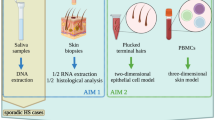
Abstract
Hidradenitis suppurativa (HS; also designated as acne inversa) is a chronic inflammatory disorder, which affects the intertriginous skin and is associated with numerous systemic comorbidities. The estimated prevalence of HS is ~1% in most studied countries. Typically starting in early adulthood, cutaneous inflamed nodules, abscesses and pus-discharging tunnels develop in axillary, inguinal, gluteal and perianal body sites. The comorbidities of HS include metabolic and cardiovascular disorders, which contribute to reduced life expectancy. A genetic predisposition, smoking, obesity and hormonal factors are established aetiological factors for HS. Cutaneous changes seem to start around hair follicles and involve activation of cells of the innate and adaptive immune systems, with pivotal roles for pro-inflammatory cytokines such as tumour necrosis factor, IL-1β and IL-17. The unrestricted and chronic immune response eventually leads to severe pain, pus discharge, irreversible tissue destruction and scar development. HS has profound negative effects on patients’ quality of life, which often culminate in social withdrawal, unemployment, depression and suicidal thoughts. The therapeutic options for HS comprise antibiotic treatment, neutralization of tumour necrosis factor and surgical intervention together with lifestyle modification. Nevertheless, there is an enormous need for awareness of HS, understanding of its pathogenesis and novel treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Saunte, D. M. L. & Jemec, G. B. E. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA 318, 2019–2032 (2017).
Ingram, J. R. et al. Population-based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br. J. Dermatol. 178, 917–924 (2018). This paper is a well-conducted registry study providing a realistic estimate of HS prevalence.
Theut Riis, P., Thorlacius, L., Knudsen List, E. & Jemec, G. B. E. A pilot study of unemployment in patients with hidradenitis suppurativa in Denmark. Br. J. Dermatol. 176, 1083–1085 (2017).
Matusiak, L., Bieniek, A. & Szepietowski, J. C. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J. Am. Acad. Dermatol. 62, 706–708 (2010).
Wolk, K. et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J. Immunol. 186, 1228–1239 (2011). This paper is the first investigation of the role of cytokines related to activated lymphocytes (for example, IL-17, IL-22, IL-26 and IFNγ) and myeloid cells (for example, IL-1β and IL-10), the elements of their pathways and their consequences in the skin of patients with HS.
Sabat, R. et al. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS One 7, e31810 (2012). This paper is a comprehensive prospective case–control study that first reported the high prevalence of the metabolic syndrome in patients with HS, an observation most striking among young patients.
Shlyankevich, J., Chen, A. J., Kim, G. E. & Kimball, A. B. Hidradenitis suppurativa is a systemic disease with substantial comorbidity burden: a chart-verified case-control analysis. J. Am. Acad. Dermatol. 71, 1144–1150 (2014).
Schneider-Burrus, S. et al. High prevalence of back pain and axial spondyloarthropathy in patients with hidradenitis suppurativa. Dermatology 232, 606–612 (2016).
Richette, P. et al. Hidradenitis suppurativa associated with spondyloarthritis — results from a multicenter national prospective study. J. Rheumatol. 41, 490–494 (2014).
Deckers, I. E. et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J. Am. Acad. Dermatol. 76, 49–53 (2017).
Egeberg, A. et al. Prevalence and risk of inflammatory bowel disease in patients with hidradenitis suppurativa. J. Invest. Dermatol. 137, 1060–1064 (2017).
Gonzalez-Lopez, M. A. et al. Increased prevalence of subclinical atherosclerosis in patients with hidradenitis suppurativa (HS). J. Am. Acad. Dermatol. 75, 329–335 (2016).
Vazquez, B. G., Alikhan, A., Weaver, A. L., Wetter, D. A. & Davis, M. D. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J. Invest. Dermatol. 133, 97–103 (2013).
Kurek, A., Johanne Peters, E. M., Sabat, R., Sterry, W. & Schneider-Burrus, S. Depression is a frequent co-morbidity in patients with acne inversa. J. Dtsch. Dermatol. Ges. 11, 743–750 (2013).
Jemec, G. B. & Kimball, A. B. Hidradenitis suppurativa: epidemiology and scope of the problem. J. Am. Acad. Dermatol. 73, S4–S7 (2015).
Ingvarsson, G. Regional variation of hidradenitis suppurativa in the Norwegian patient registry during a 5-year period may describe professional awareness of the disease, not changes in prevalence. Br. J. Dermatol. 176, 274–275 (2017).
Sung, S. & Kimball, A. B. Counterpoint: analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J. Am. Acad. Dermatol. 69, 818–819 (2013).
Kurokawa, I., Hayashi, N. & Japan Acne Research, S. Questionnaire surveillance of hidradenitis suppurativa in Japan. J. Dermatol. 42, 747–749 (2015).
Lee, J. H., Kwon, H. S., Jung, H. M., Kim, G. M. & Bae, J. M. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population-based study. J. Eur. Acad. Dermatol. Venereol. 32, 1784–1790 (2018).
