remains largely unexplained. This has led many neurologists to consider primary progressive multiple sclerosis as a separate entity, distinct from the other forms of the disease. However, the present observational study of the Lyon natural history cohort and available data from other sources in the literature allow the clinical evidence for and against this hypothesis to be reconsidered.
Comparing secondary progressive multiple sclerosis and relapsing–remitting multiple sclerosis, we found similarities in the distribution of initial symptoms during the relapsing–remitting phase, the degree of recovery from the first relapse, and the time from onset to the second neurological episode. Both populations were strikingly equally distributed according to age at onset of the relapsing–remitting phase (Table 1 and Fig. 1A). This is in perfect agreement with the results of the analysis performed on the Lyon cohort when it contained only 349 patients (Confavreux, 1977; Confavreux et al., 1980) and with other series (Fog and Linneman, 1970; Leibowitz and Alter, 1973; Poser, 1978; Minderhoud et al., 1988; Cottrell et al., 1999; Kremenchutzky et al., 1999). By contrast, the two populations of secondary progressive multiple sclerosis and relapsing–remitting multiple sclerosis in the Lyon cohort clearly differ in disease duration, which was twice as long in the secondary progressive than in the relapsing–remitting multiple sclerosis group (Table 1). Others have reached the same conclusions (Poser, 1978; Trojano et al., 1995). This being said, it has been well demonstrated that the patients with an initial exacerbating–remitting course of multiple sclerosis will naturally convert to the secondary progressive phase following an essentially linear curve (McAlpine and Compston, 1952; Confavreux, 1977; Confavreux et al., 1980; Broman et al., 1981; Vukusic and Confavreux, 2003). The rate of conversion to secondary progression is around 2–3% per annum (Vukusic and Confavreux, 2003) and the median time to secondary progression can be estimated by the Kaplan–Meier technique at around 19 years (Amato and Ponziani, 2000; Myrh et al., 2001; Eriksson et al., 2003; Vukusic and Confavreux, 2003). In other words, the longer the disease duration at the time of the survey, the higher the proportion of cases classified as secondary progressive multiple sclerosis compared with those classified as having relapsing–remitting disease. Although the relapsing–remitting and secondary progressive phases clearly represent two clinical stages of the same disease in patients with bout onset multiple sclerosis, this is an argument in favour of the hypothesis that secondary progressive multiple sclerosis is relapsing–remitting multiple sclerosis that has had ‘time to grow older’ (Confavreux, 1977; Confavreux et al., 1980).
By definition, progressive relapsing multiple sclerosis and primary progressive multiple sclerosis are distinct forms of the disease: they share the progressive onset but differ in that superimposed relapses accompany progressive relapsing but not primary progressive multiple sclerosis (Lublin and Reingold, 1996). Among the 282 patients with a progressive initial course of multiple sclerosis from our cohort, 109 (39%) exhibited ≥1 distinct relapse during progression, sometimes decades after disease onset, qualifying them for classification as progressive relapsing forms of multiple sclerosis (Lublin and Reingold, 1996). Among the 218 patients of the London, Ontario, series with an initial progressive course (Cottrell et al., 1999; Krementchutzky et al., 1999), 28% could be qualified thus. In 50%, relapses occurred in the first 10 years, and at intervals from onset up to 20 years or more for the other half. Relapses were never frequent, and most patients had but a single episode. This was usually mild and followed by good recovery (Krementchutzky et al., 1999). Our cohort shows that median age at onset was earlier in progressive relapsing than in primary progressive cases (37 versus 41 years; P = 0.02), although this is the only difference observed when comparing these two forms of multiple sclerosis according to demographic and clinical characteristics, such as gender and initial symptoms of the disease. A similar trend for age at onset was found in the London, Ontario, series (Krementchutzky et al., 1999).
