Abstract
The purpose of the current study was to develop an objective tool based on dual-task performance for screening early-stage Alzheimer’s disease (AD) and mild cognitive impairment (MCI of the Alzheimer’s type). Dual-task involved a simultaneous execution of a sensor-based upper-extremity function (UEF) motor task (normal or rapid speed) and a cognitive task of counting numbers backward (by ones or threes). Motor function speed and variability were recorded and compared between cognitive groups using ANOVAs, adjusted for age, gender, and body mass index. Cognitive indexes were developed using multivariable ordinal logistic models to predict the cognitive status using UEF parameters. Ninety-one participants were recruited; 35 cognitive normal (CN, age = 83.8 ± 6.9), 34 MCI (age = 83.9 ± 6.6), and 22 AD (age = 84.1 ± 6.1). Flexion number and sensor-based motion variability parameters, within the normal pace elbow flexion, showed significant between-group differences (maximum effect size of 1.10 for CN versus MCI and 1.39 for CN versus AD, p < 0.0001). Using these parameters, the cognitive status (both MCI and AD) was predicted with a receiver operating characteristic area under curve of 0.83 (sensitivity = 0.82 and specificity = 0.72). Findings suggest that measures of motor function speed and accuracy within a more practical upper-extremity test (instead of walking) may provide enough complexity for cognitive impairment assessment.
Similar content being viewed by others
Introduction
Cognitive impairment is a critical health problem with an increasing prevalence because of the population aging1. It is estimated that by 2040, the number of elders living with dementia will surpass nine million in the US, roughly 170% increase compared to 20012. Among different types of dementia, Alzheimer’s disease (AD) is the most common type, which influences the lives of up to 7% of the elderly population in the US and globally3. Early dementia screening provides an opportunity to begin secondary prevention, as well as planning for future care, safety concerns, and financial and legal arrangements, while decision-making capacity remains4. Unfortunately, many providers are reluctant to screen for dementia resulting in less than half of patients with AD having ever received a formal diagnosis5.
The current research was founded based on the fact that simultaneous declines in motor and cognitive performance occur with aging6,7,8. In many age-related neurodegenerative diseases, and more specifically in Alzheimer’s disease, compensatory processes in cortical and subcortical brain regions allow maintenance of motor and cognitive performance9. Assessing deficits in dual-tasking, therefore, can provide a powerful tool for screening cognitive impairments. Gait has been commonly used as the motor task component in dual-task assessments. Poor dual-task gait performance has been significantly correlated with decreased executive and neuropsychological function, and demonstrated to be associated with AD or even mild cognitive impairment (MCI)10,11,12. However, many older adults have mobility impairments, and many clinics lack adequate space to safely perform gait measures.
We have previously developed and validated an upper-extremity function (UEF) test to assess slowness, weakness, exhaustion, and flexibility, with the main purpose of frailty assessment among older adults13,14,15. The UEF test integrates low-cost sensors and data acquisition system (as low as $200), the physical assessment (including preparation/calibration) is easily performed in less than five minutes, and the post-processing is performed in less than two minutes. In previous work, we have determined strong correlations between UEF and gait speed14 and six-minute walk distance16. Further, we have compared the UEF dual-task performance (simultaneous performance of UEF and a cognitive task of counting backward) with gait dual-task performance, as well as the Montreal Cognitive Assessment (MoCA) test17,18. Within this work, a significant association has been observed between UEF speed parameters and gait speed within the dual-task trials. Also, significant correlations have been shown between MoCA and UEF dual-task motor function speed and variability.
In continuation of previous work, the purpose of the current study was to investigate the capability of UEF for discriminating clinically diagnosed elders with cognitive impairment and to develop an index for screening AD and MCI. The hypotheses were: (1) UEF dual-task performance would be significantly different between cognitive groups; (2) UEF dual-task performance would decrease with cognitive task difficulty, and this response would impact cognitive impairment predictions; and (3) instructing participants to perform the motor task component consistently (instead of rapidly) would improve the cognitive impairment predictions. If found to be valid, the proposed UEF tool would provide several advantages compared to currently available cognitive impairment screening tools such as MoCA, including19,20,21,22,23: (1) providing an objective score based on motor function; (2) capability for routinely assessment with minimum learning effects; (3) being less influenced by education and language, since the actual scoring is based on motor function performance rather than a cognitive task; and (4) providing a simple assessment protocol, without requiring the global judgment of the examiner (e.g., as it is required for clock-drawing test).