Garg, A., Kirby, J. S., Lavian, J., Lin, G. & Strunk, A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 153, 760–764 (2017).
Jemec, G. B., Heidenheim, M. & Nielsen, N. H. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J. Am. Acad. Dermatol. 35, 191–194 (1996).
Calao, M. et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One 13, e0200683 (2018). This paper is a comprehensive study describing the prevalence of HS in the general population.
Zouboulis, C. C. et al. Development and validation of the international hidradenitis suppurativa severity score system (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 177, 1401–1409 (2017). This paper describes the development of the IHS4, which has the potential to become the gold standard for the classification of patients with HS.
Deckers, I. E., van der Zee, H. H., Boer, J. & Prens, E. P. Correlation of early-onset hidradenitis suppurativa with stronger genetic susceptibility and more widespread involvement. J. Am. Acad. Dermatol. 72, 485–488 (2015).
Yang, J. H. et al. Demographic and clinical features of hidradenitis suppurativa in Korea. J. Dermatol. 45, 1389–1395 (2018).
Kromann, C. B. et al. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br. J. Dermatol. 171, 819–824 (2014).
Egeberg, A., Gislason, G. H. & Hansen, P. R. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 152, 429–434 (2016).
Ingram, J. R. The genetics of hidradenitis suppurativa. Dermatol. Clin. 34, 23–28 (2016).
von der Werth, J. M. & Williams, H. C. The natural history of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 14, 389–392 (2000).
Wang, B. et al. Gamma-secretase gene mutations in familial acne inversa. Science 330, 1065 (2010). This paper is the first study to demonstrate loss of function mutations in genes encoding γ-secretase, cumulatively found among members of Chinese families with severe HS.
Haapasalo, A. & Kovacs, D. M. The many substrates of presenilin/gamma-secretase. J. Alzheimers Dis. 25, 3–28 (2011).
Frew, J. W., Vekic, D. A., Woods, J. & Cains, G. D. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br. J. Dermatol. 177, 987–998 (2017).
Pan, Y. et al. γ-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev. Cell 7, 731–743 (2004).
O’Sullivan Coyne, G., Woodring, T. S., Lee, C. R., Chen, A. P. & Kong, H. H. Hidradenitis suppurativa-like lesions associated with pharmacologic inhibition of gamma-secretase. J. Invest. Dermatol. 138, 979–981 (2018).
Aubin-Houzelstein, G. Notch signaling and the developing hair follicle. Adv. Exp. Med. Biol. 727, 142–160 (2012).
Asano, N., Watanabe, T., Kitani, A., Fuss, I. J. & Strober, W. Notch1 signaling and regulatory T cell function. J. Immunol. 180, 2796–2804 (2008).
Ali, N. et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 169, 1119–1129 (2017).
Lowell, S., Jones, P., Le Roux, I., Dunne, J. & Watt, F. M. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10, 491–500 (2000).
Alam, M. S. et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA 107, 5943–5948 (2010).
Pang, B. et al. Elevated Notch1 enhances interleukin-22 production by CD4+ T cells via aryl hydrocarbon receptor in patients with lung adenocarcinoma. Biosci. Rep. 38, BSR20181922 (2018).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Pink, A. E. et al. Mutations in the gamma-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J. Invest. Dermatol. 132, 2459–2461 (2012).
Liu, M. et al. Genetic analysis of NCSTN for potential association with hidradenitis suppurativa in familial and nonfamilial patients. Br. J. Dermatol. 175, 414–416 (2016).
Ingram, J. R., Wood, M., John, B., Butler, R. & Anstey, A. V. Absence of pathogenic gamma-secretase mutations in a South Wales cohort of familial and sporadic hidradenitis suppurativa (acne inversa). Br. J. Dermatol. 168, 874–876 (2013).
Giatrakos, S. et al. Haplotypes of IL-12Rbeta1 impact on the clinical phenotype of hidradenitis suppurativa. Cytokine 62, 297–301 (2013).
Vural, S. et al. Association of pyrin mutations and autoinflammation with complex phenotype hidradenitis suppurativa: a case-control study. Br. J. Dermatol. 180, 1459–1467 (2019).
Revuz, J. E. et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J. Am. Acad. Dermatol. 59, 596–601 (2008).
Konig, A., Lehmann, C., Rompel, R. & Happle, R. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology 198, 261–264 (1999).
Hana, A. et al. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 80, 2214–2220 (2007).
Radek, K. A. et al. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe 7, 277–289 (2010).
Wu, Y. et al. Nicotine enhances Staphylococcus epidermidis biofilm formation by altering the bacterial autolysis, extracellular DNA releasing, and polysaccharide intercellular adhesin production. Front. Microbiol. 9, 2575 (2018).
Wittebole, X. et al. Nicotine exposure alters in vivo human responses to endotoxin. Clin. Exp. Immunol. 147, 28–34 (2007).
Boer, J. Should hidradenitis suppurativa be included in dermatoses showing Koebnerization? Is it friction or fiction? Dermatology 233, 47–52 (2017).
Alikhan, A., Lynch, P. J. & Eisen, D. B. Hidradenitis suppurativa: a comprehensive review. J. Am. Acad. Dermatol. 60, 539–561; quiz 562–563 (2009).
Riis, P. T., Ring, H. C., Themstrup, L. & Jemec, G. B. The role of androgens and estrogens in hidradenitis suppurativa — a systematic review. Acta Dermatovenerol. Croat. 24, 239–249 (2016).
Karagiannidis, I., Nikolakis, G., Sabat, R. & Zouboulis, C. C. Hidradenitis suppurativa/acne inversa: an endocrine skin disorder? Rev. Endocr. Metab. Disord. 17, 335–341 (2016).
Gauntner, T. D. Hormonal, stem cell and Notch signalling as possible mechanisms of disease in hidradenitis suppurativa: a systems-level transcriptomic analysis. Br. J. Dermatol. 180, 203–204 (2019).
Jenei, A. et al. Apocrine gland-rich skin has a non-inflammatory IL-17-related immune milieu, that turns to inflammatory IL-17-mediated disease in hidradenitis suppurativa. J. Invest. Dermatol. 139, 964–968 (2019).
Kamp, S. et al. Hidradenitis suppurativa: a disease of the absent sebaceous gland? Sebaceous gland number and volume are significantly reduced in uninvolved hair follicles from patients with hidradenitis suppurativa. Br. J. Dermatol. 164, 1017–1022 (2011).
Naik, H. B. et al. Are bacteria infectious pathogens in hidradenitis suppurativa? Debate at the symposium for hidradenitis suppurativa advances meeting, November 2017. J. Invest. Dermatol. 139, 13–16 (2019).
Guet-Revillet, H. et al. The microbiological landscape of anaerobic infections in hidradenitis suppurativa: a prospective metagenomic study. Clin. Infect. Dis. 65, 282–291 (2017). This paper investigates intracutaneous bacterial colonization in patients with HS and shows the enrichment of specific Gram-negative anaerobic species in chronic HS lesions.
Nikolakis, G. et al. Bacteriology of hidradenitis suppurativa/acne inversa: a review. J. Am. Acad. Dermatol. 73, S12–S18 (2015).
Ring, H. C. et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp. Dermatol. 24, 727–731 (2015).
Kathju, S., Lasko, L. A. & Stoodley, P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol. Med. Microbiol. 65, 385–389 (2012).
Ring, H. C. et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br. J. Dermatol. 176, 993–1000 (2017).
Ring, H. C. et al. Normal skin microbiota is altered in pre-clinical hidradenitis suppurativa. Acta Derm. Venereol. 97, 208–213 (2017).
De Vita, V. & Fabbrocini, G. Mechanical stress as a cause of hidradenitis suppurativa: a lesson from a patient with a monster hernia. Acta Dermatovenerol. Croat. 26, 260–261 (2018).
Yu, C. C. & Cook, M. G. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br. J. Dermatol. 122, 763–769 (1990). This paper describes an early histopathological study that suggests that HS develops upon dilatation and rupture of hair follicles.
Attanoos, R. L., Appleton, M. A. & Douglas-Jones, A. G. The pathogenesis of hidradenitis suppurativa: a closer look at apocrine and apoeccrine glands. Br. J. Dermatol. 133, 254–258 (1995).
Boer, J. & Weltevreden, E. F. Hidradenitis suppurativa or acne inversa. A clinicopathological study of early lesions. Br. J. Dermatol. 135, 721–725 (1996).
Jemec, G. B. & Hansen, U. Histology of hidradenitis suppurativa. J. Am. Acad. Dermatol. 34, 994–999 (1996).
von Laffert, M. et al. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp. Dermatol. 19, 533–537 (2010).
van der Zee, H. H. et al. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. Br. J. Dermatol. 164, 1292–1298 (2011).
Hunger, R. E., Surovy, A. M., Hassan, A. S., Braathen, L. R. & Yawalkar, N. Toll-like receptor 2 is highly expressed in lesions of acne inversa and colocalizes with C-type lectin receptor. Br. J. Dermatol. 158, 691–697 (2008).
Witte-Handel, E. et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J. Invest. Dermatol. 139, 1294–1305 (2019). This paper elucidates the role of IL-1β in cutaneous purulence and tissue destruction by transcriptome analyses, stimulation studies with skin-derived cells and ex vivo cytokine neutralization of HS skin.
Kelly, G. et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 173, 1431–1439 (2015).
Manfredini, M. et al. The P2X7 receptor is overexpressed in the lesional skin of subjects affected by hidradenitis suppurativa: a preliminary study. Dermatology https://doi.org/10.1159/000502026 (2019).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug. Discov. 11, 633–652 (2012).
Sedger, L. M. & McDermott, M. F. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants — past, present and future. Cytokine Growth Factor. Rev. 25, 453–472 (2014).
Wolk, K. et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J. Mol. Med. 87, 523–536 (2009).
Kimball, A. B. et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N. Engl. J. Med. 375, 422–434 (2016). This paper describes the first large RCT in HS.
van der Zee, H. H. et al. Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br. J. Dermatol. 166, 98–106 (2012). This paper is the first study presenting immunostaining data demonstrating that keratin fibres act as foreign body material in the skin of patients with HS.
List, E. K., Pascual, J. C., Zarchi, K., Nurnberg, B. M. & Jemec, G. B. E. Mast cells in hidradenitis suppurativa: a clinicopathological study. Arch. Dermatol. Res. 311, 331–335 (2019).
Platzer, C. et al. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 29, 3098–3104 (1999).
Platzer, C., Meisel, C., Vogt, K., Platzer, M. & Volk, H. D. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int. Immunol. 7, 517–523 (1995).
Sabat, R. et al. Biology of interleukin-10. Cytokine Growth Factor. Rev. 21, 331–344 (2010).
Fiorentino, D. F. et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146, 3444–3451 (1991).
Wolk, K., Docke, W., von Baehr, V., Volk, H. & Sabat, R. Comparison of monocyte functions after LPS- or IL-10-induced reorientation: importance in clinical immunoparalysis. Pathobiology 67, 253–256 (1999).
Hotz, C. et al. Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J. Invest. Dermatol. 136, 1768–1780 (2016).
Moran, B. et al. Hidradenitis suppurativa is characterized by dysregulation of the Th17:Treg cell axis, which is corrected by anti-TNF therapy. J. Invest. Dermatol. 137, 2389–2395 (2017). This paper uses multi-colour flow cytometry to investigate the cytokine production capacity of T cells derived from lesional and perilesional skin compared with unaffected HS skin. The results show the enrichment of CD4 + T cells that simultaneously secret multiple cytokines, especially in perilesional skin.
Sabat, R., Wolk, K., Loyal, L., Docke, W. D. & Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 41, 359–377 (2019).
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Van Raemdonck, K., Van den Steen, P. E., Liekens, S., Van Damme, J. & Struyf, S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor. Rev. 26, 311–327 (2015).
Vossen, A. et al. Novel cytokine and chemokine markers of hidradenitis suppurativa reflect chronic inflammation and itch. Allergy 74, 631–634 (2019).
Kao, C. Y. et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 173, 3482–3491 (2004).
Albanesi, C., Cavani, A. & Girolomoni, G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J. Immunol. 162, 494–502 (1999).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Wolk, K. et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur. J. Immunol. 39, 3570–3581 (2009).
Witte, E. et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J. Invest. Dermatol. 134, 2757–2767 (2014).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Shen, F., Ruddy, M. J., Plamondon, P. & Gaffen, S. L. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J. Leukoc. Biol. 77, 388–399 (2005).
Weiss, B. et al. Cloning of murine IL-22 receptor alpha 2 and comparison with its human counterpart. Genes Immun. 5, 330–336 (2004).
Sabat, R., Ouyang, W. & Wolk, K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat. Rev. Drug. Discov. 13, 21–38 (2014).
Mozeika, E., Pilmane, M., Nurnberg, B. M. & Jemec, G. B. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm. Venereol. 93, 301–304 (2013).
Radaeva, S., Sun, R., Pan, H. N., Hong, F. & Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39, 1332–1342 (2004).
Gimblet, C. et al. IL-22 protects against tissue damage during cutaneous leishmaniasis. PLoS One 10, e0134698 (2015).
Chestovich, P. J. et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation 93, 485–492 (2012).
Sabat, R. & Wolk, K. Deciphering the role of interleukin-22 in metabolic alterations. Cell Biosci. 5, 68 (2015).
Wang, X. et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514, 237–241 (2014).
Danby, F. W., Jemec, G. B., Marsch, W. & von Laffert, M. Preliminary findings suggest hidradenitis suppurativa may be due to defective follicular support. Br. J. Dermatol. 168, 1034–1039 (2013).
Greb, J. E. et al. Psoriasis. Nat. Rev. Dis. Prim. 2, 16082 (2016).
Thomi, R. et al. Interleukin-32 is highly expressed in lesions of hidradenitis suppurativa. Br. J. Dermatol. 177, 1358–1366 (2017).
Thomi, R., Kakeda, M., Yawalkar, N., Schlapbach, C. & Hunger, R. E. Increased expression of the interleukin-36 cytokines in lesions of hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 31, 2091–2096 (2017).
Di Caprio, R. et al. IL-36 cytokines are increased in acne and hidradenitis suppurativa. Arch. Dermatol. Res. 309, 673–678 (2017).
Hessam, S. et al. Interleukin-36 in hidradenitis suppurativa: evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br. J. Dermatol. 178, 761–767 (2018).
Foster, A. M. et al. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 192, 6053–6061 (2014).
Winkle, S. M., Throop, A. L. & Herbst-Kralovetz, M. M. IL-36gamma augments host defense and immune responses in human female reproductive tract epithelial cells. Front. Microbiol. 7, 955 (2016).
Wolk, K. et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br. J. Dermatol. 177, 1385–1393 (2017).
Abella, V. et al. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 20, 565–571 (2015).
Chakraborty, S., Kaur, S., Guha, S. & Batra, S. K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 1826, 129–169 (2012).
Thomi, R. et al. Elevated levels of the antimicrobial peptide LL-37 in hidradenitis suppurativa are associated with a Th1/Th17 immune response. Exp. Dermatol. 27, 172–177 (2018).
von Laffert, M., Stadie, V., Wohlrab, J. & Marsch, W. C. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br. J. Dermatol. 164, 367–371 (2011).
Jimenez-Gallo, D. et al. The clinical significance of increased serum proinflammatory cytokines, C-reactive protein, and erythrocyte sedimentation rate in patients with hidradenitis suppurativa. Mediators Inflamm. 2017, 2450401 (2017).
Wolk, K. & Sabat, R. Adipokines in psoriasis: an important link between skin inflammation and metabolic alterations. Rev. Endocr. Metab. Disord. 17, 305–317 (2016).
Malara, A. et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br. J. Dermatol. 178, 792–793 (2018).
Girouard, S. D., Falk, R. H., Rennke, H. G. & Merola, J. F. Hidradenitis suppurativa resulting in systemic amyloid A amyloidosis: a case report and review of the literature. Dermatol. Online J. 18, 2 (2012).
Schandorff, K. D., Miller, I. M., Krustrup, D., Jemec, G. B. & Marckmann, P. Renal amyloid A amyloidosis as a complication of hidradenitis suppurativa. Clin. Nephrol. 86, 51–54 (2016).
Damen, M., Popa, C. D., Netea, M. G., Dinarello, C. A. & Joosten, L. A. B. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis 264, 83–91 (2017).
Tsaousi, A. et al. MMP8 is increased in lesions and blood of acne inversa patients: a potential link to skin destruction and metabolic alterations. Mediators Inflamm. 2016, 4097574 (2016).
Matusiak, L., Bieniek, A. & Szepietowski, J. C. Increased serum tumour necrosis factor-alpha in hidradenitis suppurativa patients: is there a basis for treatment with anti-tumour necrosis factor-alpha agents? Acta Derm. Venereol. 89, 601–603 (2009).
Matusiak, L., Szczech, J., Bieniek, A., Nowicka-Suszko, D. & Szepietowski, J. C. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J. Am. Acad. Dermatol. 76, 670–675 (2017).
Armaka, M. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 205, 331–337 (2008).
Uluckan, O. et al. Chronic skin inflammation leads to bone loss by IL-17-mediated inhibition of Wnt signaling in osteoblasts. Sci. Transl. Med. 8, 330ra337 (2016).
Freysz, M., Jemec, G. B. & Lipsker, D. A systematic review of terms used to describe hidradenitis suppurativa. Br. J. Dermatol. 173, 1298–1300 (2015).
Revuz, J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 23, 985–998 (2009).
Schrader, A. M., Deckers, I. E., van der Zee, H. H., Boer, J. & Prens, E. P. Hidradenitis suppurativa: a retrospective study of 846 Dutch patients to identify factors associated with disease severity. J. Am. Acad. Dermatol. 71, 460–467 (2014). This paper reports on risk factors in a large representative cohort of patients with HS.
Saunte, D. M. et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br. J. Dermatol. 173, 1546–1549 (2015).
Wortsman, X., Castro, A. & Figueroa, A. Color Doppler ultrasound assessment of morphology and types of fistulous tracts in hidradenitis suppurativa (HS). J. Am. Acad. Dermatol. 75, 760–767 (2016).
Wortsman, X. et al. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol. Surg. 39, 1835–1842 (2013).
Virgilio, E., Bocchetti, T. & Balducci, G. Utility of MRI in the diagnosis and post-treatment evaluation of anogenital hidradenitis suppurativa. Dermatol. Surg. 41, 865–866 (2015).
Hurley, H. in Dermatologic Surgery: Principles and Practice (eds Roenigk, R. K., Roenigk, H. H. Jr). 729–739 (CRC Press, 1989).
Sartorius, K., Emtestam, L., Jemec, G. B. & Lapins, J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br. J. Dermatol. 161, 831–839 (2009).
Kimball, A. B. et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann. Intern. Med. 157, 846–855 (2012).
Hessam, S. et al. A novel severity assessment scoring system for hidradenitis suppurativa. JAMA Dermatol. 154, 330–335 (2018).
Kokolakis, G. & Sabat, R. Distinguishing mild, moderate, and severe hidradenitis suppurativa. JAMA Dermatol. 154, 971–972 (2018).
Horvath, B. et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group. Acta Derm. Venereol. 97, 412–413 (2017).
Chernyshov, P. V. The evolution of quality of life assessment and use in dermatology. Dermatology 235, 167–174 (2019).
Ferreira-Valente, M. A., Pais-Ribeiro, J. L. & Jensen, M. P. Validity of four pain intensity rating scales. Pain 152, 2399–2404 (2011).
Kimball, A. B. et al. Psychometric properties of the itch numeric rating scale in patients with moderate-to-severe plaque psoriasis. Br. J. Dermatol. 175, 157–162 (2016).
Pedersen, C. B. et al. Reliability and validity of the psoriasis itch visual analog scale in psoriasis vulgaris. J. Dermatol. Treat. 28, 213–220 (2017).
Spinhoven, P. et al. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol. Med. 27, 363–370 (1997).
Yao, Y., Jorgensen, A. R. & Thomsen, S. F. Work productivity and activity impairment in patients with hidradenitis suppurativa: a cross-sectional study. Int. J. Dermatol. 59, 333–340 (2019).
Wieland, C. W. et al. Myeloid marker S100A8/A9 and lymphocyte marker, soluble interleukin 2 receptor: biomarkers of hidradenitis suppurativa disease activity? Br. J. Dermatol. 168, 1252–1258 (2013).
Matusiak, L., Salomon, J., Nowicka-Suszko, D., Bieniek, A. & Szepietowski, J. C. Chitinase-3-like protein 1 (YKL-40): novel biomarker of hidradenitis suppurativa disease activity? Acta Derm. Venereol. 95, 736–737 (2015).
Grant, A., Gonzalez, T., Montgomery, M. O., Cardenas, V. & Kerdel, F. A. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J. Am. Acad. Dermatol. 62, 205–217 (2010).
Gold, D. A., Reeder, V. J., Mahan, M. G. & Hamzavi, I. H. The prevalence of metabolic syndrome in patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 70, 699–703 (2014).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2018).
van der Zee, H. H., de Winter, K., van der Woude, C. J. & Prens, E. P. The prevalence of hidradenitis suppurativa in 1093 patients with inflammatory bowel disease. Br. J. Dermatol. 171, 673–675 (2014).
Deckers, I. E., van der Zee, H. H. & Prens, E. P. Severe fatigue based on anaemia in patients with hidradenitis suppurativa: report of two cases and a review of the literature. J. Eur. Acad. Dermatol. Venereol. 30, 174–175 (2016).
Fraenkel, P. G. Anemia of inflammation: a review. Med. Clin. North. Am. 101, 285–296 (2017).
Cugno, M., Borghi, A. & Marzano, A. V. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am. J. Clin. Dermatol. 18, 555–562 (2017).
Vanlaerhoven, A. et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology 234, 232–233 (2018).
Kromann, C. B., Ibler, K. S., Kristiansen, V. B. & Jemec, G. B. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm. Venereol. 94, 553–557 (2014).
Micieli, R. & Alavi, A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int. J. Dermatol. 57, 1471–1480 (2018).
Russ, E. & Castillo, M. Lumbosacral epidural abscess due to hidradenitis suppurativa. AJR Am. J. Roentgenol. 178, 770–771 (2002).
Chapman, S., Delgadillo, D. III, Barber, C. & Khachemoune, A. Cutaneous squamous cell carcinoma complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol. Alp. Pannonica Adriat. 27, 25–28 (2018).
Tannenbaum, R., Strunk, A. & Garg, A. Association between hidradenitis suppurativa and lymphoma. JAMA Dermatol. 155, 624–625 (2019).
Wortsman, X. Imaging of hidradenitis suppurativa. Dermatol. Clin. 34, 59–68 (2016).
Sivanand, A., Gulliver, W. P., Josan, C. K., Alhusayen, R. & Fleming, P. J. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J. Cutan. Med. Surg. 24, 64–72 (2019).
Loh, T. Y., Hendricks, A. J., Hsiao, J. L. & Shi, V. Y. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology https://doi.org/10.1159/000501611 (2019).
Hunger, R. E. et al. Swiss practice recommendations for the management of hidradenitis suppurativa/acne inversa. Dermatology 233, 113–119 (2017).
Megna, M. et al. Hidradenitis suppurativa: guidelines of the Italian society of dermatology and venereology (SIDeMaST) for the use of anti-TNF-α agents. G. Ital. Dermatol. Venereol. 150, 731–739 (2015).
Zouboulis, C. C. et al. S1 guideline for the treatment of hidradenitis suppurativa/acne inversa * (number ICD-10 L73.2) [German]. J. Dtsch. Dermatol. Ges. 10, S1–S31 (2012).
Zouboulis, C. C. et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J. Eur. Acad. Dermatol. Venereol. 29, 619–644 (2015).
Sabat, R. et al. Acne inversa/hidradenitis suppurativa: an update. Hautarzt 68, 999–1006 (2017).
Dauden, E. et al. Recommendations for the management of comorbidity in hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 32, 129–144 (2018).
Nielsen, R. M., Lindso Andersen, P., Sigsgaard, V., Theut Riis, P. & Jemec, G. B. Pain perception in patients with hidradenitis suppurativa. Br. J. Dermatol. 182, 166–174 (2019).
WHO. Scoping document for WHO guidelines for the pharmacological treatment of persisting pain in adults with medical illnesses. https://www.who.int/medicines/areas/quality_safety/Scoping_WHO_GLs_PersistPainAdults_webversion.pdf?ua=1 (2012).
Clemmensen, O. J. Topical treatment of hidradenitis suppurativa with clindamycin. Int. J. Dermatol. 22, 325–328 (1983).
Fischer, A. H., Haskin, A. & Okoye, G. A. Patterns of antimicrobial resistance in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 76, 309–313 (2017).
Jemec, G. B. & Wendelboe, P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 39, 971–974 (1998).
Matusiak, L., Bieniek, A. & Szepietowski, J. C. Bacteriology of hidradenitis suppurativa — which antibiotics are the treatment of choice? Acta Derm. Venereol. 94, 699–702 (2014).
Scheinfeld, N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol. Online J. 19, 1 (2013).
Mendonca, C. O. & Griffiths, C. E. Clindamycin and rifampicin combination therapy for hidradenitis suppurativa. Br. J. Dermatol. 154, 977–978 (2006).
Bettoli, V., Join-Lambert, O. & Nassif, A. Antibiotic treatment of hidradenitis suppurativa. Dermatol. Clin. 34, 81–89 (2016).
Join-Lambert, O. et al. Dramatic reduction of clindamycin plasma concentration in hidradenitis suppurativa patients treated with the rifampin-clindamycin combination. Eur. J. Dermatol. 24, 94–95 (2014).
Albrecht, J., Baine, P. A., Ladizinski, B., Jemec, G. B. & Bigby, M. Long-term clinical safety of clindamycin and rifampicin combination for the treatment of hidradenitis suppurativa. A critically appraised topic. Br. J. Dermatol. 180, 749–755 (2019).
Join-Lambert, O. et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J. Antimicrob. Chemother. 71, 513–520 (2016).
Join-Lambert, O. et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology 222, 49–58 (2011).
Yazdanyar, S., Boer, J., Ingvarsson, G., Szepietowski, J. C. & Jemec, G. B. Dapsone therapy for hidradenitis suppurativa: a series of 24 patients. Dermatology 222, 342–346 (2011).
Riis, P. T. et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J. Am. Acad. Dermatol. 75, 1151–1155 (2016).
Kimball, A. B. et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J. Eur. Acad. Dermatol. Venereol. 30, 989–994 (2016).
Kimball, A. B., Ganguli, A. & Fleischer, A. Reliability of the hidradenitis suppurativa clinical response in the assessment of patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 32, 2254–2256 (2018).
Zouboulis, C. C. et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J. Am. Acad. Dermatol. 80, 60–69 e62 (2019).
Tzanetakou, V. et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 152, 52–59 (2016).
Maarouf, M., Clark, A. K., Lee, D. E. & Shi, V. Y. Targeted treatments for hidradenitis suppurativa: a review of the current literature and ongoing clinical trials. J. Dermatol. Treat. 29, 441–449 (2018).
Kanni, T. et al. MABp1 targeting IL-1α for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randomized study. J. Invest. Dermatol. 138, 795–801 (2018).
Weber, P., Seyed Jafari, S. M., Yawalkar, N. & Hunger, R. E. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: a case series of 9 patients. J. Am. Acad. Dermatol. 76, 1189–1191 (2017).
Kerdel, F. R. et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J. Drugs Dermatol. 18, 170–176 (2019).
Vossen, A., van Doorn, M. B. A., van der Zee, H. H. & Prens, E. P. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J. Am. Acad. Dermatol. 80, 80–88 (2019).
van der Zee, H. H., Prens, E. P. & Boer, J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J. Am. Acad. Dermatol. 63, 475–480 (2010). This paper introduces a practical surgical technique of great clinical use in the management of HS.
Lapins, J., Marcusson, J. A. & Emtestam, L. Surgical treatment of chronic hidradenitis suppurativa: CO2 laser stripping-secondary intention technique. Br. J. Dermatol. 131, 551–556 (1994). This paper gives detailed insight into a useful management technique for HS of intermediate severity.
Blok, J. L., Spoo, J. R., Leeman, F. W., Jonkman, M. F. & Horvath, B. Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III. J. Eur. Acad. Dermatol. Venereol. 29, 379–382 (2015).
Janse, I., Bieniek, A., Horvath, B. & Matusiak, L. Surgical procedures in hidradenitis suppurativa. Dermatol. Clin. 34, 97–109 (2016).
Morgan, W. P., Harding, K. G. & Hughes, L. E. A comparison of skin grafting and healing by granulation, following axillary excision for hidradenitis suppurativa. Ann. R. Coll. Surg. Engl. 65, 235–236 (1983).
Tierney, E., Mahmoud, B. H., Hexsel, C., Ozog, D. & Hamzavi, I. Randomized control trial for the treatment of hidradenitis suppurativa with a neodymium-doped yttrium aluminium garnet laser. Dermatol. Surg. 35, 1188–1198 (2009). This paper describes the utility of Nd:YAG treatment in HS.
Saunte, D. M. & Lapins, J. Lasers and intense pulsed light hidradenitis suppurativa. Dermatol. Clin. 34, 111–119 (2016).
Matusiak, L., Bieniek, A. & Szepietowski, J. C. Psychophysical aspects of hidradenitis suppurativa. Acta Derm. Venereol. 90, 264–268 (2010).
von der Werth, J. M. & Jemec, G. B. Morbidity in patients with hidradenitis suppurativa. Br. J. Dermatol. 144, 809–813 (2001). This paper describes a cross-sectional study that broadly demonstrates the health-related quality of life measures and elucidates key factors that make HS more burdensome, emphasizing the important role of health care providers in diminishing disease burden.
Onderdijk, A. J. et al. Depression in patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 27, 473–478 (2013).
Matusiak, L., Szczech, J., Kaaz, K., Lelonek, E. & Szepietowski, J. C. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm. Venereol. 98, 191–194 (2018).
Smith, H. S., Chao, J. D. & Teitelbaum, J. Painful hidradenitis suppurativa. Clin. J. Pain. 26, 435–444 (2010).
Wolkenstein, P. et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J. Am. Acad. Dermatol. 56, 621–623 (2007).
Kaaz, K., Szepietowski, J. C. & Matusiak, L. Influence of itch and pain on sleep quality in patients with hidradenitis suppurativa. Acta Derm. Venereol. 98, 757–761 (2018).
Vossen, A., Schoenmakers, A., van Straalen, K. R., Prens, E. P. & van der Zee, H. H. Assessing pruritus in hidradenitis suppurativa: a cross-sectional study. Am. J. Clin. Dermatol. 18, 687–695 (2017).
Kurek, A. et al. Profound disturbances of sexual health in patients with acne inversa. J. Am. Acad. Dermatol. 67, 422–428 (2012).
Sampogna, F. et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm. Venereol. 97, 478–482 (2017).
Jemec, G. B., Heidenheim, M. & Nielsen, N. H. Hidradenitis suppurativa — characteristics and consequences. Clin. Exp. Dermatol. 21, 419–423 (1996).
Machado, M. O. et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. https://doi.org/10.1001/jamadermatol.2019.0759 (2019).
Thorlacius, L., Cohen, A. D., Gislason, G. H., Jemec, G. B. E. & Egeberg, A. Increased suicide risk in patients with hidradenitis suppurativa. J. Invest. Dermatol. 138, 52–57 (2018). This paper reports on a registry-based study that clearly demonstrates the increased risk of suicide among patients with HS compared with the general population.
Schneider-Burrus, S. et al. Association of hidradenitis suppurativa with body image. JAMA Dermatol. 154, 447–451 (2018).
Esmann, S. & Jemec, G. B. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm. Venereol. 91, 328–332 (2011).
Scala, E. et al. A new Th-17 cytokine in hidradenitis suppurativa: antimicrobial and pro-inflammatory role of IL-26. Br. J. Dermatol. https://doi.org/10.1111/bjd.17854 (2019).
Lima, A. L. et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 174, 514–521 (2016).
Witte, K. et al. Increased presence and differential molecular imprinting of transit amplifying cells in psoriasis. J. Mol. Med. 98, 111–122 (2020).
Ring, H. C. et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 153, 897–905 (2017).
Clark, A. K., Quinonez, R. L., Saric, S. & Sivamani, R. K. Hormonal therapies for hidradenitis suppurativa: review. Dermatol. Online J. 23, 13030/qt6383k0n4 (2017).
Golbari, N. M., Porter, M. L. & Kimball, A. B. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 80, 114–119 (2019).
Loget, J. et al. Misdiagnosis of hidradenitis suppurativa continues to be a major issue. The R-ENS Verneuil study. Ann. Dermatol. Venereol. 145, 331–338 (2018).
Canoui-Poitrine, F. et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J. Invest. Dermatol. 133, 1506–1511 (2013).
Kimball, A. B., Sundaram, M., Banderas, B., Foley, C. & Shields, A. L. Development and initial psychometric evaluation of patient-reported outcome questionnaires to evaluate the symptoms and impact of hidradenitis suppurativa. J. Dermatol. Treat. 29, 152–164 (2018).
Thorlacius, L. et al. Towards global consensus on core outcomes for hidradenitis suppurativa research: an update from the HISTORIC consensus meetings I and II. Br. J. Dermatol. 178, 715–721 (2018).
Porter, M. L., Golbari, N. M., Lockwood, S. J. & Kimball, A. B. Overview and update on biologic therapy for moderate-to-severe hidradenitis suppurativa. Semin. Cutan. Med. Surg. 37, 182–189 (2018).
Acknowledgements
The authors thank A. Vossen (Erasmus University Medical Center), G. Kokolakis (Charité – Universitätsmedizin Berlin), I. Chlebicka (Wrocław Medical University), K. van Straalen (Erasmus University Medical Center) and J. Triebus (Charité – Universitätsmedizin Berlin) for their help with this manuscript. The authors also acknowledge the support by the German Federal Ministry of Education and Research (http://www.bmbf.de/; grant 01ZX1312A to K.W. and R.S.).
Author information
Authors and Affiliations
Contributions
Introduction (R.S.); Epidemiology (G.B.E.J.); Mechanisms/pathophysiology (K.W.); Diagnosis, screening and prevention (E.P.); Management (G.B.E.J.); Quality of life (Ł.M.); Outlook (A.B.K. and R.S.); Overview of Primer (R.S.). R.S. and G.B.E.J. contributed equally and are co-first authors. E.P. and K.W. contributed equally and are co-last authors.
Corresponding author
Ethics declarations
Competing interests
R.S. is a member of Arbeitsgemeinschaft Dermatologische Forschung (ADF; