Abstract
Autistics demonstrate superior performances on several visuo-spatial tasks where local or detailed information processing is advantageous. Altered spatial filtering properties at an early level of visuo-spatial analysis may be a plausible perceptual origin for such detailed perception in Autism Spectrum Disorder. In this study, contrast sensitivity for both luminance and texture-defined vertically-oriented sine-wave gratings were measured across a range of spatial frequencies (0.5, 1, 2, 4 & 8 cpd) for autistics and non-autistic participants. Contrast sensitivity functions and peak frequency ratios were plotted and compared across groups. Results demonstrated that autistic participants were more sensitivity to luminance-defined, high spatial frequency gratings (8 cpd). A group difference in peak distribution was also observed as 35% of autistic participants manifested peak sensitivity for luminance-defined gratings of 4 cpd, compared to only 7% for the comparison group. These findings support that locally-biased perception in Autism Spectrum Disorder originates, at least in part, from differences in response properties of early spatial mechanisms favouring detailed spatial information processing.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by alterations in social communication and interaction, co-occurring with restricted, repetitive patterns of behaviour, interests or activities1. It differs from other neurodevelopmental conditions by recurrent demonstrations of superior performances on perceptual and cognitive tasks where local or detailed information processing is advantageous2,3,4,5,6.
Two neurocognitive theories have been advanced to explain the local bias in autistic perception. The Weak Central Coherence4,7 (WCC) hypothesis proposes that a decreased influence, or dysfunction, of large-scale neuro-integrative mechanisms results in a reduced global or holistic representation of perceptual information, ultimately leading to a local or detailed processing style. Although the neural basis for such reduced integrative processing in ASD has yet to be elucidated, reduced neural synchrony and/or decreased functional connectivity between cortical areas8,9,10,11,12 may plausibly underlie large-scale alterations leading to relatively more efficient local analysis.
Alternatively, the Enhanced Perceptual Functioning (EPF) Model5,6 proposes that an over-functioning of lower-level perceptual mechanisms during the completion of perceptual and cognitive tasks leads to the enhanced extraction of elementary visual and auditory information. A recent functional imaging meta-analysis examining regions involved in cognitive, language and face-processing tasks has supported this view by demonstrating that early visual areas (i.e., striate (BA 17) and extrastriate areas (BA 18, 19)) are activated to a greater degree in individuals with ASD than in those without ASD13. These results are in line with the EPF's proposal of a stronger engagement of sensory processing mechanisms in perceptual tasks, including a prominent role of perception in supporting complex cognitive operations.
While both accounts are in agreement with the notion that perception in ASD is locally-oriented and sometimes enhanced, both lack clarity in terms of their underlying neural basis. One reason for this is the fact that the response properties of visual mechanisms responsible for spatial perception in ASD at early levels of processing have not been systematically explored. Studies assessing visual acuity using either clinical screening charts14,15,16 or computer-based paradigms3,17,18,19 have for the most part demonstrated that visual acuity is unremarkable in ASD, suggesting that detailed or locally-oriented visual perception in ASD is not of ocular origin. However, it is not ideal to draw firm conclusions on early visuo-spatial processing in ASD based on acuity studies where most target stimuli are considered to be broadband (i.e., Landolt-C optotypes) and do not optimally solicit selective spatial and orientation response properties defining early visual mechanisms20. The purpose of the present study was therefore to systematically assess the integrity of neural mechanisms mediating early spatial information processing in ASD by measuring contrast sensitivity functions (CSFs) in a large and well-defined group of autistic participants.