Methods
Participants
Participants were recruited from the Banner Sun Health Research Institute (BSHRI) from September 2017 to May 2018. Eligible participants from the BSHRI cohort were vetted by the clinic director (KO) to assure eligibility for participation. Then they received a pre-clinic phone call or an in-clinic opportunity to discuss the study and enrollment process. Written informed consent according to the principles expressed in the Declaration of Helsinki24 was then obtained from eligible subjects before participation. The study was approved by the University of Arizona Institutional Review Board. Participants were selected and stratified into three categories of cognitive status including cognitive normal (CN), MCI of the Alzheimer’s type, and early-stage AD. The clinical diagnosis of cognitive status happened within a time-window of six months prior to study measurements, based on the National Institute of Aging – Alzheimer’s Association (NIA-AA) criteria25,26. Cognitive groups were frequency matched (controlled distributions) on age categories and gender to assure equivalent age and gender distribution. Inclusion criteria were: (1) age of 65 years or older; (2) ability to understand study instructions; and (3) English language proficiency. Exclusion criteria were: (1) diagnosed diseases associated with severe motor performance deficits including stroke or Parkinson’s disease; (2) severe speech disorders; and (3) severe upper-extremity disorders, including elbow bilateral fractures or rheumatoid arthritis.
Clinical measures
Neuropsychological tests were conducted as a part of prior diagnostic assessments (within a time-window of six months prior to study measurements) or collected during the study data collection. Of note, the association between UEF and neuropsychological tests is beyond the scope of the current paper and is investigated in another study. Here, only associations between UEF dual-task performance with MoCA27 and the Mini–Mental State Examination (MMSE)28 were investigated.
In addition to neuropsychological tests, we collected the following clinical measures: (1) frailty (the Fried Index)29; (2) comorbidity (Charlson comorbidity score (CCI))30; and (3) depression Patient Health Questionnaire (PHQ-9)31. These three clinical measures were added as potential confounding factors in dual-task performance. Physical frailty was assessed to check differences in function between groups, since the proposed cognitive measurement is based on motor function performance. Comorbidity was measured as it is associated with an increased risk of cognitive impairment32. Finally, we assessed the level of depression at the time of the measurement, to take into account its potential confounding effect on dual-task performance. We measured depression, since results from a recent systematic review revealed significant cognitive deficits in executive function, memory, and attention in patients with depression, compared to healthy individuals33.
UEF cognitive assessment protocol and outcomes
After completing questionnaires, each participant performed the UEF elbow flexion test with the dominant arm. The UEF task consists of six trials, including a full-combination of two speed of elbow flexion (rapid and normal self-selected pace) and three cognitive conditions (no counting, counting backward by ones, and counting backward by threes starting from a random two-digit number) (Fig. 1). Within the rapid pace, the participant flexed and extended the elbow as quickly as possible in 20 seconds. Within the self-selected pace, participants performed the task as consistently as possible in 60 seconds. We considered speed and consistency to represent delay and accuracy aspects of dual-tasking, as they have been related to cognitive aging7. The order of six UEF trials was randomized to minimize confounding fatigue and learning effects. To better represent the natural environment for daily activities, there was no instruction to prioritize either the physical or the counting task12,34,35. The cognitive task of counting numbers was selected as it involves working memory36 and, therefore, is related to executive functioning, compared to other tasks such as naming objects/animals12,37,38. Counting is also a rhythmic task that greatly interferes with the rhythmicity of repetitive elbow flexion38,39.
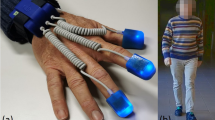
UEF procedure for identifying cognitive impairment: Two gyroscopes are attached to the wrist and upper-arm of the dominant arm to measure the elbow angular velocity. Data are presented for 60-second dual-task motor performance while counting numbers backward by threes, for participants from three cognitive groups (the same age range 72–74).
Before measurements, participants were equipped with the UEF system. A tri-axial wearable gyroscope and accelerometer sensor (sample frequency = 100 Hz, BioSensics LLC, Boston, MA, USA), was attached to the upper arm near the biceps and one to the wrist, both on one arm, using a band attached with Velcro, to estimate three-dimensional angular velocity of the upper-arm and forearm, and ultimately elbow flexion (Fig. 1).