In our series, the rates at which irreversible disability progressed, calculated from the onset of multiple sclerosis or from assignment of a given disability score, were essentially similar in progressive relapsing and primary progressive multiple sclerosis. In both cases, median survival times from onset of multiple sclerosis to reach DSS 4, DSS 6 and DSS 7 were 0, 7 and 13 years, respectively (Table 2). Taking DSS 4 as the baseline, median times to reach DSS 6 and DSS 7 were 5 and 12 years, respectively. From DSS 6, median time to reach DSS 7 was 4 years (Table 2 and Confavreux et al., 2000). These results are consistent with other series. In the London, Ontario, series, median survival times from onset of multiple sclerosis to reach DSS 3, DSS 6, DSS 8 and death were 3, 8, 18 and 35 years, both in primary progressive and progressive relapsing disease (Cottrell et al., 1999; Krementchutzky et al., 1999). Similarly, no differences between these two forms of multiple sclerosis could be discerned when calculations were made from assignment of DSS 3 to reach DSS 6, DSS 8 and death (Krementchutzky et al., 1999). In a Californian study comprising 83 cases with primary progressive multiple sclerosis and 12 with progressive relapsing multiple sclerosis, survival time from onset of multiple sclerosis to reach DSS 6 was 10.2 years and 10.9 years, respectively (Andersson et al., 1999). All these results indicate that progressive relapsing and primary progressive multiple sclerosis are, from a clinical point of view, essentially the same. Therefore, it might be appropriate to pool these cases in a single category with initial progressive course, the only difference being the subsequent experience of superimposed relapses. The occasional confusion between progressive relapsing and secondary progressive multiple sclerosis might account for the slightly earlier onset in progressive relapsing than primary progressive multiple sclerosis.
The variations in the clinical pattern between secondary progressive multiple sclerosis and cases with a progressive initial course have often been compared. The general consensus is that they are very different. The female preponderance expected in a general population of patients with multiple sclerosis is much reduced in cases with a progressive initial course, compared with those with secondary progressive multiple sclerosis (McDonnell and Hawkins, 1996, 1998; Thompson et al., 1997; Cottrell et al., 1999; Kremenchutzky et al., 1999). In our cohort, there was only a trend in that direction, not reaching statistical significance (Table 3). From the clinical perspective, the initial course and symptoms of the disease—more often related to dysfunction of long tracts in multiple sclerosis with a progressive onset than in secondary progressive disease—are very different (seeTables 1, 2 and 3). Age at onset is greater, time to assignment of irreversible disability shorter and prognosis worse in cases with progressive initial onset than in secondary progressive multiple sclerosis (Table 3). Taking account of all these considerations, the majority of clinicians consider primary progressive multiple sclerosis as distinct from secondary progressive disease.
But the distinctions are not necessarily so clear cut. In fact, comparing cases from the time when progression becomes manifest (at onset or after a period of relapses) reveals many similarities. In our series (Table 3 and Fig. 1B), age and initial symptoms at onset of the progressive phase were similar in the 496 cases with secondary progressive multiple sclerosis and the 282 cases with progressive disease from onset. As for age at onset of the progressive phase, these results are in agreement with those obtained on the Lyon cohort when it contained only 349 patients (Confavreux, 1977; Confavreux et al., 1980) and with other series (Fog and Linneman, 1970; Minderhoud et al., 1988). The proportion of cases with superimposed relapses during progression was ∼40% in both categories. However, the time course of disability accumulation during the progressive phase of the disease was more rapid and occurred earlier in secondary progressive multiple sclerosis than in individuals with a progressive onset. For instance, the median survival time from DSS 4 to DSS 6 was 4.0 years in secondary progressive multiple sclerosis and 5.4 years in multiple sclerosis with progression from onset (P = 0.001). Similarly, median age at reaching DSS 4 was 37.6 and 42.1 years in these two groups (P < 0.001) (Confavreux and Vukusic, 2006). This leads to the conclusion that, once clinical progression has started, the rate at which disability accumulates is faster in secondary progressive multiple sclerosis than in cases progressive from onset. These are not unique observations (Minderhoud et al., 1988; Cottrell et al., 1999; Kremenchutzky et al., 1999). In the London, Ontario, cohort, the median survival time from onset of progression to reach DSS 6 was 5.5 years in the 538 patients with secondary progressive multiple sclerosis and 9.5 years in the 218 patients with an initial progressive course. As for the time to reach DSS 8, the corresponding figures were around 15 and 20 years, respectively (Cottrell et al., 1999; Kremenchutzky et al., 1999). Conversely, in the Gothenburg, Sweden, cohort (Runmarker and Andersen, 1993), median survival time from the onset of progression to DSS 6 was 5.2 years for the 162 cases with secondary progressive multiple sclerosis and 6.0 years for the 36 with progression from onset, a difference that was not statistically significant.