Methods
Participants
A total of twenty-one high-functioning autistic (HFA) and fifteen typically developing (TD) adolescents and adults were recruited from the Rivière-des-Prairies Hospital database and participated in the study. Using DSM-IV21 criteria, Autistic Disorder (AD) was diagnosed using the algorithm of the Autism Diagnostic Interview-Revised (ADI-R)22 combined with the Autistic Diagnostic Observation Schedule-General (ADOS-G)23, both conducted by a trained clinician-researcher (LM) who obtained reliability on these instruments. Sixteen of the participants with AD scored above the ADI and ADOS cut-off in the three relevant areas for diagnosis (social, communication, restricted interest and repetitive behaviours). Two participants missed the cut-off score in the ADOS-Communication domain but scored above cut-off on the ADI instrument. Two additional participants received their diagnosis based only on the ADI combined with a non-standardized direct assessment based on the ADOS procedure. One participant was administered an expert clinical DSM-IV diagnosis of AD following a non-standardized direct assessment based on the ADOS procedure. All of the participants with AD had histories of language delay, immediate echolalia, stereotyped language, or pronoun reversal, as assessed by the ADI. All participants with and without AD had a Wechsler Full Scale IQ (FSIQ) greater than 80. Comparison participants and their first-degree relatives were screened with a questionnaire for history of neurological or psychiatric disorders. Autistic and non-autistic participants were matched in terms of Wechsler FSIQ and age (see Table 1). All participants had a normal or corrected-to-normal far and near vision that was assessed before testing using both near and far acuity charts (i.e. near point directional E- and C cards, Snellen letter sequence-A-new Logmar). All participants gave written, informed consent before participating. The study was conducted according to the Declaration of Helsinki and was approved by the ethics committee of Hôpital Rivière-des-Prairies in Montréal, Canada. All participants completed all experimental conditions, which were presented in a counterbalanced manner.
Apparatus and general procedure
Stimulus generation, presentation and data collection were controlled by a power Macintosh G4 computer. Stimuli were presented on an 18-inch View sonic E90FB .25 CRT monitor (1280 × 1024 pixels), refreshed at a rate of 75 Hz. Generation and animation were controlled by the VPixx (www.vpixx.com) graphics program, which produced a luminance resolution equivalent to an 11-bit video digital-to-analogue converter, resulting in 2048 luminance levels. The mean luminance of the display was 50.0 cd/m2 (x = 0.2783, y = 0.3210 in CIE (Commission Internationale de l'Eclairage) u' v' color space) where Lmin and Lmax were 0.5 and 99.50 cd/m2, respectively. In order to minimize the nonlinearities in the display, the luminance of the monitor was gamma-corrected using a color look-up table. Gamma correction was verified at a regular interval. A Minolta CS-100 Chroma Meter colorimeter was used for the calibration and luminance readings.
Stimuli
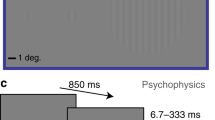
Contrast sensitivity functions (CSFs) were derived by measuring contrast detection thresholds to vertically-oriented gratings defined by both luminance (with and without noise) and texture contrast (Figure 1). The inclusion of texture-defined stimuli allowed for the assessment of early visual processing beyond the primary visual cortex, which is critical for determining whether increases in stimulus complexity influence sensitivity to detailed information. Stimuli for each condition were constructed by convolving the vertical gratings with spatial frequencies of 0.5, 1, 2, 4, 8 cycles per degree (cpd) with a circular Gaussian envelope (σ = 3.2 deg). The contrast for the luminance (no noise) condition was defined by its Michelson contrast ((Lmax − Lmin/Lmax + Lmin) × 100). Contrast detection thresholds to luminance- and texture- defined gratings were also measured, constructed by either adding (luminance contrast) or multiplying (texture contrast) a modulating sinewave to noise pattern consisting of dots (1 pixel × 1 pixel 2 arc min). Individual pixel luminance values for the noise were randomly assigned a value between 24.75 and 74.75 cd/m24,25.