Several outcome measures representing kinematics of the elbow flexion were derived using angular velocity. To extract the outcome measure, the angular velocity signal from the sensors were filtered to remove drifting (first order high pass butter-worth filter with a cutoff of 2.5 Hz), and using a peak detection algorithm maximum and minimums of the angular velocity signal, and subsequently, elbow flexion cycles were detected. Accordingly, UEF outcome measures were calculated, including: (1) speed; (2) rise time; (3) range of motion (ROM); (4) speed variability; (5) ROM variability; (6) flexion variability; and (7) flexion number (for definition of each parameter see Table 1). Of note, all the above outcomes can be calculated using an in-home MATLAB app, within less than 1 minute. These parameters were selected to present alterations in motor function execution speed and accuracy within dual-tasking, based on our previous investigations and gait dual-task studies among dementia patients12,17,18,40,41. Using these parameters, changes in motor function due to the cognitive task were determined, which included: (1) agility (speed, rise time, and flexion number, which are analogous to gait speed, stride time, and cadence); (2) flexibility (ROM, which is analogous to step length); and (3) variability (speed, ROM, and flexion variability, which are analogous to gait speed, step length, and gait cycle time variability). To assess changes in the above parameters in an individual’s performance from a single to a dual-task, dual-task “cost” was measured for each parameter as the percentage of change within two conditions.
To assess the secondary cognitive task performance (i.e., counting numbers), the number of correctly counted numbers and the number of mistakes within each arm test were considered as outcomes. These parameters represent the speed and accuracy of the secondary task within the dual-task condition42.
Statistical analysis and cognitive index development
Analysis of variance (ANOVA) models were used to evaluate the differences in all of the demographic and clinical parameters between three cognitive groups (i.e., CN, MCI, and AD), except gender, comorbidity, and frailty prevalence; chi-square χ2 tests were used to assess gender and comorbidity categories differences, and the Fisher’s exact test was used to assess frailty prevalence differences between cognitive groups.
UEF parameters for all six conditions as well as UEF dual-task cost variables were compared between groups using ANOVA models (CN vs. MCI, MCI vs. AD, and CN vs AD); age, gender, and body mass index (BMI) were considered as covariates and Cohen’s effect size (d) was estimated. Post-hoc Tukey’s honest significant difference tests were performed for three pairwise comparisons between the cognitive groups. Of note, age, gender, and BMI were selected as adjusting variables, since they have been previously associated with AD and, in general, dementia. Women are expected to have a higher risk of developing AD compared to men43. BMI as a common measure of nutritional status was considered as an adjusting variable, since BMI < 25 kg/m2 is associated with the risk of moderate-severe cognitive impairment44. Further, there is evidence that excess weight may adversely affect executive function, attention, memory, and the overall cognition45.
Two types of UEF cognitive indexes were developed similar to previous work15. The first index (UEF cognitive categorical index) was developed to predict three cognitive groups including CN, MCI, and AD (per participant categories within this study). Multivariable ordinal logistic models with the cognitive status as the dependent variable, and UEF parameters plus demographic information (i.e., age, gender, and BMI) as independent variables were used to develop the UEF categorical index (see Supplementary Information 1 for details regarding UEF categorical cognitive index and the cross-validation process). We tested the proportional odds assumption for ordinal logistic regression models using an approximate likelihood-ratio test of whether the coefficients are equal across categories.
To develop the second UEF index (UEF cognitive score) parameters selected from the categorical index were used, following methods developed for the Framingham cardiovascular risk score46. Cut-offs and weight for each of UEF parameter were determined based on mean values for each frailty group and parameter estimates from the categorical logistic models. The UEF cognitive score (from 0: CN to 1: AD) for a given participant was defined as the sum of points corresponding to performance results from UEF dual-task test (see Supplementary Information 2 for details regarding UEF cognitive score).
Finally, the association between UEF score with MoCA and MMSE were assessed using the Pearson correlation (and Spearman’s rank if not normally distributed).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Power calculation
The power calculation for this study was based on our previous work among 10 cognitively impaired and 57 cognitively intact participants18. We observed comparisons of impaired vs intact for UEF dual-task speed 864.81 ± 299.91 vs 455.05 ± 237.20 deg/s, UEF dual-task speed variability 16.30 ± 8.24 vs 41.75 ± 18.52%, and UEF dual-task ROM 100.58 ± 29.26 vs 73.60 ± 28.91 deg. Based on the variation observed in dual-task performance measures, a sample size of 30 per group (alpha = 0.05) was expected to provide 80% power to detect a difference of: (1) 200 deg/s for speed; (2) 11% for speed variability; and (3) 21 deg for ROM. Because these detectable differences were substantially below those observed in the pilot data, we expected to have adequate power to detect differences for at least these parameters between MCI and controls and between early-stage AD and controls. Of note, we observed significant differences between these parameters in our study; however, different UEF parameters emerged as the most explanatory independent variables and eventually were used in the UEF indexes.
Results
Participants
Ninety-one participants were recruited, including 35 CN (age = 83.8 ± 6.9), 34 MCI (age = 83.9 ± 6.6), and 22 AD (age = 84.1 ± 6.1) older adults. Of note, no participant was excluded because of incapability to perform the UEF test. Age, gender, height, and weight were not significantly different between these groups (p > 0.41, Table 2). Frailty status, and comorbidity and depression scores were also not significantly different between cognitive groups (p > 0.08, Table 2).
UEF dual-task performance among cognitive groups
ANOVA results showed significant differences in executing motor function between cognitive groups, especially within dual-task trials (Fig. 2). On average among two dual-task difficulties and two motor task conditions, flexion number demonstrated the largest overall effect size for between CN and MCI group comparisons (average effect size = 0.83 ± 0.23; max effect size = 1.10), and between CN and AD group comparisons (average effect size = 1.20 ± 0.18; max effect size = 1.38). For MCI and AD group comparisons, ROM variability showed, on average between all conditions, the largest overall effect size (average effect size = 0.55 ± 0.16; max effect size = 0.69).
Within the single-task trials with no cognitive task, significant differences were only observed in the agility parameters of elbow flexion, and not flexibility and variability (Tables 3 and 4). The overall effect sizes for all between group comparisons were 0.40 ± 0.20, 0.14 ± 0.08, and 0.25 ± 0.16 for agility, flexibility, and variability parameters, respectively, within the single-task condition. Differences between UEF parameters among the three cognitive groups were more noticeable within dual-task conditions; significant differences were observed for all motor function categories, including agility, flexibility, and variability of elbow flexion. The overall effect sizes for all between group comparisons were 0.57 ± 0.30, 0.23 ± 0.13, and 0.46 ± 0.30 for agility, flexibility, and variability parameters, respectively, within the dual-task counting backward by ones. The corresponding effect size values were further increased to 0.70 ± 0.42, 0.38 ± 0.15, and 0.67 ± 0.32 within the dual-task counting by threes.
Comparing dual-task trials between normal and rapid flexion condition, between groups differences in motor function agility were more pronounced within rapid elbow flexion (average effect size = 0.68 ± 0.33) compared to normal speed flexion (average effect size = 0.41 ± 0.34). On the other hand, flexibility of flexion and motor function variability were more noticeably different between cognitive groups when participants were asked to do the UEF test as consistently as possible (average effect size = 0.65 ± 0.29 for flexibility and average effect size = 0.77 ± 0.33 for variability) rather than rapidly (average effect size = 0.37 ± 0.16 for flexibility and average effect size = 0.41 ± 0.30 for variability).
Among dual-task cost parameters, rise time, flexion number, and flexion variability showed significant between group differences within the cognitive task of counting backward by threes (average effect size = 0.49 ± 0.37 and 0.44 ± 0.37 for normal and rapid elbow flexion, respectively). Overall, similar to our previous findings17, we observed higher effect sizes and less between-subject variability within dual-task trials compared to dual-task costs, and therefore, we used dual-task variables for further UEF cognitive index development.
UEF cognitive index
Based on graph inspections and Shapiro-Wilk tests, UEF parameter distributions appeared normal, with the exception of rise time, for which logarithmic transformation was used to provide normal distribution. Within rapid flexion and counting backward by threes, univariate logistic models revealed that all UEF parameters were significantly associated with the cognitive status (p < 0.03), except for ROM (p = 0.46). For rapid flexion with the easier cognitive task of counting backward by ones, in addition to ROM, speed and flexion variability were also not significantly associated with the cognitive status (p > 0.15). Within the normal flexion condition, for both cognitive task difficulty, all UEF variables were significantly associated with cognition status (p < 0.001), except for ROM and speed parameters (p > 0.06).
A forward stepwise approach for selection of UEF parameters as independent variables resulted in flexion number and ROM variability as included parameters under rapid flexion condition, and additionally flexion variability under normal self-selected pace flexion condition (Table 5). Age, gender, and BMI, as well as parameters related to cognitive task performance were not included in the models, since, in addition to UEF parameters, they were not significantly associated with the cognitive status. Using the model with the whole sample, highest values for receiver operating characteristic (ROC) area under curves were achieved within normal self-selected pace UEF (ROC area under curve of 0.83 in predicting MCI and AD for both cognitive conditions, Table 5). Accordingly, we performed the 10-fold cross validation only for UEF normal speed tests. Results from 10-fold cross-validation for normal pace counting backward by ones showed mean values of 0.83 ± 0.01 and 0.83 ± 0.02 for ROC area under curve for MCI and AD prediction within the training sets, and sensitivity and specificity of 0.77 ± 0.17 and 0.71 ± 0.23 for predicting cognitive impairment (MCI and early AD) within the testing sets, respectively. Corresponding values were 0.83 ± 0.03 and 0.82 ± 0.03, 0.80 ± 0.21 and 0.75 ± 0.21 for normal pace elbow flexion while counting backward by threes. Using the ordinal logistic regression models, two probability equations (categorical cognitive indexes) were derived for each of normal pace UEF tests, from parameter estimates (Supplementary Information 1). Similarly, two UEF cognitive scores were established for each normal self-selected pace UEF tests (Supplementary Information 2). Results showed significant correlation between the UEF cognitive score with both MoCA and MMSE (p < 0.0001, r = 0.61 for MoCA and r = 0.42 for MMSE on average).
Discussion
UEF cognitive index for screening alzheimer’s disease
As hypothesized, we were able to discriminate MCI and AD with maximum sensitivity and specificity of 0.82 and 0.72 (ROC area under curve = 0.83). Within our sample, MMSE provided a sensitivity and specificity of 33% and 94% for identifying the combined group of MCI and AD, using a previously established cutoff of below 24 for cognitive impairment. Interestingly, when only AD participants were considered as the cognitively impaired group, sensitivity and specificity of 100% and 38% were achieved. These findings were in agreement with previous work suggesting MMSE provide better sensitivity in identifying more progressed stages of cognitive impairment, rather than MCI28. Using MoCA with a cutoff of below 26 for cognitive impairment, within our sample, combined groups of MCI and AD were identified with a sensitivity and specificity of 96% and 67%. The sensitivity of MoCA increased to 100% when only AD participants were considered as the cognitively impaired group; while specificity dropped to 38%. These values of sensitivity and specificity were similar but lower than previously reported values for MoCA, which were 90% sensitivity and 87% specificity27. Overall, these findings suggest that MoCA provides a very sensitive and rather specific tool for screening MCI and early AD. Comparing UEF with MMSE, UEF may be advantageous in discriminating between MCI and CN. Further, UEF prediction accuracy was found to be comparable to MoCA for identifying MCI and AD.
Among several parameters related to motor performance the agility, measured by the flexion number, and the variability, measured by ROM and flexion variability were the only predicting parameters included in cognitive indexes. This is in agreement with our previous findings17,18, as well as previous research on gait dual-task; gait variability and stride velocity within the dual-task condition have been reported as the most sensitive parameters for assessing cognitive impairment, compared to other spatial-temporal gait parameters12,38,40,47.
Results from ANOVA and logistic models showed that dual-task performance provides a better distinction of cognitive status compared to dual-task cost, especially when performing normal self-selected pace testing. The calculation of dual-task cost was included here to account for musculoskeletal deficits, by normalizing the dual-task performance for each participant using the single-task baseline. However, dual-task cost parameters could not discriminate as well here, and therefore, only dual-task parameters were included in the UEF cognitive models. We believe performing normal speed dual-task instead of rapid elbow flexion could minimize the influence of musculoskeletal deficits on cognitive task performance. Further, the main goal of this study was to develop a clinically “quick” screening tool for cognitive assessment. Accordingly, employing an index that involves only one 60-second trial (instead of two 60-second trials) would be advantageous for busy clinical settings. Also, unlike walking that can be excessively influenced by muscle strength, reflexive performance, and dynamic balance deficits, elbow flexion is a less musculoskeletal demanding task, which may be more suitable for cognitive assessment.
Parameters related to the performance of the secondary task were not selected in the index model because they did not improve models of cognitive status with motor performance. Although results also showed significant differences in cognitive task performance among cognitive groups, for between-group comparisons, higher effect sizes were achieved for UEF motor task parameters (Tables 3 and 4).
The effect of motor and cognitive task conditions
Interestingly, unlike what was hypothesized, increasing the cognitive task difficulty did not noticeably influence the cognitive status predictions, especially within normal self-selected pace of elbow flexion. Although, within rapid motor task function, increasing the cognitive task difficulty slightly enhanced the UEF index cognitive prediction (by 4%), this influence was not observed within normal speed elbow flexion. Previous studies suggested that in dual-task testing the cognitive task should be sufficiently challenging to bring individuals near the limit of their ability48. Within this study, for the first time, we investigated the execution of an uncommon motor function and asked participants to perform this task as consistently as possible. Results from the current study suggest performing an uncommon motor task (rather than a motor task that is performed on daily basis such as walking), may require some skill-learning factors and consequently provide higher degrees of brain cortex challenges49. Accordingly, working memory may primarily get involved in learning this new motor task as well as counting numbers50,51, and therefore, even a simpler task of counting backward by ones could push MCI and early AD participants to show motor function deficits within dual-tasking. Nonetheless, the hypothesis of skill-learning factors within UEF should be confirmed in future research using brain imaging evidence (e.g., fMRI).
Overall, asking participants to perform the motor task consistently, rather than rapidly, improved the MCI and AD predictions by roughly 9%. Due to the imposed risk of falling within the rapid gait, especially when it is combined with a cognitive task, limited research exists to investigate differences in dual-task performance with respect to motor task execution speed in gait studies52. More specifically, to the best of our knowledge, no study exists to compare the quality of cognitive impairment predictions in older adults using both normal and rapid speed walking53,54. Current findings, for the first time, provide evidence that performing dual-task consistently with a desired speed can be advantageous over rapid motor task execution for cognitive impairment assessment among elders.
Limitations and future direction
Several confounding parameters, including age, depression, comorbidity, and physical frailty were considered for adjustment within the current study. However, other potential confounding variables inherently may exist for performing UEF as a cognitive screening tool, such as the level of education and severe elbow arthritis. Within the current approach, an index was developed that only relies on motor function performance, and therefore, minimizes the influence of education level in cognitive screening. Further, previous research and our previous UEF motor function assessment showed most elders with arthritis are still able to perform UEF as it involves elbow flexion (rather than other joints prone to injury such as shoulder)15.
Due to the selection criteria, the generalizability of the current findings is limited to MCI of the Alzheimer’s type and early AD (groups chosen to reduce between-subject variability within our small sample size). However, as AD is the most common type of dementia, we believe current findings could provide a promising screening tool for assessing cognitive impairment in most older adults, with potential future use for other dementias. In follow-up studies to the current study, we will address this limitation by recruiting participants with other definitively diagnosed types of dementia.
Also, the current study lacks intra- and inter-rater reliability assessments; however, UEF was tested four times within the current experimental setup, and consistent results were observed within ANOVA models and UEF index development. Further, within several studies, the UEF motor task has been validated for frailty assessment among larger samples of older adults within different experimental settings. Of note, in continuation of this research, we will incorporate the UEF cognitive index into the original UEF frailty score.
Within the current study the UEF is validated to provide a sensitivity and specificity of 0.82 and 0.72, respectively. Although the specificity of UEF for identifying AD is not high, we believe, for the purpose of screening AD, UEF would provide acceptable sensitivity. Although acceptable sensitivity was achieved using UEF parameters and findings support consistency in current findings, the accuracy of cognitive status prediction may further be improved using dynamic analysis of elbow flexion motion. Within gait trials, several approaches have been implemented previously for regularity (repeatability) and stability assessment of dynamic motion within gait trials. The same approaches can be implemented within the current setup, which will be addressed in our future studies.
Finally, the association between UEF cognitive score (and its components) with neuropsychological tests has not been investigated here. In future research, we will assess the association between the developed UEF cognitive index with different components of cognitive impairments within neuropsychological tests, including working memory, attention, and executive functioning deficits within our cohort.
Conclusion
Within the current study, for the first time, we assess the association between UEF dual-task among elders with clinically diagnosed cognitive impairments. Findings suggest that both speed and accuracy of motor function performance provide physiologically meaningful outcomes for screening cognitive impairment among older adults, especially when individuals are asked to perform the motor function as consistently as possible. Further, within the current sample, no noticeable difference was observed in predicting the cognitive status by implementing more challenging cognitive task (counting backward by threes versus ones). Overall, the current study showed promise in implementing UEF cognitive index as a simple and quick tool for screening cognitive impairment, which can make the motor function task less demanding and more suitable for elders with mobility impairments.
References
Lyketsos, C. G. et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. Jama 288, 1475–1483 (2002).
Ferri, C. P. et al. Global prevalence of dementia: a Delphi consensus study. The lancet 366, 2112–2117 (2006).
Takizawa, C., Thompson, P. L., van Walsem, A., Faure, C. & Maier, W. C. Epidemiological and economic burden of Alzheimer’s disease: a systematic literature review of data across Europe and the United States of America. Journal of Alzheimer’s disease 43, 1271–1284 (2015).
Bradford, A., Kunik, M. E., Schulz, P., Williams, S. P. & Singh, H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer disease and associated disorders 23, 306 (2009).
Athilingam, P., Visovsky, C., Elliott, A. F. & Rogal, P. J. Cognitive Screening in Persons With Chronic Diseases in Primary Care Challenges and Recommendations for Practice. American journal of Alzheimer’s disease and other dementias 30, 547–558 (2015).
Volkow, N. D. et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. American Journal of psychiatry 155, 344–349 (1998).
Verhaeghen, P., Steitz, D. W., Sliwinski, M. J. & Cerella, J. Aging and dual-task performance: a meta-analysis. Psychology and aging 18, 443 (2003).
Blazer, D. G., Yaffe, K. & Liverman, C. T. Cognitive Aging:: Progress in Understanding and Opportunities for Action. (National Academies Press, 2015).
Laurin, D., Verreault, R., Lindsay, J., MacPherson, K. & Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Archives of neurology 58, 498–504 (2001).
Sheridan, P. L., Solomont, J., Kowall, N. & Hausdorff, J. M. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. Journal of the American Geriatrics Society 51, 1633–1637 (2003).
Camicioli, R., Howieson, D., Lehman, S. & Kaye, J. Talking while walking The effect of a dual task in aging and Alzheimer’s disease. Neurology 48, 955–958 (1997).
Montero-Odasso, M., Muir, S. W. & Speechley, M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Archives of physical medicine and rehabilitation 93, 293–299 (2012).
Toosizadeh, N. et al. Assessing upper-extremity motion: an innovative, objective method to identify frailty in older bed-bound trauma patients. Journal of the American College of Surgeons 223, 240–248 (2016).
Toosizadeh, N., Mohler, J. & Najafi, B. Assessing upper extremity motion: an innovative method to identify frailty. Journal of the American Geriatrics Society 63, 1181–1186 (2015).
Toosizadeh, N., Wendel, C., Hsu, C.-H., Zamrini, E. & Mohler, J. Frailty assessment in older adults using upper-extremity function: index development. BMC geriatrics 17, 117 (2017).
Toosizadeh, N. et al. Assessing upper-extremity motion: An innovative method to quantify functional capacity in patients with chronic obstructive pulmonary disease. PloS one 12, e0172766 (2017).
Ehsani, H. et al. The association between cognition and dual-tasking among older adults: the effect of motor function type and cognition task difficulty. Clinical interventions in aging 14, 659–669 (2019).
Toosizadeh, N. et al. Upper-extremity dual-task function: an innovative method to assess cognitive impairment in older adults. Frontiers in aging neuroscience 8, 167 (2016).
Borson, S., Scanlan, J., Brush, M., Vitaliano, P. & Dokmak, A. The Mini-Cog: a cognitive’vital signs’ measure for dementia screening in multi-lingual elderly. International journal of geriatric psychiatry 15, 1021–1027 (2000).
Teng, E. L. et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. International Psychogeriatrics 6, 45–58 (1994).
Leopold, N. A. & Borson, A. J. An alphabeticalWORLD’A new version of an old test. Neurology 49, 1521–1524 (1997).
Wind, A. W. et al. Limitations of the Mini‐Mental State Examination in diagnosing dementia in general practice. International journal of geriatric psychiatry 12, 101–108 (1997).
Mungas, D., Marshall, S., Weldon, M., Haan, M. & Reed, B. Age and education correction of Mini-Mental State Examination for English-and Spanish-speaking elderly. Neurology 46, 700–706 (1996).
Association, G. A. o. t. W. M. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. The Journal of the American College of Dentists 81, 14 (2014).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 7, 280–292 (2011).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–939 (1984).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 53, 695–699 (2005).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 12, 189–198 (1975).
Fried, L. P. et al. Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 56, M146–M157 (2001).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases 40, 373–383 (1987).
Kroenke, K. & Spitzer, R. L. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric annals 32, 509–515 (2002).
Vassilaki, M. et al. Multimorbidity and risk of mild cognitive impairment. Journal of the American Geriatrics Society 63, 1783–1790 (2015).
Rock, P., Roiser, J., Riedel, W. & Blackwell, A. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological medicine 44, 2029–2040 (2014).
Hittmair-Delazer, M., Semenza, C. & Denes, G. Concepts and facts in calculation. Brain 117, 715–728 (1994).
Weiss, E. et al. Brain activation pattern during a verbal fluency test in healthy male and female volunteers: a functional magnetic resonance imaging study. Neuroscience Letters 352, 191–194 (2003).
Lee, K.-M. & Kang, S.-Y. Arithmetic operation and working memory: Differential suppression in dual tasks. Cognition 83, B63–B68 (2002).
Smith, E. E. et al. The neural basis of task-switching in working memory: effects of performance and aging. Proceedings of the National Academy of Sciences 98, 2095–2100 (2001).
Beauchet, O., Dubost, V., Aminian, K., Gonthier, R. & Kressig, R. W. Dual-task-related gait changes in the elderly: does the type of cognitive task matter? Journal of motor behavior 37, 259 (2005).
