-
PDF
- Split View
-
Views
-
Cite
Cite
M. Martinussen, B. Fischl, H. B. Larsson, J. Skranes, S. Kulseng, T. R. Vangberg, T. Vik, A.-M. Brubakk, O. Haraldseth, A. M. Dale, Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method, Brain, Volume 128, Issue 11, November 2005, Pages 2588–2596, https://doi.org/10.1093/brain/awh610
Close - Share Icon Share
Abstract
Infants with low birth weight are at increased risk of perinatal brain injury. Disruption of normal cortical development may have consequences for later motor, behavioural and cognitive development. The aim of this study was to measure cerebral cortical thickness, area and volume with an automated MRI technique in 15-year-old adolescents who had low birth weight. Cerebral MRI for morphometric analysis was performed on 50 very low birth weight (VLBW, birth weight ≤1500 g), 49 term small for gestational age births (SGA, birth weight <10th percentile at term) and 58 control adolescents. A novel method of cortical surface models yielded measurements of cortical thickness and area for each subject's entire brain and computed cross-subject statistics based on cortical anatomy. The cortical surface models demonstrated regional thinning of the parietal, temporal and occipital lobes in the VLBW group, whereas regional thickening was demonstrated in the frontal and occipital lobes. The areas of change were greatest in those with the shortest gestational age at birth and lowest birth weight. Cortical surface area and cortical volume were lower in the VLBW than in the Control group. Within the VLBW group, there was an association between surface area and estimation of the intelligence quotient IQ (IQest) and between cortical volume and IQest. Furthermore, cortical grey matter as a proportion of brain volume was significantly lower in the VLBW, but not in the SGA group compared with Controls. This observed reorganization of the developing brain offers a unique opportunity to investigate any relationship between changes in cortical anatomy and cognitive and social impairments, and the increase in psychiatric disorders that have been found in VLBW children and adolescents.
Introduction
Prematurely born infants with very low birth weight (VLBW: birth weight ≤1500 g) are at increased risk of brain injury. These infants are delivered at a critical time when their brain architecture has not been fully developed (Volpe, 2001a). Infants who are born small for gestational age (SGA) are at increased risk for neurological and intellectual dysfunction (Strauss et al., 1998; Roth et al., 1999; Lundgren et al., 2001), suggesting their intrauterine condition was not optimal for maintaining brain development. Despite its major clinical and social significance, the biological mechanisms underlying the neurodevelopmental deficits in VLBW and SGA infants remain unclear. The key to understanding brain development in these infants lies in careful examination of the structural and functional organization of the brain. Only recently have medical imaging methods, such as MRI, been used to quantitatively assess neuroanatomical development following brain injury in a scientific manner (Inder et al., 1999; Ajayi-Obe et al., 2000; Peterson et al., 2000; Allin et al., 2004).
Methods that accurately measure the cortical thickness may provide new insights into the disruption of normal cortical development in these infants. In vivo, the cerebral cortex has been difficult to study due to its complex folding patterns and regional variability, and due to lack of reliable methods. MRI-based morphometric analysis assessing the cortex has resulted in new knowledge of normal cortical development and how disease affects the cerebral cortex (Ashburner et al., 2000; Sowell et al., 2001; Rosas et al., 2002; Herbert et al., 2003). The main aim of our study was to accurately measure the thickness and surface area of the cerebral cortex in a follow-up study of adolescents with low birth weight. Cortical thickness was measured across the entire brain, and cross-subject statistics were generated in a coordinate system based on cortical anatomy (Dale et al., 1999; Fischl et al., 1999a, b). Our main hypothesis was that infants with low birth weight would demonstrate, as adolescents, decreased thickness and small volume of cerebral cortex.
Materials and methods
Study design
This is a population-based follow-up study. The VLBW children were admitted to the neonatal intensive care unit at the University Hospital in Trondheim (the referral hospital) in 1986–1988. A subgroup of children born in 1988 had been assessed thoroughly at 1 and 6 years of age (Skranes et al., 1997, 1998a, b).
The SGA and control children were the second or third births of mothers living in the Trondheim area. They were enrolled before gestational week 20 in a multicentre study between January 1986 and March 1988, and followed prospectively through pregnancy (Bakketeig et al., 1993; Vik et al., 1997).
All the births in a 10% random sample of mothers and all the SGA births were included for follow-up (Vik et al., 1996). In the present study, MRI was carried out between February 2001 and September 2003.
Study population
VLBW adolescents
VLBW was defined as a birth weight ≤1500 g. A total of 99 children were admitted to the neonatal intensive care unit in 1986–1988. Of these, 23 died, one child with trisomy 21 was excluded, 6 moved out of the area and 13 did not want to participate in the study. In addition to the remaining 56 VLBW adolescents who entered the study, 8 other VLBW subjects who were not part of the original cohort were included. However, they were not different from the others in terms of gestational age, birth weight or head circumference at birth. In all, 55 VLBW adolescents underwent MRI. Due to image artefacts from dental braces, the MRI of 5 adolescents were excluded, leaving 50 such investigations (26 males and 24 females) for the morphometric brain analysis: 9 individuals had a birth weight <1000 g, 21 were born small for gestational age and 11 were born as a twin. Cerebral palsy (i.e. 5 diplegia and 1 hemiplegia, all but 1 could walk) was diagnosed in 6 of the VLBW adolescents who underwent MRI. There were no major visual or hearing impairments in any of the included adolescents.
