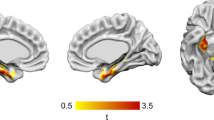
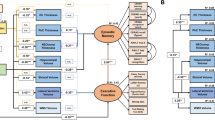
Abstract
Detection of incipient Alzheimer disease (AD) pathophysiology is critical to identify preclinical individuals and target potentially disease-modifying therapies towards them. Current neuroimaging and biomarker research is strongly focused in this direction, with the aim of establishing AD fingerprints to identify individuals at high risk of developing this disease. By contrast, cognitive fingerprints for incipient AD are virtually non-existent as diagnostics and outcomes measures are still focused on episodic memory deficits as the gold standard for AD, despite their low sensitivity and specificity for identifying at-risk individuals. This Review highlights a novel feature of cognitive evaluation for incipient AD by focusing on spatial navigation and orientation deficits, which are increasingly shown to be present in at-risk individuals. Importantly, the navigation system in the brain overlaps substantially with the regions affected by AD in both animal models and humans. Notably, spatial navigation has fewer verbal, cultural and educational biases than current cognitive tests and could enable a more uniform, global approach towards cognitive fingerprints of AD and better cognitive treatment outcome measures in future multicentre trials. The current Review appraises the available evidence for spatial navigation and/or orientation deficits in preclinical, prodromal and confirmed AD and identifies research gaps and future research priorities.
Key points
-
Episodic memory has limited utility as a diagnostic and outcome measure for preclinical Alzheimer disease (AD).
-
Spatial navigation deficits have the potential to detect underlying pathophysiology in preclinical AD.
-
The brain areas affected earliest by AD pathophysiology are key nodes in the spatial navigation network.
-
Genetically at-risk individuals show altered spatial navigation patterns before any episodic memory symptom onset.
-
Spatial navigation is emerging as a potential cost-effective cognitive biomarker to detect AD in the preclinical stages, which has important implications for future diagnostics and treatment approaches.
-
Future spatial navigation benchmarks and standardization of spatial navigation tests are needed to realize this goal.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blennow, K., de Leon, M. J. & Zetterberg, H. Alzheimer’s disease. Lancet 368, 387–403 (2006).
Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 11, 459–509 (2015).
Habchi, J. et al. An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic Aβ42 aggregates linked with Alzheimers disease. Sci. Adv. 2, e1501244–e1501244 (2016).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Yang, T. et al. Small molecule, non-peptide p75 ligands inhibit Abeta-induced neurodegeneration and synaptic impairment. PLoS ONE 3, e3604 (2008).
Vauzour, D. et al. Nutrition for the ageing brain: towards evidence for an optimal diet. Ageing Res. Rev. 35, 222–240 (2017).
Dubois, B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014).
Rajah, M. N. et al. Family history and APOE4 risk for Alzheimer’s disease impact the neural correlates of episodic memory by early midlife. NeuroImage Clin. 14, 760–774 (2017).
Bellassen, V., Igloi, K., de Souza, L. C., Dubois, B. & Rondi-Reig, L. Temporal order memory assessed during spatiotemporal navigation as a behavioral cognitive marker for differential alzheimer’s disease diagnosis. J. Neurosci. 32, 1942–1952 (2012).
Birrer, R. B. & Vemuri, S. P. Depression in later life: a diagnostic and therapeutic challenge. Am. Fam. Physician 69, 2375–2382 (2004).
Bronnick, K., Emre, M., Tekin, S., Haugen, S. B. & Aarsland, D. Cognitive correlates of visual hallucinations in dementia associated with Parkinson’s disease. Mov. Disord. 26, 824–829 (2011).
Pennington, C., Hodges, J. R. & Hornberger, M. Neural correlates of episodic memory in behavioral variant frontotemporal dementia. J. Alzheimers Dis. 24, 261–268 (2011).
Flanagan, E. C. et al. False recognition in behavioral variant frontotemporal dementia and Alzheimer’s disease-disinhibition or amnesia? Front. Aging Neurosci. 8, 1–11 (2016).
Tu, S. et al. Lost in spatial translation - a novel tool to objectively assess spatial disorientation in Alzheimer’s disease and frontotemporal dementia. Cortex 67, 83–94 (2015).
Yew, B., Alladi, S., Shailaja, M., Hodges, J. R. & Hornberger, M. Lost and forgotten? Orientation versus memory in Alzheimer’s disease and frontotemporal dementia. J. Alzheimers Dis. 33, 473–481 (2013).
Fu, H. et al. Tau pathology induces excitatory neuron loss, grid cell dysfunction, and spatial memory deficits reminiscent of early Alzheimer’s Disease. Neuron 93, 533–541 (2017).
Serino, S., Morganti, F., Di Stefano, F. & Riva, G. Detecting early egocentric and allocentric impairments deficits in Alzheimer’s disease: an experimental study with virtual reality. Front. Aging Neurosci. 7, 1–10 (2015).
Lithfous, S., Dufour, A. & Després, O. Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: insights from imaging and behavioral studies. Ageing Res. Rev. 12, 201–213 (2013).
Templer, V. & Hampton, R. Episodic memory in nonhuman animals. Curr. Biol. 23, 801–806 (2013).
Allison, S. L., Fagan, A. M., Morris, J. C. & Head, D. Spatial navigation in preclinical Alzheimer’s disease. J. Alzheimers Dis. 52, 77–90 (2016).
Kunz, L. et al. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science 350, 430–433 (2015).
Jack Jr, C. R. et al. Introduction to revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer Association Workgroups. Alzheimers Dement. 7, 257–262 (2011).
Medina, M. & Avila, J. New perspectives on the role of tau in Alzheimer’s disease. Implications for therapy. Biochem. Pharmacol. 88, 540–547 (2014).
Nelson, P. T. et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 71, 362–381 (2012).
Knopman, D. S. et al. Neuropathology of cognitively normal elderly. J. Neuropathol. Exp. Neurol. 62, 1087–1095 (2003).
Chételat, G. et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. NeuroImage Clin. 2, 356–365 (2013).
Morris, G. P., Clark, I. A. & Vissel, B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s Disease. Acta Neuropathol. Commun. 2, 1–21 (2014).
Galton, C. J., Patterson, K., Xuereb, J. H. & Hodges, J. R. Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123, 484–498 (2000).
Braak, H. & Del Tredici, K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138, 2814–2833 (2015).
Hartshorne, J. K. & Germine, L. T. When does cognitive functioning peak? The asynchron. rise fall different cognitive abilities across life span. Psychol. Sci. 26, 433–443 (2015).
Park, H. L., O’Connell, J. E. & Thomson, R. G. A systematic review of cognitive decline in the general elderly population. Int. J. Geriatr. Psychiatry 18, 1121–1134 (2003).
Brayne, C. et al. Estimating the true extent of cognitive decline in the old old. J. Am. Geriatr. Soc. 47, 1283–1288 (1999).
Brailean, A. et al. Cohort differences in cognitive aging in the longitudinal aging study Amsterdam. J. Gerontol. B Psychol. Sci. Soc. Sci. https://doi.org/10.1093/geronb/gbw129 (2016).
Bertoux, M. et al. Two distinct amnesic profiles in behavioral variant frontotemporal dementia. Biol. Psychiatry 75, 582–588 (2014).
Hornberger, M., Piguet, O., Graham, A. J., Nestor, P. J. & Hodges, J. R. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology 74, 472–479 (2010).
Hornberger, M. & Piguet, O. Episodic memory in frontotemporal dementia: a critical review. Brain 135, 678–692 (2012).
Wong, S., Flanagan, E., Savage, G., Hodges, J. R. & Hornberger, M. Contrasting prefrontal cortex contributions to episodic memory dysfunction in behavioural variant frontotemporal dementia and alzheimer’s disease. PLoS ONE 9, e87778 (2014).
Cerman, J. et al. Subjective spatial navigation complaints - a frequent symptom reported by patients with subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 15, 219–228 (2017).
Tu, S., Spiers, H. J., Hodges, J. R., Piguet, O. & Hornberger, M. Egocentric versus allocentric spatial memory in behavioral variant frontotemporal dementia and Alzheimer’s disease. J. Alzheimers Dis. 59, 883–892 (2017).
Killian, N. J. & Buffalo, E. A. Grid cells map the visual world. Nat. Neurosci. 21, 161–162 (2018).
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005).
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I. & Moser, M. B. Path integration and the neural basis of the ‘cognitive map’. Nat. Rev. Neurosci. 7, 663–678 (2006).
Fuhs, M. C. & Touretzky, D. S. A. Spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. 26, 4266–4276 (2006).
Boccia, M., Nemmi, F. & Guariglia, C. Neuropsychology of environmental navigation in humans: review and meta-analysis of fMRI studies in healthy participants. Neuropsychol. Rev. 24, 236–251 (2014).
Wolbers, T., Weiller, C. & Büchel, C. Neural foundations of emerging route knowledge in complex spatial environments. Brain Res. Cogn. Brain Res. 21, 401–411 (2004).
Hartley, T., Maguire, E. A., Spiers, H. J. & Burgess, N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron 37, 877–888 (2003).
O’Keefe, John & Nadel, L. The Hippocampus as a Cognitive Map (Oxford University Press, Oxford, 1978).
Loomis, J. M., Golledge, R. G. & Klatzky, R. L. Navigation system for the blind: auditory display modes and guidance. Presence Teleoperators virtual Environments 7, 193–203 (1998).
Loomis, J. M. et al. Nonvisual navigation by blind and sighted: assessment of path integration ability. J. Exp. Psychol. Gen. 122, 73–91 (1993).
Spiers, H. J. & Barry, C. Neural systems supporting navigation. Curr. Opin. Behav. Sci. 1, 47–55 (2015).
Byrne, P., Becker, S. & Burgess, N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 114, 340–375 (2007).
Chiu, T. C. et al. Alpha modulation in parietal and retrosplenial cortex correlates with navigation performance. Psychophysiology 49, 43–55 (2012).
Gaffan, D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. J. Cogn. Neurosci. 6, 305–320 (1994).
King, J. A., Trinkler, I., Hartley, T., Vargha-Khadem, F. & Burgess, N. The hippocampal role in spatial memory and the familiarity—recollection distinction: a case study. Neuropsychology 18, 405–417 (2004).
Feigenbaum, J. D. & Morris, R. G. Allocentric versus egocentric spatial memory after unilateral temporal lobectomy in humans. Neuropsychology 18, 462–472 (2004).
Parslow, D. M. et al. Allocentric spatial memory activation of the hippocampal formation measured with fMRI. Neuropsychology 18, 450–461 (2004).
Ekstrom, A. D. et al. Cellular networks underlying human spatial navigation. Nature 425, 184–188 (2003).
Maguire, E. a et al. Knowing where and getting there: a human navigation network. Science 280, 921–924 (1998).
Auger, S. D., Mullally, S. L. & Maguire, E. A. Retrosplenial cortex codes for permanent landmarks. PLoS ONE 7, e43620 (2012).
Auger, S. D. & Maguire, E. A. Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. Cortex 49, 2904–2913 (2013).
Moffat, S. D., Kennedy, K. M., Rodrigue, K. M. & Raz, N. Extrahippocampal contributions to age differences in human spatial navigation. Cereb. Cortex 17, 1274–1282 (2007).
Aggleton, J. P., Pralus, A., Nelson, A. J. D. & Hornberger, M. Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain 139, 1877–1890 (2016).
Aggleton, J. P. & Nelson, A. J. D. Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits? Neurosci. Biobehav. Rev. 54, 131–144 (2015).
Doeller, C. C. F., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature 463, 657–661 (2010).
Burgess, N., Barry, C. & O’Keefe, J. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007).
Taube, J. S., Muller, R. U. & Ranck, J. B. Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations.J. Neurosci. 70, 436–447 (1990).
