-
PDF
- Split View
-
Views
-
Cite
Cite
Joseph P. McGowan, Sanjiv S. Shah, Camelia E. Ganea, Steve Blum, Jerome A. Ernst, Kathleen L. Irwin, Noemi Olivo, Paul J. Weidle, Risk Behavior for Transmission of Human Immunodeficiency Virus (HIV) among HIV-Seropositive Individuals in an Urban Setting, Clinical Infectious Diseases, Volume 38, Issue 1, 1 January 2004, Pages 122–127, https://doi.org/10.1086/380128
Close - Share Icon Share
Abstract
We conducted interviews with 256 human immunodeficiency virus (HIV)-infected patients who attended an HIV clinic in New York City to assess ongoing risk behaviors for HIV transmission. After learning that the result of an HIV test was positive, 106 subjects (41%) had unprotected sex, 63 (25%) had a new sexually transmitted disease diagnosis, and 38 (15%) used injection drugs. Unprotected sex was reported by 50% of women, 29% of heterosexual men (P = .006, compared with women), and 42% of men who have sex with men, and it was reported more often by persons with a history of trading sex for money or drugs (P < .001). In multivariate analysis, unprotected sex was associated with a history of trading sex for money or drugs (adjusted odds ratio [AOR], 4.0; 95% confidence interval [CI], 2.2–7.0) and use of highly active antiretroviral therapy (AOR, 1.8; 95% CI, 1.1–3.1). Ongoing risk-reduction counseling and substance abuse treatment for HIV-infected persons are needed to reduce behaviors associated with HIV transmission.
Because HIV-seropositive persons are the source of transmission of HIV, it is important to characterize the type and extent of their risk behaviors for HIV transmission. It has been estimated that approximately one-third of HIV-infected persons in the United States are unaware of their HIV serostatus [1, 2]. Although these individuals may indeed be the source of transmission for many new cases of HIV infection, HIV-seropositive individuals who are aware of their infection and who engage in high-risk activity may also pose a significant risk for transmission in the community. Indeed, recent outbreaks of sexually transmitted diseases (STDs) among persons with long-standing HIV infection suggest that there are ongoing risk behaviors among some groups [3–5]. Because many HIV-infected persons are receiving antiretroviral therapy, risk behavior for HIV transmission among HIV-seropositive persons may not only lead to the spread of HIV infection but would likely account for the transmission of drug-resistant HIV [6, 7].
Ongoing risk behavior for HIV transmission has been described for men who have sex with men (MSM) and for other populations reporting injection drug use (IDU) as a risk factor for HIV transmission [8, 9]. We report high-risk behavior in a population of HIV-seropositive persons among whom the predominant risk factors for HIV acquisition were heterosexual contact and IDU. The objectives of this study were (1) to assess ongoing risk behaviors for transmission of HIV among HIV-seropositive persons in primary care who received HIV risk-reduction counseling and education as part of routine practice in our clinic, and (2) to identify associations with ongoing risk behavior.
Methods
Patients were recruited from the Infectious Diseases Clinic at the Bronx-Lebanon Hospital Center in the Bronx, New York. Data were collected as part of a study of HIV transmission in the Bronx [10]. Patients were included in the study if they had a documented date of their first positive HIV serological test result, were ≥18 years of age, lived in the United States since at least the age of 15 years, and spoke English, Spanish, French, or 1 of 3 West African languages (Twi, Ga, or Hausa). Attempts were made to recruit equal numbers of men and women within 3 strata defined by the time since their first positive HIV antibody test result (<1 year, 1–4 years, or ≥5 years).
It was the policy and standard practice at the Bronx-Lebanon Hospital Center Infectious Diseases Clinic, before and during the study period, to provide HIV risk-reduction counseling and education to each patient by the clinical care team (which consisted of ≥1 of the following: the primary care clinician, nursing staff, social workers, and trained health educators) on an ad hoc basis but at least annually as part of a required comprehensive visit. The practice was to ask structured questions about the presence or absence of current sexual activity and drug use that facilitated discussions about risk reduction.
After obtaining informed consent, a single, confidential interview designed to ascertain HIV risk behaviors was conducted in the patients' native language by an interviewer who was not on the clinic staff. Patients were not compensated other than for transportation costs. Data collected from the time before knowledge of HIV-positive serostatus included the number and sex of lifetime sexual partners, use of condoms, IDU (including the sharing of needles, cotton, cookers, rinse water, or other injection equipment), trade of sex for money or drugs without use of a condom (hereafter, “sex for money or drugs”), and diagnosis of STDs. For diagnosis of STDs, the patient was asked whether, before or at the time of their first positive HIV test result,“… a doctor, nurse, or other health care provider ever tell you that you had a sexually transmitted disease, venereal disease (VD) like gonorrhea (clap), syphilis (bad blood), sores on your genitals from herpes or chancroid, chlamydia, or [for women only] pelvic inflammatory disease (pus tubes), or [for men only] a drip from your penis?” The question regarding diagnosis of an STD did not distinguish between newly diagnosed and recurrent STDs, nor was the specific diagnosis available.
Information collected for the period after the person knew he/she was HIV seropositive included data on the use of antiretroviral therapy, sexual contacts and use of condoms, reported diagnoses of STDs (for the time after his/her first positive HIV test result, as above), and IDU (for the time after his/her first positive HIV test result, as above). Knowledge of their partner's HIV serostatus was not elicited for the period after the patient had learned of his/her positive HIV test result. HAART was defined as any antiretroviral combination that included a nonnucleoside analogue reverse-transcriptase inhibitor or a protease inhibitor in combination with ≥1 nucleoside analogue reverse-transcriptase inhibitor. Patients were classified as having had unprotected sex (UPS) after they learned of their HIV test result if they answered affirmatively to one or both of 2 questions about the lack of condom use during risky sexual practices (defined as receptive vaginal and/or anal intercourse for women; defined as insertive vaginal and/or anal intercourse or receptive anal intercourse for men) and/or reported diagnosis of an STD.
Categorical data were analyzed using χ2 or Fisher's exact test, and continuous data were analyzed using Student's t test or the nonparametric Kruskal-Wallis test for 2 groups. In a forward, stepwise, multivariate, logistic regression analysis, we considered UPS after knowledge of a positive HIV test result as the outcome variable and sex, age, IDU after diagnosis of HIV, time since positive HIV test result, male-male sex, report of a history of trading sex for money or drugs, and any use of HAART as predictor variables. All statistical tests were performed using SAS, version 6.0 (SAS Institute). The study was approved by the Institutional Review Board for the protection of human subjects at Bronx-Lebanon Hospital Center.
Results
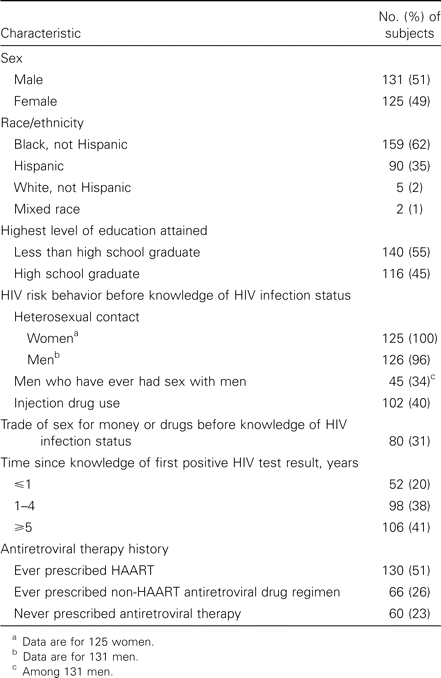
Four hundred twenty-six patients were evaluated for study eligibility, of whom 256 persons (median age, 40 years; range 20–66 years) were recruited into the study during the period of September 1997 through February 1998 (table 1). Eighty-seven persons did not meet eligibility criteria, and 46 persons were eligible but were not needed in the sampling strata that remained unfilled. Of those persons who were eligible and needed for sample requirements, 26 refused to enroll and 11 did not return for an interview.
Demographic characteristics of 256 subjects in a study of risk behavior for transmission of HIV among HIV-1—seropositive individuals in an urban setting.
All patients reported having had UPS before knowing that they were HIV seropositive. Of note, heterosexual contact was the only reported risk behavior for 118 patients (46%).
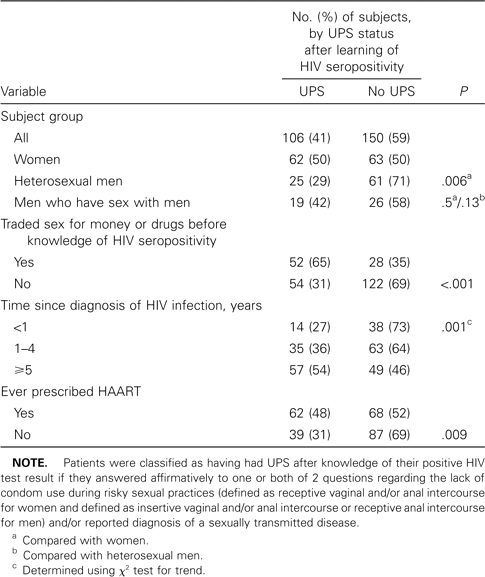
UPS after known HIV seropositivity. One hundred six (41%) of 256 patients reported having had UPS after they learned of their positive HIV test result. UPS after knowledge of HIV positivity was reported (1) more by women than by heterosexual men (but not more than by MSM), (2) more by patients who reported trading sex for money or drugs than by those who did not, (3) more by those who knew their serostatus longer, and (4) more by those who had ever been prescribed HAART than by those who had not (table 2). Those who reported having had UPS after learning of a positive HIV test result knew their serostatus an average of 17 months longer than those who did not (P = .001) and had more sex partners before testing positive (median, 40 partners; range, 1–1500 partners) than did those who did not (median, 12 partners; range, 1–1000 partners; P = .002). Report of UPS after knowledge of a positive HIV test result was not related to level of education or age category (<35 years, 35–49 years, or >49 years).