The next step is to compare cases with an exacerbating–remitting initial course to those with a progressive initial course. It serves little purpose to restate the differences with respect to sex ratio, age and symptoms at onset or survival times from onset or between disability landmarks because these are essentially similar to what has already been discussed. Our objective here is to compare, within a general cohort of patients having multiple sclerosis, all cases with an exacerbating–remitting onset (i.e. ‘relapsing–remitting multiple sclerosis’ and ‘secondary progressive multiple sclerosis’) and those with a progressive onset (i.e. ‘progressive relapsing multiple sclerosis’ and ‘primary progressive multiple sclerosis’) with respect to the time course of disability. In the 1562 patients with an exacerbating–remitting initial course and the 282 patients with progression from onset in our cohort (Table 4) (Confavreux et al., 2000), the time from assignment of DSS 4 to reach DSS 6 and DSS 7, and the time from DSS 6 to DSS 7, appeared strikingly similar. Furthermore, age at the time of assigning disability landmarks could be viewed as not substantially influenced by the initial course, be it exacerbating–remitting or progressive (Table 4 and Confavreux and Vukusic, 2006). Therefore, the more rapid accumulation of disability generally observed in our series (Table 3) and in others' (Minderhoud et al., 1988; Cottrell et al., 1999; Kremenchutzky et al., 1999), and the earlier age at disability milestones observed in our series (Table 3) in secondary progressive multiple sclerosis compared with individuals with a progressive onset, more likely reflect limited disease duration at the time of the survey. Indeed, as discussed above, the proportion of cases with an exacerbating–remitting onset converting to secondary progression follows a somewhat linear curve during the course of multiple sclerosis. The shorter the disease duration, the fewer the cases with secondary progressive multiple sclerosis within the population of cases having an initial exacerbating–remitting course. The subgroup of individuals with an exacerbating–remitting onset, already having converted to secondary progressive multiple sclerosis at the time of any survey, is made up of the most severe group from the cohort of all cases with an exacerbating–remitting onset. It is therefore not surprising that, the longer the disease lasts, the more estimates for the time course of disability accumulation slow down and approximate to those seen in the population with progressive multiple sclerosis from onset. In the Gothenburg, Sweden, cohort, where the proportion of secondary progressive multiple sclerosis (77% of cases with an exacerbating–remitting onset) and the duration of the disease (>25 years) were both high, accumulation of disability was similar in progressive onset and secondary progressive multiple sclerosis (Runmarker and Andersen, 1993). Therefore, from a clinical perspective, secondary and primary progression share much more than they differ.