SGA adolescents
Of 1200 eligible women, 104 (9%) gave birth at full term to an SGA child, defined as a birth weight <10th percentile adjusted for gestational age, gender and parity (Vik et al., 1997). At follow-up, 12 children had moved away. Of the remaining 92, 32 did not consent to participate in our study. Of the 60 remaining SGA adolescents, 50 had an MRI investigation. Due to image artefacts, one SGA subject was excluded, leaving 49 MRI investigations (20 males and 29 females) for the morphometric analysis of the brain. Cerebral palsy (diplegia, could walk) was diagnosed in one SGA adolescent who underwent MRI.
Control adolescents
The control group comprised 120 subjects with birth weight ≥10th percentile for gestational age, born at term to mothers in the 10% random sample. At follow-up, 10 had moved away. Of the remaining 110, 27 did not consent, leaving 83 who participated. Of these, 65 had a MRI investigation. Due to image artefacts from braces, 7 MRI scans were excluded, leaving 58 MRI investigations (22 males and 36 females) for the morphometric studies.
Non-participants
There were no differences in maternal age at childbirth, length of gestation or the infants birth weight, body length and head circumference between those who participated and those who did not consent in any of the groups.
Data collected prenatally and at birth
In the VLBW group, 25 mothers had received steroids for foetal lung maturation, 33 had premature rupture of the membranes <24 h before labour and 14 had prolonged ruptures. Persistent ductus arteriosus was diagnosed in 10 of the 50 infants. Twenty-six newborns needed ventilatory support (range: 1–63 days). It took 3–39 days before they regained their birth weight.
Estimation of intelligence quotient
Estimation of the intelligence quotient (IQest) was derived from the vocabulary and block design subtests of Wechsler Intelligence Scale for Children-Third edition (Wechsler, 1999). Low IQest was defined as <2 SD of the Control group mean value.
MR imaging
MRI was performed at St Olav's Hospital, University Hospital of Trondheim, Norway on a 1.5 Tesla Siemens Symphony Sonata, Siemens AG, Erlangen, Germany.
The imaging for the morphometric analysis was a 3D inversion recovery prepared fast low flip angle gradient echo sequence (MPRAGE), with 128 sagittal partitions, 1.33 mm slice thickness, TR between inversion pulses of 2730 ms; TR/TE/flip angle/TI, 7.1 ms/3.45 ms/7°/1000 ms; acquisition matrix of 256 × 192 × 128; square FOV of 256 mm; NEX 1; and acquisition duration 8.5 min. These acquisition parameters were chosen for optimal contrast between grey matter, white matter and CSF. The sequence was acquired twice during the same image session for each adolescent.
Image analysis
In order to create a single image with high contrast-to-noise, two separate MPRAGE acquisitions were motion-corrected and averaged by subject. These 3D structural scans were used to construct models of each individual's cortical surface, and for generating cross-subject statistics in a cortical surface-based coordinate system (Dale et al., 1999; Fischl et al., 1999a, b). As the method uses both intensity and continuity information in the segmentation and deformation procedures to measure cortical thickness, the thickness is not limited to the voxel resolution of the image (Fischl et al., 2002).
Briefly, cortical thickness measurements were obtained by reconstructing the grey/white matter boundary and the pial surface of the cortex, segmenting cortical white matter by classifying all white matter voxels in the MRI volume and tessellation of the grey/white matter boundary. Subsequently, this reconstructed surface was deformed by surface algorithm to conform to the grey/white and pial surfaces (Fischl et al., 2002).
Cortical thickness was defined as the shortest distance between the grey/white matter boundary and pial surface models, at each surface location. Thickness measures were mapped to the inflated surface of the reconstructed brain of each adolescent. This procedure allows visualization of data across the entire cortical surface without being obscured by cortical folding.
In order to present cortical thickness across subjects, a procedure of accurate matching of morphologically homologous cortical locations was as follows: each reconstructed brain was morphed to an average spherical surface representation that optimally aligned sulcal and gyral features across subjects, while minimizing metric distortion (Fischl et al., 1999a, b). Further, to remove noise-induced variations in measurements, a small surface-based Gaussian blurring kernel with a standard deviation of 11 mm was applied to smoothe the thickness estimates. Total brain volume was calculated by the volume of all brain labels, according to the automated method of Fischl et al. (2002).