Procedure
All experiments were conducted in a dimly lit room where participants comfortably viewed the stimuli from a distance of 57 cm, with head movements minimized using a chinrest. Verbal instructions were given to each participant prior to each experimental block. Practice trials were completed to familiarize the participants with fixation, stimuli presentation and responding. Participants were reminded to fixate on the center of each pattern and encouraged to rest if tired or distracted throughout the testing session. The experimenter remained present throughout testing and initiated successive trials while monitoring fixation and fatigue; no time limit was imposed. Each trial began with the appearance of a small white fixation dot (0.15 deg in diameter) presented centrally on a monitor. A two-interval temporal forced-choice paradigm was then initiated with a press of the spacebar and participants indicated which of two subsequently presented intervals contained the target grating (non-target intervals consisted of uniform grey background). Participant responded by pressing one of two keys on the keyboard. Intervals lasted 500 milliseconds (ms), separated by a 250 ms fixation. Thresholds were obtained for each condition (5 spatial frequency levels × 3 grating attribute types) within a single adaptive staircase (Harvey's ML-PEST) procedure26. The staircase used fit a new psychometric function to the data after each trial and ended after a 90% confidence level so that the threshold estimate for all three stimuli fell within 0,1 log units of the true threshold measure. Contrast sensitivity was defined as the inverse of the luminance threshold for the luminance-defined gratings and the inverse of texture-contrast threshold (texture modulation depth) for the texture defined gratings contrast24,25. Sensitivity for each attribute condition was then plotted as a function of spatial frequency in order to derive a CSF.
Data Analysis
The data are presented and analyzed in three different ways for each attribute condition (luminance-defined with and without noise and texture defined conditions). First, a mixed analysis of variance was conducted on absolute sensitivity (1/luminance or texture contrast) using a mixed analysis of variance, with Group as a between subject factor and Spatial Frequency as a within group subject factor. The effect size was estimated by the calculation of eta and, in accordance with Cohen27, it was considered small if eta = .01, medium if eta = .06 and large if eta = .14. Second, sensitivity measures were normalized for each participant by dividing the sensitivity of each spatial frequency (Si) by the highest sensitivity (Sh) for each attribute condition (Si/Sh). This was done to reduce the variability to absolute differences in sensitivity between participants, allowing more emphasis to be placed on differential sensitivity to spatial frequency for each participant. Student t-tests were also used to test our a priori hypothesis that the AD groups would be more sensitive to high-spatial frequency gratings when defined by luminance6,24. Finally, categorical data defined by the sensitivity level where participants' highest, or peak sensitivity was obtained and examined using a Pearson Chi-square (χ2) test for each attribute condition. The data from one autistic participant was excluded from statistical analysis in the luminance-defined with and without noise attribute conditions because thresholds were unattainable on at 2 of the 5 spatial frequencies assessed in each condition.
Results
Descriptive statistics for age, Wechsler Full Scale Intelligence Quotient (FSIQ), Verbal IQ (VIQ) and Performance IQ (PIQ) for all participants are presented in Table 1. Preliminary analyses were conducted to ensure that FSIQ, VIQ and PIQ were not acting as potential confounding factors. Specifically, we first examined whether significant group differences were present for either VIQ or PIQ. Results from these analyses revealed no significant group differences for any measures of IQ (Table 1). We then examined whether IQ may be correlated with sensitivity across spatial frequency conditions. No significant correlations were found for the AD group. Two significant correlations were found between IQ and sensitivity in the control group. In the first order, no noise condition, sensitivity for 8 cpd was correlated with FSIQ (r (15) = .563, p = .029) and PIQ r (15) = .549, p = .029). In the second order, no noise condition, sensitivity for 4 cpd was correlated with, FSIQ (r (15) = .566, p = .028 and VIQ (r (15) = .614, p = .015). Given these moderate, yet limited correlations, we found no clear confounding effect of PIQ or VIQ. For this reason, IQ was therefore not used as a covariate during statistical analyses.
Luminance-defined stimuli (without noise) condition
Figure 2 shows the mean sensitivity on the y-axis for the control (black line) and AD (dashed line) groups presented as a function of spatial frequencies (SF) plotted on the x-axis. The sensitivity of each individual participant in both control and AD groups is shown in Figure 3. The interaction of Group and Spatial Frequency was significant, F (4, 30) = 2.877, p = .040, eta = .277. We conducted reverse Helmert comparisons to progressively contrast increasing levels of spatial frequencies to their next higher level (0.5 vs 1 cpd; pooled 0.5 and 1 cpd vs 2 cpd; pooled 0.5, 1 and 2 cpd vs 4 cpd; pooled 0.5, 1, 2 and 4 cpd vs 8 cpd). These comparisons revealed that ASD participants had an increased sensitivity for high SFs in comparison to lower SFs (pooled lower frequencies 0.5, 1, 2, 4 cpd) vs highest frequency (8 cpd), F (1, 33) = 4.183, p = .049, eta = .112. Moreover, individual contrasts of low and high SFs (i.e., 0.5 cpd vs 8 cpd; 1 cpd vs 8 cpd; 2 cpd vs 8 cpd; 4 cpd vs 8 cpd) using a conservative Bonferonni corrected alpha level of .0125 (0.05/4) revealed an increased sensitivity in the AD relative to the control group with increasing spatial frequency (F (1, 33) = 4.183, p = .007, eta = .199). We conducted an hypothesis driven analysis comparing sensitivities of the ASD and TD groups for the highest spatial frequency tested (8 cpd). An independent samples t-test revealed a significant effect, t (1, 33) = 2.209, p = .034, eta = .098, indicating a superior sensitivity for the AD group than the control group at a spatial frequency of 8 cpd. We observed the same pattern of results when data were normalized (Figure 4).
A Pearson Chi-square (χ2) test was computed to compare peak distribution between our two groups. The results of this analysis revealed a significant between group difference for the peak distribution: χ2 (2) = 7.09, p = .029 (Figure 5). Specifically, 35% of autistic participants reached peak sensitivity for gratings of 4 cpd compared to 7% in the comparison group. The remaining 93% of participants in the comparison group reached peak sensitivity for gratings of either 1 or 2 cpd.
Noise conditions
Luminance-defined stimuli with noise
The sensitivity of each individual participant in both control and AD groups is shown in Figure 3. A significant Group x Spatial Frequency interaction was not found for this attribute condition, F (4, 30) = 1.549, p = .214, eta = .171. We observed a significant main effect of spatial frequency, F (4, 30) = 86.85, p < .0001, eta = .921. Multiple comparisons contrasting levels of spatial frequencies demonstrated several significant differences: between the 2 cpd and the pooled means of .05 and 1 cpd (F (1, 33) = 4.178, p = .049, eta = .112); between 4 cpd and the pooled mean of the lower frequencies (.05, 1 and 2) (F (1, 33) = 148.586, p < .0001, eta = .818); and finally, between the highest spatial frequency (8 cpd) and the pooled mean of all the lower frequencies (0.5, 1, 2 and 4 cpd), F (1, 33) = 4.178, p = .049, eta = .112. The data analysis conducted with the normalized sensitivity showed the same pattern of results. A Pearson Chi-square (χ2) test was conducted to compare peak distribution between the groups. No between group difference was observed for the peak distribution: χ2 (2) = .0895, p = .639.
Texture-defined stimuli (or second-order gratings)
Thresholds for the highest spatial frequency gratings (8 cpd) were not measured for the texture-defined condition, since pilot studies demonstrated that these stimuli were not consistently visible to all participants. The sensitivity of each individual participant in both control and AD groups is shown in Figure 3. Analyses failed to reveal a significant interaction between group and spatial frequency, F (3, 32) = .160, p = .923, eta = .015. Instead, a main effect of spatial frequency was found, F (3, 32) = 44.84, p < .0001, eta = .808. Multiple comparisons contrasting low to high spatial frequencies revealed a significant difference between sensitivity for 2 cpd and the pooled sensitivity to frequencies of 0.5 and 1 cpd, F (1, 33) = 39.85, p < .0001 .001, eta = .555. A significant difference was observed between the 4 cpd and the pooled mean sensitivity for lower frequencies (0.5, 1 and 2 cpd), F (1, 33) = 132.513, p < .0001, eta = .805. A similar pattern of results was observed when data were normalized. A Pearson Chi-square (χ2) test was conducted to compare peak distribution between our two groups. No between group differences were observed for the peak distribution: χ2 (2) = .264, p = .607. In sum, group-differences were not found for conditions where spatial stimuli contained noise, whether luminance- or texture-defined.