These observational data on the natural history of multiple sclerosis suggest that the clinical phenotype and course of multiple sclerosis are age dependent. Relapsing–remitting disease can be regarded as multiple sclerosis in which insufficient time has elapsed for the conversion to secondary progression; secondary progressive forms as relapsing–remitting multiple sclerosis that has ‘grown older’; and progressive from onset disease as multiple sclerosis ‘amputated’ from the usual preceding relapsing–remitting phase. At the population level, times to reach disability milestones, and the ages at which these landmarks are reached, follow a predefined schedule not obviously influenced by relapses, whenever they may occur, or by the initial course of the disease, whatever its phenotype. The emergence of the progressive phase of multiple sclerosis might just be an effect of age, rather than the effect of a change in the pathogeny of the disease. This leads to a unifying concept of the disease in which primary and secondary progression might be regarded as essentially similar. This concept is supported by observations from familial cases of multiple sclerosis. The diversity of clinical phenotypes within families with multiple sclerosis and the prevalence of familial forms are essentially similar for index cases with a relapsing–remitting multiple sclerosis and cases with a progressive from onset multiple sclerosis (Weinshenker et al., 1990; Robertson et al., 1996; Cottrell et al., 1999). From the clinical and statistical positions, there are arguments in favour of considering multiple sclerosis as one disease with different clinical phenotypes rather than an entity encompassing several distinct diseases, each having a different aetiology and mechanism—the position of complexity rather than true heterogeneity.
This unitary hypothesis is somewhat provocative when the clinical course is so obviously two-staged, a relapsing–remitting phase being followed by a progression in the majority of the patients with multiple sclerosis. Furthermore, the analysis of the Lyon cohort has already shown that the influence of clinical variables observed at baseline, or soon thereafter, on the accumulation of irreversible disability is limited to the time from onset of multiple sclerosis to the assignment of DSS 4 (Confavreux et al., 2003). The same clinical variables do not influence the course beyond this point and into the upper echelons of disability. Therefore, the clinical natural history of multiple sclerosis is characterized by an initial phase, of variable duration, influenced by these clinical variables; and a second phase, which proceeds independently. This suggests that when a detectable threshold of irreversible disability has been reached, the disease enters a final common pathway, where subsequent accumulation of disability becomes a self-perpetuating process, amnesic to the prior clinical history of the disease. Interestingly, the ‘amnesic phenomenon’ is observed wherever the detectable threshold for irreversible disability is set (Confavreux et al., 2003; Coustans et al., 2004), and whether or not the phase of relapses and remissions has passed (Fog and Linnemann, 1970; Patzold and Pocklington, 1982) and laboratory evidence for neurodegeneration is in place (Rudick et al., 1999; Fox et al., 2000; Filippi et al., 2003, 2004; Ingle et al., 2003). Therefore, we might speculate that, at first in the disease course, neurodegeneration is clinically invisible but detectable using laboratory methods that provide more sensitivity; later, diffuse neurodegeneration dominates and this is expressed as irreversible and progressive disability. This suggests that multiple sclerosis, instead of being two-staged, is a one-stage disorder, with a tight intermingling of acute focal recurrent inflammation and diffuse chronic progressive neurodegeneration since the outset of the disease.
We consider it timely to offer a more comprehensive classification of the evolution of multiple sclerosis (Confavreux and Vukusic, 2002; Confavreux and Compston, 2006). The current position has great merits and makes the logical distinction between cases with primary and secondary progression (Lublin and Reingold, 1996). However, this classification gathers individuals with and without relapses in the category of secondary progression, but splits them (progressive relapsing multiple sclerosis versus primary progressive multiple sclerosis) in the category of primary progression. When comparing cases with a progressive onset and secondary progressive multiple sclerosis, most authors tend to select all the cases of secondary progressive multiple sclerosis, that is with or without relapses superimposed on progression, but only cases with primary progressive multiple sclerosis stricto sensu, that is excluding cases with progressive relapsing multiple sclerosis. It may be speculated that this classification asymmetry has led to conclusions that are more related to the level of acute recurrent focal inflammation than the timing of progression. Therefore we suggest that multiple sclerosis is categorized as having two types of onset (‘exacerbating–remitting ’ or ‘progressive’) and three main forms of evolution (‘relapsing-remitting’, ‘secondary progressive’ or ‘primary progressive’). This results in five subtypes depending on whether or not the progressive phase (itself primary or secondary) develops with or without relapses (‘relapsing’ versus ‘non-relapsing’).