Statistics
Thickness maps from one study group were averaged using the high-resolution surface-based averaging techniques and compared with the thickness from a second study group whose average was ascertained similarly (Fischl et al., 2000). Mean cortical thickness and variance were calculated at each location (data not shown). Statistically significant thickness difference maps were generated using t-tests between groups, i.e. at each vertex using a random effect model across both cortical hemispheres. As the VLBW group was heterogeneous in terms of perinatal data, we also generated thickness difference maps between subgroups (by gestational age, birth weight and head circumference) of VLBW and the Control adolescents.
Global cortical area, mean cortical thickness and cortical volume in the three study groups were compared by use of one-way ANOVA, while applying Scheffes post hoc test. Simple linear regression was employed to analyse clinical data (gestational age, birth weight, and head circumference at birth and head circumference at one year of age). Also, perinatal data (prenatal steroids, rupture of membranes, ductus arteriosus status, intra-ventricular haemorrhage, days on ventilator and days before birth weight was regained) were analysed by simple linear regression. When more than one of these variables significantly predicted the global measures, multiple regression analysis was performed. Correlations between cortical thickness and IQest of the adolescents were calculated using Pearson's moment correlation coefficient and corresponding two-tailed significance levels. P-values <0.05 were considered statistically significant.
Ethics
The regional committee for medical research ethics approved the study protocol. Written informed consent was obtained from adolescents and parents.
Results
Group characteristics of the adolescents who took part are presented in Table 1. Gestational age was shorter and birth weight and head circumferences were lower in the VLBW than in the two other groups. The SGA group had lower weight and head circumference at birth than in the control group. The VLBW group was slightly younger, 3–4 months, at MRI investigation compared to the two other groups. IQest scores were lower in the VLBW group than in the Control group. Ten adolescents in the VLBW group had low IQest defined as 2 SD below the control group mean, and three of these also had cerebral palsy. Among the 26 VLBW with gestational age ≤28 weeks, 6 adolescents had low IQest.
Group characteristics of the adolescents
| Group characteristics . | n . | VLBW . | n . | SGA . | n . | Control . |
|---|---|---|---|---|---|---|
| Gestational age in weeks | 50 | 29.1 ± 2.7*,# | 49 | 39.5 ± 1.1 | 58 | 39.6 ± 1.1 |
| Birth weight in grams | 50 | 1195 ± 239*,# | 49 | 2915 ± 216* | 58 | 3707 ± 486 |
| Head circumference at birth in cm | 49 | 27.0 ± 2.3*,# | 49 | 33.8 ± 1.2* | 58 | 35.4 ± 1.2 |
| Head circumference at 1 year in cm | 31 | 45.3 ± 2.9*,# | 45 | 46.7 ± 1.3 | 52 | 47.3 ± 1.1 |
| MRI age in years | 50 | 15.2 ± 0.6*,# | 49 | 15.6 ± 0.6 | 58 | 15.5 ± 0.5 |
| IQest scores | 49 | 87 ± 20* | 49 | 95 ± 15 | 54 | 98 ± 15 |
| Group characteristics . | n . | VLBW . | n . | SGA . | n . | Control . |
|---|---|---|---|---|---|---|
| Gestational age in weeks | 50 | 29.1 ± 2.7*,# | 49 | 39.5 ± 1.1 | 58 | 39.6 ± 1.1 |
| Birth weight in grams | 50 | 1195 ± 239*,# | 49 | 2915 ± 216* | 58 | 3707 ± 486 |
| Head circumference at birth in cm | 49 | 27.0 ± 2.3*,# | 49 | 33.8 ± 1.2* | 58 | 35.4 ± 1.2 |
| Head circumference at 1 year in cm | 31 | 45.3 ± 2.9*,# | 45 | 46.7 ± 1.3 | 52 | 47.3 ± 1.1 |
| MRI age in years | 50 | 15.2 ± 0.6*,# | 49 | 15.6 ± 0.6 | 58 | 15.5 ± 0.5 |
| IQest scores | 49 | 87 ± 20* | 49 | 95 ± 15 | 54 | 98 ± 15 |
Mean ±SD;
P < 0.01 compared to Control group;
P < 0.01 compared to SGA group.