Muller, R. U., Ranck Jr., J. B. & Taube, J. S. Head direction cells: properties and functional significance. Curr. Opin. Neurobiol. 6, 196–206 (1996).
Shine, J. P., Valdes-Herrera, J. P., Hegarty, M. & Wolbers, T. The human retrosplenial cortex and thalamus code head direction in a global reference frame. J. Neurosci. 36, 6371–6381 (2016).
Lever, C., Burton, S., Jeewajee, A., Keefe, J. O. & Burgess, N. Europe PMC funders group boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. 29, 9771–9777 (2010).
Mahmood, O., Adamo, D., Briceno, E. & Moffat, S. D. Age differences in visual path integration. Behav. Brain Res. 205, 88–95 (2009).
Alexander, A. S. & Nitz, D. A. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci. 18, 1143–1151 (2015).
Czajkowski, R. et al. Encoding and storage of spatial information in the retrosplenial cortex. Proc. Natl Acad. Sci. USA 111, 8661–8666 (2014).
Bird, C. M., Keidel, J. L., Ing, L. P., Horner, A. J. & Burgess, N. Consolidation of complex events via reinstatement in posterior cingulate cortex. J. Neurosci. 35, 14426–14434 (2015).
Dhindsa, K. et al. Examining the role of the temporo-parietal network in memory, imagery, and viewpoint transformations. Front. Hum. Neurosci. 8, 1–13 (2014).
Vass, L. K. & Epstein, R. A. Abstract representations of location and facing direction in the human brain. J. Neurosci. 33, 6133–6142 (2013).
Clark, B. J., Brown, J. E. & Taube, J. S. Head direction cell activity in the anterodorsal thalamus requires intact supragenual nuclei. J. Neurophysiol. 108, 2767–2784 (2012).
Knight, R. & Hayman, R. Allocentric directional processing in the rodent and human retrosplenial cortex. Front. Hum. Neurosci. 8, 1–5 (2014).
Chersi, F. & Pezzulo, G. Using hippocampal-striatal loops for spatial navigation and goal-directed decision-making. Cogn. Process. 13, 125–129 (2012).
Sheynikhovich, D., Chavarriaga, R., Strösslin, T., Arleo, A. & Gerstner, W. Is there a geometric module for spatial orientation? Insights from a rodent navigation model. Psychol. Rev. 116, 540–566 (2009).
Elduayen, C. & Save, E. The retrosplenial cortex is necessary for path integration in the dark. Behav. Brain Res. 272, 303–307 (2014).
Mullally, S. L. & Maguire, E. A. A. New role for the parahippocampal cortex in representing space. J. Neurosci. 31, 7441–7449 (2011).
Iaria, G., Palermo, L., Committeri, G. & Barton, J. J. S. Age differences in the formation and use of cognitive maps. Behav. Brain Res. 196, 187–191 (2009).
Moffat, S. D. et al. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav. Neurosci. 116, 851–859 (2002).
Gazova, I. et al. Spatial navigation in young versus older adults. Front. Aging Neurosci. 5, 1–8 (2013).
Moffat, S. D. Aging and spatial navigation: what do we know and where do we go? Neuropsychol. Rev. 19, 478–489 (2009).
Heo, S. et al. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 1315, 119–127 (2010).
Bach, M. E. et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl Acad. Sci. USA 96, 5280–5285 (1999).
Driscoll, I. et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72, 1906–1913 (2009).
Lalonde-Parsi, M.-J. & Lamontagne, A. Perception of self-motion and regulation of walking speed in young-old adults. Motor Control 19, 191–206 (2015).
Holden, H. M. & Gilbert, P. E. Less efficient pattern separation may contribute to age-related spatial memory deficits. Front. Aging Neurosci. 4, 1–6 (2012).
Lester, A. W., Moffat, S. D., Wiener, J. M., Barnes, C. A. & Wolbers, T. The aging navigational system. Neuron 95, 1019–1035 (2017).
Carpenter, H. E., Kelly, K. B., Bizon, J. L. & Frazier, C. J. Age-related changes in tonic activation of presynaptic versus extrasynaptic γ-amniobutyric acid type B receptors in rat medial prefrontal cortex. Neurobiol. Aging 45, 88–97 (2016).
Rodgers, M. K., Sindone, J. A. & Moffat, S. D. Effects of age on navigation strategy. Neurobiol. Aging 33, 202.e15–202.e22 (2012).
Zheng Bian & George Andersen, J. Aging and the perception of egocentric distance. Psychol. Aging 28, 813–825 (2013).
Norman, J. F., Adkins, O. C., Norman, H. F., Cox, A. G. & Rogers, C. E. Aging and the visual perception of exocentric distance. Vision Res. 109, 52–58 (2015).
Vandenberg, S. G. & Kuse, A. R. Mental rotations, a group test of three-dimensional spatial visualization. Percept. Mot. Skills 47, 599–604 (1978).
Money, J. et al. A standardized road-map test of direction sense; Manual (Johns Hopkins Press, Baltimore, MD, 1965).
Mitolo, M. et al. Relationship between spatial ability, visuospatial working memory and self-assessed spatial orientation ability: a study in older adults. Cogn. Process. 16, 165–176 (2015).
Schinazi, V. R., Nardi, D., Newcombe, N. S., Shipley, T. F. & Epstein, R. A. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus 23, 515–528 (2013).
Mapstone, M., Steffenella, T. M. & Duffy, C. J. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD. Neurology 60, 802–808 (2003).
Cushman, L. A. & Duffy, C. J. Virtual reality identifies navigational defects in Alzheimer disease and cognitive aging. Nat. Clin. Pract. Neurol. 4, 638–639 (2008).
Cogné, M. et al. The contribution of virtual reality to the diagnosis of spatial navigation disorders and to the study of the role of navigational aids: a systematic literature review. Ann. Phys. Rehabil. Med. 60, 164–176 (2017).
Pengas, G. et al. The relationship of topographical memory performance to regional neurodegeneration in Alzheimer’s disease. Front. Aging Neurosci. 4, 1–10 (2012).
Jheng, S. S. & Pai, M. C. Cognitive map in patients with mild Alzheimer’s disease: a computer-generated arena study. Behav. Brain Res. 200, 42–47 (2009).
Serino, S. & Riva, G. Getting lost in Alzheimer’s disease: a break in the mental frame syncing. Med. Hypotheses 80, 416–421 (2013).
Irish, M. et al. Scene construction impairments in Alzheimer’s disease - a unique role for the posterior cingulate cortex. Cortex 73, 10–23 (2015).
Padurariu, M., Ciobica, A., Mavroudis, I., Fotiou, D. & Baloyannis, S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr. Danub. 24, 152–158 (2012).
Weniger, G., Ruhleder, M., Lange, C., Wolf, S. & Irle, E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia 49, 518–527 (2011).
Tan, R. H., Wong, S., Hodges, J. R., Halliday, G. M. & Hornberger, M. Retrosplenial cortex (BA 29) volumes in behavioral variant frontotemporal dementia and alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 35, 177–182 (2013).
Morganti, F., Stefanini, S. & Riva, G. From allo- to egocentric spatial ability in early Alzheimer’s disease: a study with virtual reality spatial tasks. Cogn. Neurosci. 4, 171–180 (2013).
Hort, J. et al. Spatial navigation deficit in amnestic mild cognitive impairment. Proc. Natl Acad. Sci. USA 104, 4042–4047 (2007).
Jack, C. R. et al. Suspected non-Alzheimer disease pathophysiology—concept and controversy. Nat. Rev. Neurol. 12, 117–124 (2016).
DeIpolyi, A. R., Rankin, K. P., Mucke, L., Miller, B. L. & Gorno-Tempini, M. L. Spatial cognition and the human navigation network in AD and MCI. Neurology 69, 986–997 (2007).
Dubois, B. & Albert, M. L. Amnestic MCI or prodromal Alzheimer’s disease? Lancet Neurol. 3, 246–248 (2004).
Laczó, J., Andel, R., Vyhnalek, M. & Vlcek, K. APOE and spatial navigation in amnestic MCI: results from a computer-based test. Neuropsychology 28, 676–684 (2014).
Julkunen, V. et al. Cortical thickness analysis to detect progressive mild cognitive impairment: a reference to Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 28, 404–412 (2009).
Mokrisova, I. et al. Real-space path integration is impaired in Alzheimer’s disease and mild cognitive impairment. Behav. Brain Res. 307, 150–158 (2016).
Laczó, J. et al. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav. Brain Res. 202, 252–259 (2009).
Genin, E. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol. Psychiatry 16, 903–907 (2011).
Scahill, R. I., Schott, J. M., Stevens, J. M., Rossor, M. N. & Fox, N. C. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc. Natl Acad. Sci. USA 99, 4703–4707 (2002).
Pengas, G., Hodges, J. R., Watson, P. & Nestor, P. J. Focal posterior cingulate atrophy in incipient Alzheimer’s disease. Neurobiol. Aging 31, 25–33 (2010).
Fennema-Notestine, C. et al. Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Hum. Brain Mapp. 30, 3238–3253 (2009).
Hämäläinen, A. et al. Voxel-based morphometry to detect brain atrophy in progressive mild cognitive impairment. Neuroimage 37, 1122–1131 (2007).
Whitwell, J. L. et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 71, 743–749 (2008).
van Groen, T. & Michael Wyss, J. Connections of the retrosplenial granular a cortex in the rat. J. Comp. Neurol. 300, 593–606 (1990).
Tan, C. C., Yu, J. T. & Tan, L. Biomarkers for preclinical alzheimer’s disease. J. Alzheimers Dis. 42, 1051–1069 (2014).
Patel, K. T. et al. Default mode network activity and white matter integrity in healthy middle-aged ApoE4 carriers. Brain Imaging Behav. 7, 60–67 (2013).
Pihlajamaki, M. et al. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 24, 28–36 (2010).
Skoog, I. et al. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dement. Geriatr. Cogn. Disord. 15, 169–176 (2003).
Villemagne, V. L., Fodero-Tavoletti, M. T., Masters, C. L. & Rowe, C. C. Tau imaging: early progress and future directions. Lancet Neurol. 14, 114–124 (2015).
Johnson, K. A. et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79, 110–119 (2016).
Weston, P. S. J. et al. Presymptomatic cortical thinning in familial Alzheimer disease: a longitudinal MRI study. Neurology 87, 2050–2057 (2016).
Yasen, A. L., Raber, J., Miller, J. K. & Piper, B. J. Sex, but not apolipoprotein E polymorphism, differences in spatial performance in young adults. Arch. Sex. Behav. 44, 2219–2226 (2015).
Bunce, D. et al. APOE genotype and cognitive change in young, middle- aged, and older adults living in the community. J. Gerontol. A Biol. Sci. Med. Sci. 69, 379–386 (2014).
Salvato, G., Patai, E. Z., McCloud, T. & Nobre, A. C. Apolipoprotein ε4 breaks the association between declarative long-term memory and memory-based orienting of spatial attention in middle-aged individuals. Cortex 82, 206–216 (2016).
Greenwood, P. M., Lambert, C., Sunderland, T. & Parasuraman, R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle - aged adults: results from the National Institute of Mental Health ’s BIOCARD Study. Neuropsychology 2, 199–211 (2015).
Parasuraman, R., Greenwood, P. M. & Sunderland, T. The apolipoprotein E gene, attention, and brain function. Neuropsychology 16, 254–274 (2002).
Evans, S. et al. Cognitive and neural signatures of the APOE E4 allele in mid-aged adults. Neurobiol. Aging 35, 1615–1623 (2014).
Berteau-Pavy, F., Park, B. & Raber, J. Effects of sex and APOE epsilon 4 on object recognition and spatial navigation in the elderly. Neuroscience 147, 6–17 (2007).
Bott, J.-B. et al. APOE sensitive cholinergic sprouting compensates for hippocampal dysfunctions due to reduced entorhinal input. J. Neurosci. 36, 10472–10486 (2016).
Risacher, S. L. et al. APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 11, 1417–1429 (2015).
Amariglio, R. E. et al. Specific SMC in older persons may indicate poor cognitive function. J. Am. Geriatr. Soc. 59, 1612–1617 (2011).
Amariglio, R. E. et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886 (2012).
Hort, J. et al. Effect of donepezil in alzheimer disease can be measured by a computerized human analog of the morris water maze. Neurodegener. Dis. 13, 192–196 (2014).
Laczó, J. et al. Scopolamine disrupts place navigation in rats and humans: a translational validation of the hidden goal task in the morris water maze and a real maze for humans. Psychopharmacol. 234, 535–547 (2016).
Coutrot, A. et al. Global determinants of navigation ability. Preprint at https://www.biorxiv.org/content/early/2017/09/14/188870.1 (2018).
Coutrot, A. et al. Virtual navigation tested on a mobile app (Sea Hero Quest) is predictive of real-world navigation performance: preliminary data. Preprint at https://www.biorxiv.org/content/early/2018/04/22/305433 (2018).
Coughlan, G. et al. Impact of sex and APOE status on spatial navigation in pre- symptomatic Alzheimer’s disease. Preprint at https://www.biorxiv.org/content/early/2018/03/23/287722 (2018).
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006).
Vann, S. D., Aggleton, J. P. & Maguire, E. A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 10, 792–802 (2009).
Author information
Authors and Affiliations
Contributions
M.H. and G.C. contributed to all aspects of the manuscript. J.L. and J.H. contributed to reviewing and editing the manuscript before submission. A.-M.M. contributed to writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Neurology thanks T. Brandt, K. Possin and T. Wolbers for their contribution to the peer review of this work.
Related link
Sea Hero Quest: www.seaheroquest.com
Glossary
- Episodic memory
-
One’s memory of events represented by aspects of the past not present in other memories, such as the time, place or social context.
- Mild cognitive impairment
-
(MCI). Prodromal or intermediate stage between the expected cognitive decline of normal ageing and the more serious decline of dementia.
- Egocentric navigation strategies
-
Egocentric self-centred navigation frames encode spatial information from the viewpoint of the navigator.
- Allocentric navigation strategies
-
Allocentric strategies are based on the navigator’s perception of landmark positions relative to other landmarks.
- Morris water maze
-
A test of spatial learning examining rodent ability to navigate from different starting locations around an open swimming arena to locate a submerged escape platform using only distal cues. For more information, see elsewhere149.
- Significant memory concerns
-
(SMCs). Self-experienced persistent declines in cognitive abilities in comparison with a prior normal status; occur in the absence of objective impairment on standardized neuropsychological tests.
Rights and permissions
About this article
Cite this article
Coughlan, G., Laczó, J., Hort, J. et al. Spatial navigation deficits — overlooked cognitive marker for preclinical Alzheimer disease?. Nat Rev Neurol 14, 496–506 (2018). https://doi.org/10.1038/s41582-018-0031-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-018-0031-x
This article is cited by
-
3D convolutional neural networks uncover modality-specific brain-imaging predictors for Alzheimer’s disease sub-scores
Brain Informatics (2024)
-
Shorter self-reported sleep duration is associated with worse virtual spatial navigation performance in men
Scientific Reports (2024)
-
Comparing episodic memory outcomes from walking augmented reality and stationary virtual reality encoding experiences
Scientific Reports (2024)
-
Effects of older age on visual and self-motion sensory cue integration in navigation
Experimental Brain Research (2024)
-
Virtual Reality and Serious Videogame-Based Instruments for Assessing Spatial Navigation in Alzheimer’s Disease: A Systematic Review of Psychometric Properties
Neuropsychology Review (2024)