The authors are indebted to the patients for their participation in the Lyon Multiple Sclerosis Database; Drs Gilbert Aimard, Michel Devic, Thibault Moreau, Jean-Jacques Ventre, Jean-François de Saint Victor, Iuliana Achiti-Ionescu, Sandrine Blanc, Françoise Bouhour, Patricia Tourniaire, Françoise Durand-Dubief, Laurence Gignoux, Jérôme Grimaud, and Georges Riche for their contribution to the development of the database; Mr Albert Biron, Dr Bernard Frangoulis and Mrs Catherine Vidal for maintaining the database; and Mrs France-Isabelle Pairel for assistance in preparing the manuscript. This work was supported by contracts with the Commission of the European Communities Directorate General XII (BMH1-CT93–1529, CIPD-CT94–0227 and BMH4-CT96–0064), by funds from the Ligue Française contre la Sclérose En Plaques (L.F.S.E.P.), the Association pour la Recherche sur la Sclérose en Plaques (A.R.S.E.P.), les Compagnons du Beaujolais, the Lions Club ‘Village Beaujolais’ and Bouygues Telecom, and by unconditional grants from Biogen Idec France, Schering SA, Serono France, and Teva Pharma France.
References
Amato MP, Ponziani G. A prospective study on the prognosis of multiple sclerosis.
Andersson PB, Waubant E, Gee L, Goodkin DE. Multiple sclerosis that is progressive from the time of onset. Clinical characteristics and progression of disability.
Arnold DL. Magnetic resonance spectroscopy: imaging axonal damage in MS.
Brex PA, Jenkins R, Fox NC, Crum WR, O'Riordan JL, Plant GT, et al. Detection of ventricular enlargement in patients at the earliest clinical stage of MS.
Broman T, Andersen O, Bergmann L. Clinical studies on multiple sclerosis. I. Presentation of an incidence material from Gothenburg.
Ciccarelli O, Werring DJ, Wheeler-Kingshott CAM, Barker GJ, Parker GJ, Thompson AJ, et al. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations.
Confavreux C. L'histoire naturelle de la sclérose en plaques. Etude par informatique de 349 observations. Thèse de Médecine. Lyon: Université Claude Bernard;
Confavreux C, Aimard G, Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients.
Confavreux C, Compston A. The natural history of multiple sclerosis. In: Compston A, editor. ‘McAlpine's multiple Sclerosis. 4th edn.’ London: Churchill Livingstone Elsevier; 2006. p. 183–272.
Confavreux C, Compston DAS, Hommes OR, McDonald WI, Thompson AJ. EDMUS, a European Database for Multiple Sclerosis.
Confavreux C, Vukusic S. Natural History of Multiple Sclerosis: implications for counseling and therapy.
Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis.
Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process.
Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis.
Cottrell DA, Kremenchutzky M, Rice GPA, Koopman WJ, Hader W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis.
Coustans M, Leray E, Le Page E, Chaperon J, Edan G. Both relapsing-remitting and primary progressive multiple sclerosis are a two-stage disease, suggesting two consecutive mechanisms underlying the progression of disability in multiple sclerosis.
Eriksson M, Andersen O, Runmarker B. Long-term follow-up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis.
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis.
Filippi M, Campi A, Martinelli V, Pereira C, Scotti G, Comi G. Transitional progressive multiple sclerosis: MRI and MTI findings.
Filippi M, Bozzali M, Rovaris M, Gonen O, Kesavadas C, Ghezzi A, et al. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis.
Filippi M, Rovaris M, Inglese M, Barkhof F, De Stefano N, Smith S, et al. Interferon beta-1a for brain tissue loss in patients at presentation with syndromes suggestive of multiple sclerosis: a randomized, double-blind, placebo-controlled trial.