Characteristics associated with unprotected sex (UPS) and sexually transmitted diseases diagnosed after knowledge of HIV seropositivity.
STDs. One hundred seventy-one (67%) of 256 persons reported having had an STD diagnosed before or after knowledge of their positive HIV test result. Diagnosis of an STD after knowledge of a positive HIV test result was reported by 63 patients (25%), of whom 48 reported diagnosis of an STD both before and after their positive HIV test result, and 15 patients reported an STD diagnosis only after their positive HIV test result. Diagnosis of an STD after knowledge of HIV seropositivity was reported (1) more by women (30%) than by heterosexual men (15%; P = .02) but not more than by MSM (27%; P = .8, compared with women; P = .11, compared with heterosexual men); (2) more by those who reported trading sex for money or drugs (45%) than by those who did not (16%; P < .001); and (3) more by those who knew their HIV serostatus longer (for <1 year, 12%; for 1–4 years, 21%; for ≥5 years, 34%; P = .003, by χ2 test for trend). Patients who reported receiving an STD diagnosis after learning about their positive HIV test result knew their serostatus an average of 18 months longer than did those who did not report an STD diagnosis during this period (P = .003).
IDU. IDU before knowledge of a positive HIV test result was reported by 104 (41%) of 256 patients, of whom 80 (77%) reported sharing needles. IDU after knowledge of a positive HIV test result was reported by 38 (15%) of 256 patients, of whom 5 (13%) reported sharing needles and/or other injection equipment. Among women, trading sex for money or drugs before testing HIV positive was associated with IDU after knowledge of HIV infection: 12 (67%) of 18 women who reported IDU after knowledge of their positive HIV test result had reported a history of trading sex for money or drugs before they knew they were HIV infected, compared with 41 (40%) of 103 women who denied IDU after their positive HIV test result (P = .03).
Multivariate analysis. After adjusting for the factors described in the Methods, a history of trading sex for money or drugs (adjusted OR [AOR], 4.0) and receipt of HAART (AOR, 1.8) were both statistically associated with UPS after knowledge of HIV status (table 3). A second model that included a first-order interaction term between a history of trading sex for money or drugs and HAART use showed that, compared with the subgroup of patients who denied past trading of sex for money or drugs and who had not been prescribed HAART, the patients who reported a history of trading sex for money or drugs and who had been prescribed HAART were more likely to report UPS after they learned that they were HIV seropositive (AOR, 11.0; 95% CI, 4.0–31.5).
Association of reported unprotected sex after knowledge of positive HIV test result with history of trade of sex for money or drugs and HAART use.
Discussion
In this study of HIV-infected persons whose primary risk behavior for acquiring HIV was heterosexual contact or IDU, sexual behavior that places persons at high risk for HIV transmission to their sex partner was reported by >40% of persons, despite being in ongoing clinical care after they knew they were HIV infected. Proportionately more women than heterosexual men (but not men who have sex with men) reported UPS and diagnosis of an STD after they learned of their positive HIV test result. Our analysis demonstrates that, in this population, this association was largely because women and men who have sex with men were more likely to report a history of trading sex for money or drugs than were heterosexual men.
History of trade of sex for money or drugs was only obtained for the period before knowledge of a first positive HIV test result; however, study participants may have continued this behavior after they tested positive for HIV. This assumption is consistent with the findings of other investigators that trading sex for money or drugs is difficult to change, despite a person's knowledge of HIV infection [11–13]. Substance use, especially use of crack cocaine, is highly prevalent in this community [14, 15] and, along with poverty and economic needs (“survival sex”), it may be driving much of the ongoing HIV risk behavior—particularly among women—through the exchange of sex for money or drugs [11, 16].