Taga, G., Yamaguchi, Y. & Shimizu, H. Self-organized control of bipedal locomotion by neural oscillators in unpredictable environment. Biological cybernetics 65, 147–159 (1991).
Al-Yahya, E. et al. Cognitive motor interference while walking: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 35, 715–728 (2011).
IJmker, T. & Lamoth, C. J. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait & posture 35, 126–130 (2012).
Hauer, K. et al. Cognitive impairment decreases postural control during dual tasks in geriatric patients with a history of severe falls. Journal of the American Geriatrics Society 51, 1638–1644 (2003).
Gao, S., Hendrie, H. C., Hall, K. S. & Hui, S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Archives of general psychiatry 55, 809–815 (1998).
Coin, A. et al. Nutritional predictors of cognitive impairment severity in demented elderly patients: the key role of BMI. The journal of nutrition, health & aging 16, 553–556 (2012).
Volkow, N. D. et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity 17, 60–65 (2009).
Sullivan, L. M., Massaro, J. M. & D’Agostino Sr, R. B. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Statistics in medicine 23, 1631–1660 (2004).
Muir, S. W. et al. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait & posture 35, 96–100 (2012).
Hunter, S. W., Divine, A., Frengopoulos, C. & Odasso, M. M. A framework for secondary cognitive and motor tasks in dual-task gait testing in people with mild cognitive impairment. BMC geriatrics 18, 202 (2018).
Kawai, R. et al. Motor Cortex Is Required for Learning but Not for Executing a Motor Skill. Neuron 86, 800–812, https://doi.org/10.1016/j.neuron.2015.03.024 (2015).
Hikosaka, O., Nakamura, K., Sakai, K. & Nakahara, H. Central mechanisms of motor skill learning. Curr Opin Neurobiol 12, 217–222 (2002).
McLean, J. F. & Hitch, G. J. Working memory impairments in children with specific arithmetic learning difficulties. J Exp Child Psychol 74, 240–260, https://doi.org/10.1006/jecp.1999.2516 (1999).
Cullen, S. et al. Guidelines for gait assessments in the Canadian Consortium on Neurodegeneration in Aging (CCNA). Canadian Geriatrics Journal 21, 157 (2018).
Eggenberger, P., Tomovic, S., Münzer, T. & de Bruin, E. D. Older adults must hurry at pedestrian lights! A cross-sectional analysis of preferred and fast walking speed under single-and dual-task conditions. PLoS one 12, e0182180 (2017).
Smith, E., Cusack, T. & Blake, C. The effect of a dual task on gait speed in community dwelling older adults: A systematic review and meta-analysis. Gait & posture 44, 250–258 (2016).
Acknowledgements
This project was supported by an award from the National Institute of Aging (NIA) (award number: 1 R21 AG055852-01). The findings of this manuscript are those of the authors and do not necessarily represent the official views of NIA. Thanks to Sarah Fakhoury, Daniel Gaytan-Jenkins, and Anthony Lopez for data collection, and to Banner Sun Health Research Institute’s Longevity Study participants for their generous participation.
Author information
Authors and Affiliations
Contributions
N.T.: final approval of the version to be submitted, study concept and design, data collection, data analysis and interpretation of data, and preparation of the manuscript. H.E.: final approval of the version to be submitted, study concept and design, data analysis and interpretation of data, and preparation of the manuscript. C.W.: final approval of the version to be submitted, study design, data analysis and interpretation of data, and preparation of manuscript. E.Z.: final approval of the version to be submitted, data analysis and interpretation of data, and preparation of the manuscript. K.O.: final approval of the version to be submitted, data analysis and interpretation of data, acquisition of subjects, and preparation of the manuscript. J.M.: final approval of the version to be submitted, study concept and design, data analysis and interpretation of data; and preparation of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toosizadeh, N., Ehsani, H., Wendel, C. et al. Screening older adults for amnestic mild cognitive impairment and early-stage Alzheimer’s disease using upper-extremity dual-tasking. Sci Rep 9, 10911 (2019). https://doi.org/10.1038/s41598-019-46925-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46925-y
This article is cited by
-
Investigating the associations between upper limb motor function and cognitive impairment: a scoping review
GeroScience (2023)
-
Response-code conflict in dual-task interference and its modulation by age
Psychological Research (2023)
-
A computational musculoskeletal arm model for assessing muscle dysfunction in chronic obstructive pulmonary disease
Medical & Biological Engineering & Computing (2023)
-
Innovative motor and cognitive dual-task approaches combining upper and lower limbs may improve dementia early detection
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.