Discussion
The goal of this set of experiments was to measure contrast sensitivity functions (CSFs) for luminance- and texture-defined gratings in a group of autistic participants. A between group difference was found for luminance-defined gratings, with the autistic group demonstrating an increased sensitivity to luminance-defined, high spatial frequency gratings (i.e., 8 cpd). Moreover, there were group differences for the peak distribution of sensitivity for gratings of 4 cpd: 35% of autistic participants reached peak sensitivity at this frequency compared to 7% in the comparison group. Between-group differences in either absolute or peak distribution sensitivity were not found for gratings containing noise, whether defined by either luminance or texture. The former findings are consistent with those of Behrmann et al.28 who reported that autistic sensitivity to stimuli of different spatial frequencies (0.13, 0.42, 1.26, 4.19 and 12.6 cpd) defined by luminance modulations embedded in noise was similar to that of a control group. The latter results, however, differ from previous demonstrations of lower sensitivity to static information defined by texture in ASD24,29. Methodological differences may explain why this occurred as the present investigation examined detection thresholds for sensitivity to vertically-oriented static gratings of varying spatial frequencies, whereas others explored the detection of grating orientations (vertical and horizontal) and object-boundaries with fixed spatial frequency information. The use of noise in our stimuli may moreover have influenced findings related to the texture condition, given that this stimulus attribute it is not best suited to assess mechanisms selective to spatial frequency.
A skewing of peak sensitivity towards higher spatial frequency information in our AD group is consistent with the results of de Jonge et al.3, who found a trend toward relatively increased spatial frequency sensitivity for mid- to high-spatial frequencies (6 to 18 cpd) in a group of ASD participants using a Vistech contrast sensitivity chart30. More recently, Jemel and colleagues31 assessed the contrast sensitivity response properties of early visual-evoked potentials to sine-wave gratings of low, medium and high spatial frequencies in autistic and neurotypical adults matched on IQ. While mid- and high-frequency gratings elicited segregated brain responses in the control group, similar responses to mid and high frequency information were evidenced in the ASD group. They interpret this finding as evidence for altered functional segregation of early spatial filtering mechanisms in ASD, with mid-spatial frequency information being processed by those selective for high-frequency information, ultimately resulting in bias towards detailed visual processing.
In the only previous direct assessment of CSF in ASD, Koh et al.32 measured contrast sensitivity functions (CSF) in individuals with and without ASD across a wider range of spatial frequencies. These authors found no group differences on any of the four CSF measures assessed (i.e., visual acuity, peak spatial frequency, peak contrast sensitivity and contrast sensitivity at a low spatial frequency). However, Koh et al.32 used horizontally oriented sinusoidal gratings to derive CSFs, whereas our study used vertically-oriented gratings, the orientation most often used when assessing CSFs in both typical and clinical populations. Second, their study included a rather small (n = 10) and clinically heterogeneous ASD sample including participants diagnosed with autistic disorder (n = 1), Asperger's Syndrome (n = 7) and Pervasive Developmental Disorder - Not Otherwise Specified (n = 2). In contrast, all of the autistic participants in the present study strictly and similarly satisfied DSM IV criteria for Autistic Disorder (AD). This later distinction may underlie the differences between the two studies. Accordingly, speech delay, present in AD and absent in Asperger Syndrome, aggregates with perceptual ability peaks in both visual and auditory domains: speech onset delay predicts higher performance in visuospatial peaks of ability, as measured by the Block Design subtest of the Wechsler intelligence tests33. Similarly, within the auditory modality, superior pitch processing (enhanced pitch discrimination for simple tones) is often manifested in participants with a diagnosis of Autistic Disorder, but not Asperger Syndrome34,35.