Our study found that UPS after notification of a positive HIV test result was more commonly reported by patients who received HAART than by those who did not receive HAART. The use of HAART may contribute to a misconception that risk of HIV transmission is eliminated, especially if the HIV-1 plasma load is low [17]. Although clinical studies indicate that HIV transmission rates are positively associated with plasma virus load, there is no threshold below which transmission never occurs [18, 19]. Furthermore, identification of drug-resistant strains of HIV in newly infected patients who have never received antiretroviral therapy (primary drug resistance) have been increasingly described in communities where antiretroviral therapy is commonly prescribed [6, 7, 20]. Although the lack of adherence to HAART may generate drug resistance, it is ongoing risk behavior that leads to transmission of primary drug-resistant HIV strains. Alternatively, because data about protected sex were not collected, it is possible that persons receiving HAART who benefit from an improved health status may generally engage in increased sexual activity, both protected and unprotected, that may not be related to their perception of transmission risk.
The association between use of HAART and report of UPS after learning that HIV test results were positive showed that there was a strong interaction with a history of trade of sex for money or drugs. Subjects who both used HAART and had a history of trade of sex for money or drugs had an AOR of 11 for UPS after learning of HIV-positive serostatus, compared with those who had neither characteristic. Although persons who engage in trade of sex for money or drugs may want to decrease their risk of transmission of HIV with use of HAART, the lack of condom use among these persons illustrates a potential misunderstanding of the current prevention messages that advocate continued condom use even if HAART is being administered. Alternatively, the same factors that drive continued commercial sex trade, such as maintaining a drug habit, may lead some patients to sell their HAART and other HIV-related medications to other individuals or to pharmacies [21].
Genital ulcer diseases, such as herpes simplex and syphilis, and nonulcerative STDs, such as gonorrhea and chlamydia, can increase HIV viral shedding in the genital tract and have been associated with an increased risk of HIV transmission [22–26]. We found that approximately one-fourth of patients, including nearly one-third of the women, reported diagnosis of an STD after they had learned that they were HIV infected. Diagnosis and treatment of STDs in HIV-infected patients should play a critical role in strategies to reduce transmission of HIV [22].
The prevalence of risk behavior for HIV transmission was higher among patients who knew their HIV positive test result longer. This may be the result of a cumulative effect related to increased opportunity, with time, for risky behavior and/or a waning of motivation for risk-behavior modification (“prevention fatigue”) over time that leads to recidivism of unsafe sex practices. However, our study did not assess the frequency and/or recentness of risk-reduction counseling.
It was encouraging that reporting of IDU and needle and paraphernalia sharing was much lower for individuals after they had learned of their positive HIV test result than before. Data on enrollment of patients in methadone maintenance, needle exchange, and other substance abuse treatment programs were not collected as part of the study, but participation in such programs, along with risk-reduction counseling by health care providers, may have had an impact on these findings.
A limitation of the study is our inability to distinguish behavior, such as UPS or needle sharing, that is likely to cause HIV transmission (to a partner who is HIV uninfected) from behavior that is likely to have no impact on transmission (to a partner who the patient knew was also HIV infected). Also, this study relied on self-reported risk behavior, which may underestimate actual risk behavior [27]. STD data were based on participant's reports of STD diagnoses by health care providers. An STD that did not prompt a patient to see a health care provider may not have been reported, and STDs that may be relatively asymptomatic, such as gonorrhea and chlamydia, may be underdiagnosed, especially among women. Some STDs mentioned in the questionnaire, such as herpes simplex virus, are chronic and may relapse over time; therefore, a newly diagnosed STD may not always represent a newly acquired STD. Lastly, because of its cross-sectional design that reviewed past behaviors, our study was not able to examine the exact temporal relationship between knowledge of HIV serostatus, onset of particular risk behaviors after notification of test result, onset of HAART use, or exact timing of patient knowledge of changes in virus load, factors that might influence ongoing risk behavior. Recall of risk behaviors by study subjects may be affected by the time elapsed since they first learned of a positive HIV test result. A prospective study is needed to address more fully these important issues.
Our findings demonstrate that individuals who know that they are HIV infected may be an important source for transmission of HIV and STDs in an urban setting. This study highlights that trade of sex for money or drugs is an important factor associated with transmission risk behavior in HIV-seropositive persons, especially women. Periodic risk assessment and screening for STDs should be a routine part of HIV primary care, and, when STDs are identified, there should be a discussion with the patient about sexual practices. Patients should be cautioned that the use of antiretroviral therapy is not a guarantee against virus transmission. Resources should be mobilized for routine screening for STDs, for behavioral modification to reinforce safe sex practices, and for improved access to drug dependency programs.
References
Presented in part: 8th Conference on Retroviruses and Opportunistic Infections, Chicago, Illinois, February 2001 (abstract 263).
Financial support: Cooperative agreement (UC64/CCU213430-01) between the Centers for Disease Control and Prevention and the Bronx-Lebanon Hospital Center.






Comments