Fog T, Linneman F. The course of multiple sclerosis in 73 cases with computer designed curves.
Fox NC, Jenkins R, Lary SM, Stevenson VL, Losseff NA, Crum WR, et al. Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI.
Fu L, Matthews PM, De Stefano N, Worsley KJ, Narayanan S, Francis GS, et al. Imaging axonal damage of normal-appearing white matter in multiple sclerosis.
Gayou A, Brochet B, Dousset V. Transitional progressive multiple sclerosis: a clinical and imaging study.
Ingle GT, Stevenson VL, Miller DH and Thompson AJ. Primary progressive multiple sclerosis: a 5-year clinical and MR study.
Kremenchutzky M, Cottrell D, Rice G, Hader W, Baskerville J, Koopman W, et al. The natural history of multiple sclerosis: a geographically based study. 7. Progressive-relapsing and relapsing-progressive multiple sclerosis: a re-evaluation.
Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS).
Leibowitz U, Alter M. Multiple sclerosis: clues to its cause. Amsterdam: North-Holland Publishing Company; 1973. p. 1–373.
Losseff NA, Wang L, Lai HM, Yoo DS, Gawne-Cain ML, McDonald WI, et al. Progressive cerebral atrophy in multiple sclerosis. A serial MRI study.
Losseff NA, Webb SL, O'Riordan JI, Page R, Wang L, Barker GJ, et al. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey.
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination.
McAlpine D, Compston ND. Some aspects of the natural history of disseminated sclerosis: incidence, course and prognosis; factors affecting onset and course.
McDonnell GV, Hawkins SA. Primary progressive multiple sclerosis:a distinct syndrome?
McDonnel GV, Hawkins SA. Clinical study of primary progressive multiple sclerosis in Northern Ireland, UK.
Minderhoud JM, Van der Hoeven JH, Prange AJA. Course and prognosis of chronic progressive multiple sclerosis. Results of an epidemiological study.
Myhr KM, Riise T, Vedeler C, Nortvedt MW, Gronning R, Midgard R, et al. Disability and prognosis in multiple sclerosis: demographic and clinical variables important for the ability to walk and awarding of disability pension.
Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976–1980.
Poser S. Multiple sclerosis. An analysis of 812 cases by means of electronic data processing. Berlin: Springer-Verlag; 1978.
Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols.
Robertson NP, Clayton D, Fraser M, Deans J, Compston DA. Clinical concordance in sibling pairs with multiple sclerosis.
Rudick RA, Fischer E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group.
Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up.
Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in MS: report by the panel on the evaluation of experimental trials of therapy in MS.
Stevenson VL, Miller DH, Rovaris M, Barkhof F, Brochet B, Dousset V, et al. Primary and transitional progressive MS. A clinical and MRI cross-sectional study.
Stevenson VL, Miller DH, Leary SM, Rovaris M, Barkhof F, Brochet B, et al. One year follow-up study of primary and transitional progressive multiple sclerosis.
Thompson AJ, Kermode AG, Wicks D, MacManus DG, Kendall BE, Kingsley DP, et al. Major differences in the dynamics of primary and secondary progressive multiple sclerosis.
Thompson AJ, Polman CH, Miller DH, McDonald WI, Brochet B, Filippi M, et al. Primary progressive multiple sclerosis.
Tortorella C, Viti B, Bozzali M, Sormani MP, Rizzo G, Gilardi MF, et al. A magnetization transfer histogram study of normal-appearing brain tissue in MS.
Traboulsee A, Dehmeshki J, Brex PA, Dalton CM, Chard D, Barker GJ, et al. Normal-appearing brain tissue MTR histograms in clinically isolated syndromes suggestive of MS.
Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis.
Weinshenker BG. Progressive forms of MS: classification streamlined or consensus overtuned?
Weinshenker BG, Bulman D, Carriere W, Baskerville J, Ebers GC. A comparison of sporadic and familial multiple sclerosis.