Group characteristics of the adolescents
| Group characteristics . | n . | VLBW . | n . | SGA . | n . | Control . |
|---|---|---|---|---|---|---|
| Gestational age in weeks | 50 | 29.1 ± 2.7*,# | 49 | 39.5 ± 1.1 | 58 | 39.6 ± 1.1 |
| Birth weight in grams | 50 | 1195 ± 239*,# | 49 | 2915 ± 216* | 58 | 3707 ± 486 |
| Head circumference at birth in cm | 49 | 27.0 ± 2.3*,# | 49 | 33.8 ± 1.2* | 58 | 35.4 ± 1.2 |
| Head circumference at 1 year in cm | 31 | 45.3 ± 2.9*,# | 45 | 46.7 ± 1.3 | 52 | 47.3 ± 1.1 |
| MRI age in years | 50 | 15.2 ± 0.6*,# | 49 | 15.6 ± 0.6 | 58 | 15.5 ± 0.5 |
| IQest scores | 49 | 87 ± 20* | 49 | 95 ± 15 | 54 | 98 ± 15 |
| Group characteristics . | n . | VLBW . | n . | SGA . | n . | Control . |
|---|---|---|---|---|---|---|
| Gestational age in weeks | 50 | 29.1 ± 2.7*,# | 49 | 39.5 ± 1.1 | 58 | 39.6 ± 1.1 |
| Birth weight in grams | 50 | 1195 ± 239*,# | 49 | 2915 ± 216* | 58 | 3707 ± 486 |
| Head circumference at birth in cm | 49 | 27.0 ± 2.3*,# | 49 | 33.8 ± 1.2* | 58 | 35.4 ± 1.2 |
| Head circumference at 1 year in cm | 31 | 45.3 ± 2.9*,# | 45 | 46.7 ± 1.3 | 52 | 47.3 ± 1.1 |
| MRI age in years | 50 | 15.2 ± 0.6*,# | 49 | 15.6 ± 0.6 | 58 | 15.5 ± 0.5 |
| IQest scores | 49 | 87 ± 20* | 49 | 95 ± 15 | 54 | 98 ± 15 |
Mean ±SD;
P < 0.01 compared to Control group;
P < 0.01 compared to SGA group.
Cortical thickness regional data
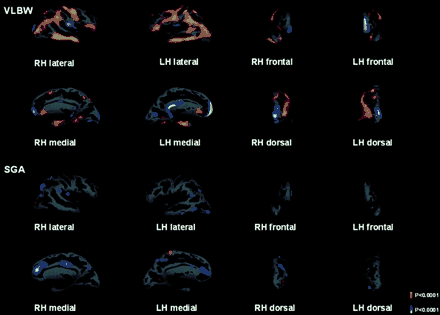
Cortical thickness measures were mapped to the inflated surface of each subject's brain reconstruction and statistical difference maps between the three groups were generated. Figure 1 presents the maps that compare VLBW and controls, and those comparing SGA and controls. The brain areas with statistically significant differences between groups are coloured, in which the colour scale shows the dynamic range of statistically significant changes, i.e. thinning from red (P < 0.05) to yellow (P < 0.0001), and thickening from dark (P < 0.05) to light blue (P < 0.0001).
The average statistical map of 50 VLBW subjects compared with the average map of 58 Control subjects. The average statistical map of 49 SGA subjects compared with the average map of 58 Control subjects. Statistical maps are overlaid on the surface reconstruction with inflated brains, right hemisphere (RH), left hemisphere (LH). Dark grey areas correspond to sulci and light grey areas correspond to gyri. Non-neocortical regions that are not part of the cortical mantle (such as corpus callosum and thalamus) have been excluded from the analysis. The brain areas with statistically significant difference between groups are shown in colour, and the colour scale shows the dynamic range of the statistically significant changes, red to yellow represents a thinning of the cortex, and full yellow corresponds to a statistical difference in cortical thickness with a P value of 0.0001. Blue represents a statistically significant thickening of the cortex; light blue corresponds to a statistical difference in cortical thickness with a P value of 0.0001.
When the VLBW group was compared with Controls, significant cortical thinning was present in the parietal lobe (pre- and post-central gyrus and the supra-marginal part of the parietal inferior gyrus) and in the temporal lobe (temporal middle gyrus, intermedius primus Jensen sulcus, occipito-temporal medial gyrus, Parahippocampal part and temporal middle gyrus). Significant cortical thickening was present on the medial surface of the hemispheres (pericollasal sulcus) and in the frontal lobe (frontal superior gyrus, orbital gyrus and rectus gyrus). We could also demonstrate cortical thickening in the temporal region (circular insula superior sulcus) and in the occipital lobe (occipital superior gyrus).
When comparing the VLBW with the SGA group, we found similar areas of thinning and thickening (data not shown). Moreover, we observed some thicker areas among SGA than Control subjects.
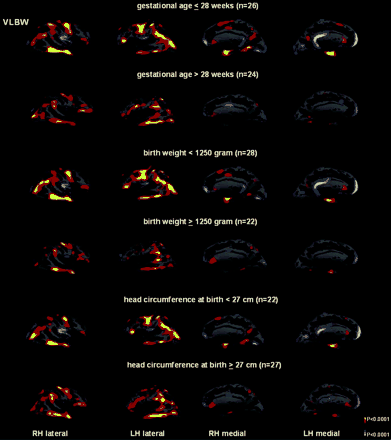
We also compared cortical thickness in subgroups of VLBW adolescents with the Controls (Fig. 2). Although areas of significant focal cortical thinning could be demonstrated in VLBW adolescents born after 28 gestational weeks, they were less than the differences below or equal to that age. Similar differences were demonstrated in birth weight subgroups (BW ≥1250 g, BW <1250 g) and in head circumference at birth subgroups (HC ≥ 27 cm, HC < 27 cm).
Statistical maps showing areas of significant differences in cortical thickness between the 58 control subjects and the VLBW adolescents subgrouped according to gestational age, birth weight and head circumference at birth. The colour scale shows the dynamic range of changes, yellow represents statistically significant thinning (P < 0.0001), whereas light blue represents statistically significant thickening (P < 0.0001).