Different hypotheses have been advanced to explain why autistics are selectively biased toward detailed visual information. Arguments have focused on various, even complementary alterations of local connectivity within neural assemblies mediating sensory processing and long-range connectivity between functional brain regions36,37. Specifically, the locally-oriented and sometimes enhanced autistic perception can be interpreted as reflecting atypical local connectivity affecting the response properties or tuning of visual spatial filters24,38. At a neural level, the response properties of early visual mechanisms responsible for the encoding of elementary visual information, such as orientation and spatial frequency, are modulated by the balance of excitatory/inhibitory activity39. Animal and human studies have also demonstrated that GABA mediates this balance in both visual and auditory modalities40,41. There is also evidence that increased concentrations of GABA in humans are related to lower line orientation39 and tactile discrimination thresholds42. This supports the hypothesis that GABAergic mechanisms play an important role in sensory discrimination. An alteration of these specific mechanisms may be responsible for increased sensitivity to high-spatial frequency information in ASD43 and possibly, in other sensory modalities where GABAergic transmission is involved in shaping the response properties of perceptual mechanisms (i.e., auditory cortex).
Until recently, low-level perception has been overlooked as contributing to ASD's cognitive and behavioural phenotype36. Little is known as yet to whether differences in elementary perception, exemplified by higher sensitivity to high SFs (or to enhanced pitch perception in the auditory modality) are related to deficits in social perception in ASD witht he later are most often interpreted as a reflection of socially-related behaviours44. However, some studies have started to assess possible links between elementary and social perception in ASD within a developemntal context45. For example, Vlamings et al46 demonstrated a processing bias for high-spatial frequency gratings subserving detailed information, concurrent with a detailed-driven approach to facial-expression perception in a group of 3- to 4-year-old children with ASD. These results indicate that an atypical early bias for detailed spatial information in ASD may affect the development of neural mechanisms involved in face processing, with consequences regarding emotion processing and/or social interaction. Local neural alterations mediating perception at the early stages of processing, such as a biased sensitivity to high-spatial frequency information, may therefore be involved in differential autistic performance on higher-level cognitive tasks, whether social in nature or not.
In conclusion, the results of the present study provide a plausible perceptual and biological explanation for superior autistic performance on perceptual tasks that are performed optimally using a detailed-oriented approach. These results are most consistent with local neural models of autistic perception5,6,43 that emphasize atypical extraction of elementary visual information by neural systems operating within early sensory brain areas47,48,49. Such models are most consistent with ASD's perceptual phenotype being defined by the altered activity of early visual mechanisms, rather than the collateral consequence of a reduced global representation of non-social or social information.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th ed. (Arlington, VA: American Psychiatric Publishing, 2013).
Behrmann, M. et al. Configural processing in autism and its relationship to face processing. Neuropsychologia 44, 110–129 (2006).
de Jonge, M. V. et al. Visual information processing in high-functioning individuals with autism spectrum disorders and their parents. Neuropsychology 21, 65–73 (2007).
Happe, F. & Frith, U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord 36, 5–25 (2006).
Mottron, L. & Burack, J. Enhanced perceptual functioning in the development of autism. in The development of autism: Perspectives from theory and research (ed. Burack, C., Yir- miya, & Zelazo) 131–148 (Mahwah, NJ: Erlbaum, 2001).
Mottron, L., Dawson, M., Soulieres, I., Hubert, B. & Burack, J. Enhanced perceptual functioning in autism: an update and eight principles of autistic perception. J Autism Dev Disord 36, 27–43 (2006).
Frith, U. & Happe, F. Autism: beyond “theory of mind”. Cognition 50, 115–132 (1994).
Brock, J., Brown, C. C., Boucher, J. & Rippon, G. The temporal binding deficit hypothesis of autism. Dev Psychopathol 14, 209–224 (2002).
Grice, S. J. et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport 12, 2697–2700 (2001).
Rippon, G., Brock, J., Brown, C. & Boucher, J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int J Psychophysiol 63, 164–172 (2007).
Castelli, F., Frith, C., Happe, F. & Frith, U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125, 1839–1849 (2002).
Just, M. A., Cherkassky, V. L., Keller, T. A. & Minshew, N. J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127, 1811–1821 (2004).
Samson, F., Mottron, L., Soulières, I. & Zeffiro, T. A. Enhanced visual functioning in autism: An ALE meta-analysis. Hum Brain Mapp 33, 1553–1581 (2012).
de Jonge, M. V., Kemner, C. & van Engeland, H. Superior disembedding performance of high-functioning individuals with autism spectrum disorders and their parents: the need for subtle measures. J Autism Dev Disord 36, 677–683 (2006).
Falkmer, M. et al. Visual acuity in adults with Asperger's syndrome: no evidence for “eagle-eyed” vision. Biol Psychiatry 70, 812–816 (2011).
Milne, E., Griffiths, H., Buckley, D. & Scope, A. Vision in children and adolescents with autistic spectrum disorder: evidence for reduced convergence. J Autism Dev Disord 39, 965–975 (2009).
Bach, M. & Dakin, S. C. Regarding “Eagle-eyed visual acuity: an experimental investigation of enhanced perception in autism”. Biol Psychiatry 66, e19–20; author reply e23–14 (2009).
Tavassoli, T., Latham, K., Bach, M., Dakin, S. C., Baron-Cohen, S. Psychophysical measures of visual acuitr in autism spectrum conditions. Vision Res 51, 1778–17780 (2011).
Kéïta, L., Mottron, L. & Bertone, A. Far visual acuity is unremarkable in autism: do we need to focus on crowding? Autism Res 3, 333–341 (2010).
Kothe, A. C. & Regan, D. Crowding depends on contrast. Optom Vis Sci 67, 283–286 (1990).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed. (Arlington, VA: American Psychiatric Publishing, 1994).
Lord, C., Rutter, M. & Le Couteur, A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24, 659–685 (1994).
Lord, C. et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30, 205–223 (2000).
Bertone, A., Mottron, L., Jelenic, P. & Faubert, J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain 128, 2430–2441 (2005).
Ledgeway, T. & Smith, A. T. Changes in perceived speed following adaptation to first-order and second-order motion. Vision Res 37, 215–224 (1997).
Harvey, L. J. Efficient estimation of sensory thresholds with ML-PEST. Spat Vis 11, 121–128 (1997).
Cohen, J. Statistical power analysis for the behavioral sciences, (Lawrence Erlbaum Associates Inc., HillsdaleNJ, 1988).
Behrmann, M., Thomas, C. & Humphreys, K. Seeing it differently: visual processing in autism. Trends Cogn Sci 10, 258–264 (2006).
Vandenbroucke, M. W. G., Scholte, H. S., van Engeland, H., Lamme, V. A. F. & Kemner, C. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain 131, 1013–1024 (2008).
Vistech contrast test system (VCTS) application manual, (Vistech Consultants, Inc, Dayton, OH, 1988).
Jemel, B., Mimeault, D., Saint-Amour, D., Hosein, A. & Mottron, L. VEP contrast sensitivity responses reveal reduced functional segregation of mid and high filters of visual channels in autism. J Vis 10, 13 (2010).
Koh, H. C., Milne, E. & Dobkins, K. Spatial contrast sensitivity in adolescents with autism spectrum disorders. J Autism Dev Disord 40, 978–987 (2010).
Geoffray, M. M., Soulières, I. & Berthiaume, C. Peaks of Ability Combined with Speech Onset Identify Subgroups within Autism Spectrum. in 10th Annual International Meeting for Autism Research (IMFAR) (San Diego California, may 12th-14 2011).
Bonnel, A. et al. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia 48, 2465–2475 (2010).
Jones, C. R. et al. Auditory discrimination and auditory sensory behaviours in autism spectrum disorders. Neuropsychologia 47, 2850–2858 (2009).
Belmonte, M. K. et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry 9, 646–663 (2004).
Muller, R. A. From loci to networks and back again: anomalies in the study of autism. Ann N Y Acad Sci 1145, 300–315 (2008).
Ferster, D. & Miller, K. D. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci 23, 441–471 (2000).
Edden, R. A., Muthukumaraswamy, S. D., Freeman, T. C. & Singh, K. D. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci 29, 15721–15726 (2009).
Razak, K. A. & Fuzessery, Z. M. GABA shapes a systematic map of binaural sensitivity in the auditory cortex. J Neurophysiol 104, 517–528 (2010).
Fatemi, S. H. et al. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord 40, 743–750 (2010).
Puts, N. A., Edden, R. A., Evans, C. J., McGlone, F. & McGonigle, D. J. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci 31, 16556–16560 (2011).
Bertone, A., Hanck, J., Kogan, C., Chaudhuri, A. & Cornish, K. Associating Neural Alterations and Genotype in Autism and Fragile X Syndrome: Incorporating Perceptual Phenotypes in Causal Modeling. J Autism Dev Disord 40, 1541–1548 (2010).
Pelphrey, K. A., Shultz, S., Hudac, C. M. & Vander Wyk, B. C. Research review: Constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry 52, 631–644 (2011).
Leonard, H. C., Karmiloff-Smith, A. & Johnson, M. H. The development of spatial frequency biases in face recognition. J Exp Child Psychol 106, 193–207 (2010).
Vlamings, P. H., Jonkman, L. M., van Daalen, E., van der Gaag, R. J. & Kemner, C. Basic abnormalities in visual processing affect face processing at an early age in autism spectrum disorder. Biol Psychiatry 68, 1107–1113 (2010).
Kéïta, L., Mottron, L., Dawson, M. & Bertone, A. Atypical lateral connectivity: a neural basis for altered visuospatial processing in autism. Biol Psychiatry 70, 806–811 (2011).
Latham, K., Chung, S. T., Allen, P. M., Tavassoli, T. & Baron-Cohen, S. Spatial localisation in autism: evidence for differences in early cortical visual processing. Mol Autism 4, 4 (2013).
Snijders, T. M., Milivojevic, B., Kemner, C. Atypical excitation–inhibition balance in autism captured by the gamma response to contextual modulation. Neuroimage Clin 3, 65–72 (2013).
Acknowledgements
This study was supported by funding from an Autism Speaks mentor-based (L.M. and A.B.) fellowship to L.K. and a Canadian Institute for Health Research operating Grant to L.M. and A.B. We thank all the participants for their involvement in this project.
Author information
Authors and Affiliations
Contributions
L.K. and A.B. designed experiments. L.K., A.B., J.G. and L.M. wrote the main manuscript text. L.K., C.B. and A.B. analyzed the data. L.K. and A.B. prepared Figures 1 through 5 and Table 1. All of the authors (K.L., J.G., C.B., L.M. and A.B.) reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kéïta, L., Guy, J., Berthiaume, C. et al. An early origin for detailed perception in Autism Spectrum Disorder: biased sensitivity for high-spatial frequency information.. Sci Rep 4, 5475 (2014). https://doi.org/10.1038/srep05475
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05475
This article is cited by
-
Sex modulation of faces prediction error in the autistic brain
Communications Biology (2024)
-
Association between Autism Spectrum Disorder (ASD) and vision problems. A systematic review and meta-analysis
Molecular Psychiatry (2023)
-
Convergent Validity of Behavioural and Subjective Sensitivity in Relation to Autistic Traits
Journal of Autism and Developmental Disorders (2022)
-
Is It Fear? Similar Brain Responses to Fearful and Neutral Faces in Infants with a Heightened Likelihood for Autism Spectrum Disorder
Journal of Autism and Developmental Disorders (2021)
-
Clinically relevant autistic traits predict greater reliance on detail for image recognition
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.