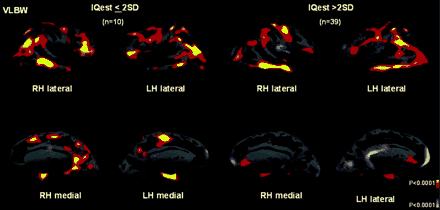
Low IQest, defined as 2 SD below the control group mean, was associated with thinner cortex in the parahippocampal region in VLBW adolescents, whereas normal IQest was associated with thicker cortex in the frontal areas in this group compared with Controls (Fig. 3).
Statistical maps showing areas of significant differences in cortical thickness between the 58 control subjects and the VLBW adolescents subgrouped according to IQest scores. The colour scale shows the dynamic range of changes, yellow represents statistically significant thinning (P < 0.0001), whereas light blue represents statistically significant thickening (P < 0.0001).
Cortical thickness global data
In both left and right hemisphere, cortical surface area and volume were lower in the VLBW compared with the Control group (Table 2). For the right hemisphere, cortical surface area was significantly lower in the SGA than among Controls. Although cortical volume was lower in the SGA than in the Control group, this did not reach statistical significance. In none of the groups were there any significant differences in mean cortical thickness.
Global measures of cortical thickness, cortical surface area and cortical volume
| . | Right hemisphere . | . | . | Left hemisphere . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | VLBW . | SGA . | Control . | VLBW . | SGA . | Control . | ||||
. | n = 50 . | n = 49 . | n = 57 . | n = 50 . | n = 49 . | n = 57 . | ||||
| Cortical surface area (cm2) | 815 ± 96* | 842 ± 82* | 885 ± 81 | 814 ± 96* | 843 ± 82 | 879 ± 81 | ||||
| Cortical thickness (mm) | 2.13 ± 0.09 | 2.17 ± 0.10 | 2.16 ± 0.09 | 2.13 ± 0.09 | 2.16 ± 0.10 | 2.17 ± 0.09 | ||||
| Cortical volume (cm3) | 173 ± 21* | 183 ± 19 | 191 ± 20 | 174 ± 22* | 182 ± 19 | 190 ± 20 | ||||
| . | Right hemisphere . | . | . | Left hemisphere . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | VLBW . | SGA . | Control . | VLBW . | SGA . | Control . | ||||
. | n = 50 . | n = 49 . | n = 57 . | n = 50 . | n = 49 . | n = 57 . | ||||
| Cortical surface area (cm2) | 815 ± 96* | 842 ± 82* | 885 ± 81 | 814 ± 96* | 843 ± 82 | 879 ± 81 | ||||
| Cortical thickness (mm) | 2.13 ± 0.09 | 2.17 ± 0.10 | 2.16 ± 0.09 | 2.13 ± 0.09 | 2.16 ± 0.10 | 2.17 ± 0.09 | ||||
| Cortical volume (cm3) | 173 ± 21* | 183 ± 19 | 191 ± 20 | 174 ± 22* | 182 ± 19 | 190 ± 20 | ||||
Mean ± SD;
P < 0.05 compared to Control group.
Global measures of cortical thickness, cortical surface area and cortical volume
| . | Right hemisphere . | . | . | Left hemisphere . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | VLBW . | SGA . | Control . | VLBW . | SGA . | Control . | ||||
. | n = 50 . | n = 49 . | n = 57 . | n = 50 . | n = 49 . | n = 57 . | ||||
| Cortical surface area (cm2) | 815 ± 96* | 842 ± 82* | 885 ± 81 | 814 ± 96* | 843 ± 82 | 879 ± 81 | ||||
| Cortical thickness (mm) | 2.13 ± 0.09 | 2.17 ± 0.10 | 2.16 ± 0.09 | 2.13 ± 0.09 | 2.16 ± 0.10 | 2.17 ± 0.09 | ||||
| Cortical volume (cm3) | 173 ± 21* | 183 ± 19 | 191 ± 20 | 174 ± 22* | 182 ± 19 | 190 ± 20 | ||||
| . | Right hemisphere . | . | . | Left hemisphere . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | VLBW . | SGA . | Control . | VLBW . | SGA . | Control . | ||||
. | n = 50 . | n = 49 . | n = 57 . | n = 50 . | n = 49 . | n = 57 . | ||||
| Cortical surface area (cm2) | 815 ± 96* | 842 ± 82* | 885 ± 81 | 814 ± 96* | 843 ± 82 | 879 ± 81 | ||||
| Cortical thickness (mm) | 2.13 ± 0.09 | 2.17 ± 0.10 | 2.16 ± 0.09 | 2.13 ± 0.09 | 2.16 ± 0.10 | 2.17 ± 0.09 | ||||
| Cortical volume (cm3) | 173 ± 21* | 183 ± 19 | 191 ± 20 | 174 ± 22* | 182 ± 19 | 190 ± 20 | ||||
Mean ± SD;
P < 0.05 compared to Control group.
In addition, the total brain volume was significantly lower in the VLBW and SGA groups than in the Control group (VLBW, 1470 ± 163; SGA 1468 ± 129; Control 1548 ± 118 cm3; P < 0.02). Cortical grey matter, as a proportion of brain volume, was significantly lower in the VLBW group (VLBW, 23.7 ± 2.1%; Control, 24.9 ± 1.7%; P < 0.01), but, not in the SGA group compared to Controls.
Perinatal data as predictors for global measures
In all three groups, head circumference at one year of age predicted brain volume measured at age 15 (VLBW group beta 0.55; P < 0.001, SGA group beta 0.65; P < 0.0001 and Controls beta 0.45; P < 0.0001).
There was an increase in cortical surface area with increasing head circumference at one year in the VLBW (beta 0.57; P < 0.001) and the SGA group (beta 0.49; P < 0.0001). In contrast, there was an increasing cortical surface area with increasing head circumference at birth in the Control group (beta 0.33; P < 0.02). Neither gestational age, birth weight nor head circumference at birth or at one year of age predicted mean global cortical thickness. However, in the VLBW group, number of days on ventilator and days before the birth weight was regained also predicted total surface area (beta 0.34; P < 0.03, beta 0.35; P < 0.015).
Association between global measures and IQest
Significant correlations were found only in the VLBW group, both between IQest and cortical area (right hemisphere, r = 0.509; P < 0.0001, left hemisphere, r = 0.346; P < 0.05), and between IQest and cortical volume (right hemisphere, r = 0.483; P < 0.0001, left hemisphere, r = 0.304; P < 0.05).
Discussion
In this study, we used a novel method of surface reconstruction to accurately measure cortical thickness on cerebral MRI. The method has been validated by performing analysis on post-mortem brains (Rosas et al., 2002), and by manual measurements (Kuperberg et al., 2003). All surface models in our study were visually inspected for accuracy. The question we addressed was whether adolescents with low birth weight show deviations in cerebral cortical thickness and area compared with adolescents with normal birth weight. Although the mean cortical thickness was similar, there were still specific areas of statistically significant differences in cortical thickness between the study groups (see Fig. 1). In particular, there were areas of thinner cortex in VLBW births and most of them were located in the parietal and the temporal lobes.
Among previously published papers, Peterson et al. (2000) reported reduced cortical volumes in 8-year-old prematurely born infants. As a result of their measurement methods, however, they were unable to exactly localize the changes, or distinguish grey from white matter tissue. Grey matter abnormality is probably highly correlated to both presence and severity of white matter abnormality (Inder et al., 2003). Furthermore, grey matter abnormality seems to persist during maturation. Thus in adolescents who were born prematurely, reduced cortical grey matter and enlarged ventricles have been demonstrated (Nosarti et al., 2002).
The specific areas of thinner cortex we found in adolescents born prematurely could be a consequence of primary cortical neuronal damage or secondary to white matter damage. We speculate that these findings may be a consequence of white matter damage, as the regions of thinner cortex have a distribution which seems to reflect the shape of the ventricles. This might represent an association between the areas of thinner cortex and underlying white matter injury due to periventricular leukomalacia. Axonal disruption is a classic feature of periventricular leucomalacia (Dammann et al., 2001). Marin-Padialla et al. (1997) employed histopathological methods and detected distinct alterations in the morphology and organization of neurons in the cerebral cortex overlying periventricular leukomalacia.
We recently reported a high prevalence of qualitative abnormal MRI findings at 15 years of age in the VLBW adolescents included in the present study (Skranes et al., 2005), and the combination of white matter reduction, ventricular dilation and reduced thickness of the corpus callosum found in >50% of the adolescents were interpreted as sequelae following periventricular leucomalacia. Some of the VLBW adolescents in the present study (the 1988 cohort) also participated in two previous studies at our institution with 1-year and 6-year follow-up (Skranes et al., 1993, 1997). Cerebral ultrasound scans in the first days after birth were reported in Skranes et al. (1993) and were normal in 79% of these VLBW births. However, late ultrasound scans necessary for the diagnosis of periventricular leucomalacia were not performed and assessment of periventricular leucomalacia in the neonatal period might have been missed in many cases. Skranes et al. (1997) also found that in the infants with more extensive MRI abnormalities at 1 year of age, at 6 years of age periventricular correlates, such as periventricular gliosis and peritrigonal atrophy with localized ventricular dilation were reported in 50% of the cases. We interpret this as indication of a large proportion of periventricular malacia in the VLBW adolescents in the present study.
Surprisingly, although cortical volume and total surface area were lower in the VLBW group than in the Control group, we found some areas of thicker cortex in VLBW compared with the Controls, especially in the frontal lobe. There are no data in the present study to explain this finding. Exposure to hypoxia during weeks of postnatal life has been shown to decrease cortical volumes, but increase the number of neurons in animal models (Stewart et al., 1997). As an explanation for this finding, it was suggested that the pattern of programmed apoptosis in the neocortex may be altered by hypoxia. We speculate whether our finding of thicker frontal areas might represent a disruption in such programmed cell death in the developing cortex. However, late spatial and temporal patterns of brain growth in humans have also been reported (Sowell et al., 1999). Brain development includes regionally specific cortical maturation (Giedd et al., 1999), and grey matter reduction may reflect more mature organization that supports improved cognitive functioning. Cortical surface grey matter reduction between childhood and adolescence was reported to be most prominent in the frontal lobes (Sowell et al., 2001). Whether the thicker frontal area in our VLBW group may represent delayed maturation, and may be associated with executive functions operating at a less mature level, would need further investigation.
When the VLBW group was sub-grouped according to gestational age, birth weight and head circumference (see Fig. 2), we found indications of a maturation-dependent vulnerability of brain development; the shorter the gestational age and lower the birth weight and head circumference at birth, the larger the regions of cortical thinning. This vulnerability was reported by Nosarti et al. (2002) who found that the more prematurely the infant was born, the smaller the white matter volume at 15 years of age. Peterson et al. (2000) reported that brain volume reductions were associated with poorer cognitive outcome. They also found significant associations between regional brain volumes and gestational age at birth, suggesting that disturbance in brain development is proportional to degree of immaturity.
In our VLBW group, smaller cortical volume was associated with smaller cortical area, but not to global cortical thinning (see Table 2). Thus, not only was regional cortical thinning present among the premature births, but also global decrease in cortical area. We speculate whether this may be due to disturbed global cortical maturation. Ajayi-Obe et al. (2000) found that preterm infants, who were imaged when they reached their term age, had less complex cortical folding and reduced cortical surface area than term births. Inder et al. (1999) found that cerebral cortical grey matter volume was more reduced in preterms with periventricular leucomalacia than those without. This may also indicate some disturbance in cortical neuronal development caused by impairment of neuronal connections to and from the cerebral cortex due to white matter injury.
We found that the adolescent cortical surface area of VLBW births was predicted by head circumference at 1 year of age, even if it was not by head circumference at birth. The newborn period of the premature infant is one of relative malnutrition. The effects of nutrition on cognitive development suggest that nutritional factors have an impact on organizational events of brain maturation (Brandt et al., 2003). High energy intake is correlated with catch-up growth of head circumference, and is associated with increased developmental and intelligence quotients (Frisk et al., 2002). An association between cortical development and cognitive function is suggested also in our study since IQest correlated with cortical surface area and cortical volume. Significant associations of morphological disturbances in brain development and cognitive function have been reported in 8-year-old infants (Peterson et al., 2000), and in 15–16-year-old adolescents (Abernethy et al., 2002). Peterson et al. (2000) found that regional cortical volumes correlated with IQ. However, their method could not discriminate whether the reduced cortical volumes in preterm children preferentially involved either cortical grey or adjacent white matter. The method used in our study does, however, allow us to localize the differences to the cortical surface. Statistically significant thinning of the parahippocampal cortex in the VLBW with low IQest (see Fig. 3) may be supported by the findings of Abernethy et al. (2002) of an association between low hippocampal formation volumes and low IQ in VLBW teenagers.
We found that an increasing number of days with ventilatory support and days before the birth weight was regained predicted smaller cortical surface area at 15 years of age. Days in ventilator is linked to lung injury in the preterm infant, and oxygen deprivation is suggested to be a major cause of neurodevelopmental disability in preterm infants (Volpe, 2001b). One might speculate that unfavourable conditions in the newborn period, e.g. hypoxic episodes and malnutrition, permanently impair cortical growth resulting in neurodevelopmental disability.
The total brain volume was significantly reduced among SGA births, which indicates that intrauterine growth restriction impairs cerebral development. There is neuropathological evidence for a deleterious effect of malnutrition on brain development, a reduction in cell number and cell size, and an overall lower brain weight (Rees et al., 1988; Mallard et al., 1998).
Prematurely born SGA infants have demonstrated definite neurostructural differences in cortical grey matter volume at term age (Tolsa et al., 2004). At 15 years of age, the cortical volume was lower in our SGA group than the controls, albeit non-significantly. The difference between the study of Tolsa et al., and ours might be explained by the postnatal catch-up growth that occurred in our adolescents. However, growth restriction might have an additional impact on late brain development in premature births who were also small for dates.
Our results may seem to be of importance for delineating the relation between structural and functional consequences of low birth weight. Disorganization or reorganization of the developing brain including the cerebral cortex may thus be responsible for cognitive and social impairments and the increase in psychiatric disorders in the prematurely born (Stewart et al., 1999; Indredavik et al., 2004).
The study was supported by the Department of Laboratory Medicine Children's and Women's Health, Faculty of Medicine, Norwegian University of Science and Technology, and by St Olav's Hospital, University Hospital of Trondheim, Norway. The study was also supported by the US National Institutes of Health (NICHD contract No. 1-HD-4-2803 and No. 1-HD-1-3127; R01-NS39581, R01-RR16594, P41-RR14075 and R01-RR13609), the Mental Illness and Neuroscience Discovery (MIND) Institute, and in part by the Biomedical Informatics Research Network Project (BIRN, http://www.nbirn.net), which is funded by the National Center for Research Resources at the National Institutes of Health (NCRR BIRN Morphometric Project BIRN002).
References
Abernethy LJ, Palaniappan M, Cooke RW. Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight.
Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants.
Allin M, Henderson M, Suckling J, Nosatri C, Rushe T, Fearon P, et al. Effects of very low birthweight on brain structure in adulthood.
Bakketeig LS, Jacobsen G, Hoffman HJ, Lindmark G, Bergsjo P, Molne K. Pre-pregnancy risk factors of small for-gestational age birth among parous women in Scandinavia.
Brandt I, Sticker EJ, Lentze MJ. Catch-up growth of head circumference of very low birth weight, small for gestational age preterm infants and mental development to adulthood.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I. Segmentation and surface reconstruction.
Dammann O, Hagberg H, Leviton A. Is periventricular leuckomalacia an axonopathy as well as an oligopathy?
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis II. Inflation, flattening, a surface based coordinate system.
Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution inter-subject averaging and a coordinate system for the cortical surface.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain.
Fischl B, Dale AM. Measuring the thickness of the human cortex from magnetic resonance images.
Frisk V, Amsel R, Whyte HEA. The Importance of head growth patterns in predicting the cognitive abilities and literacy skills of small-for-gestational-age children.
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study.
Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys.
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical grey matter volume at term.
Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: A qualitative magnetic resonance imaging study.
Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk A-M. Psychatric symptoms and disorders in adolescents with low birth weight.
Kuperberg GR, Broome MR, Mc Guire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia.
Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Intellectual and psychological performance in males born small for gestational age with and without catch-up growth.
Mallard CE, Rees S, Stringer M, Cock ML, Harding R. Effects of chronic placental insufficiency on brain development in fetal sheep.
Marin-Padialla M. Developmental neuropathology and impact of perinatal brain damage. II: white matter lesions of the neocortex.
Nosarti C, Al-Asady MHS, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes.
Peterson SB, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants.
Rees S, Bocking AD, Harding R. Structure of the fetal sheep brain in experimental growth retardation.
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington's disease.
Roth S, Chang TC, Robson S, Spencer JAD, Wyatt JS, Stuart AL. The neurodevelopmental outcome of term infants with different intrauterine growth characteristics.
Skranes JS, Vik T, Nilsen G, Smevik O, Andersson HW, Rinck P, Brubakk A-M. Cerebral magnetic resonance imaging (MRI) and mental and motor function of very low birth weight infants at one year of corrected age.
Skranes JS, Vik T, Nilsen G, Smevik O, Andersson HW, Brubakk A-M. Cerebral magnetic resonance imaging and mental and motor function of very low birth weight children at six years of age.
Skranes J, Vik T, Nilsen G, Smevik O, Andersson HW, Brubakk A-M. Can cerebral MRI at age 1 predict motor and intellectual outcomes in very-low-birthweight children?
Skranes J, Nilsen G, Smevik O, Vik T, Brubakk AM. Cerebral MRI of very low birth weight children at 6 years of age compared with the findings at 1 year.
Skranes JS, Martinussen M, Smevik O, Myhr G, Indredavik M, Vik T, Brubakk AM. Cerebral MRI findings in very-low-birth-weight and small-for-gestational-age children at 15 years of age.
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions.
Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation.
Strauss RS. Growth and development of term children born with low birth weights: effects of genetic and environmental factors.
Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, et al. Brain structure and neurocognitive and behavioral function in adolescents who were born very preterm.
Stewart WB, Ment L, Schwartz M. Chronic postnatal hypoxia increases the number of cortical neurons.
Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, et al. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction.
Vik T, Markestad T, Ahlsten G, Gebre-Medhin M, Jacobsen G, Hoffman HJ. Body proportions and early neonatal morbidity in small for gestational age infants of successive births.
Vik T, Jacobsen G, Vatten L, Bakketeig LS. Pre- and post-natal growth in children of women who smoked in pregnancy.
Volpe JJ. Neurology of the newborn. W.B. Saunders Company, Philadelphia, Pennsylvania, USA;
Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection.
Author notes
1Nuclear Magnetic Resonance Center, Massachusetts General Hospital, Harvard Medical School and 2MIT Artificial Intelligence Laboratory, MIT, Boston, MA, Departments of 3Neuroscience and 4Radiology, University of California, San Diego, CA, USA, Departments of 5Circulation and Medical Imaging, 6Public Health and General Practice and 7Laboratory Medicine, Children's and Women's Health, Faculty of Medicine, Norwegian University of Science and Technology, 8Department of Medical Imaging, St Olav's Hospital, Trondheim, Norway and 9Functional and Diagnostic MR unit, Glostrup University Hospital, Denmark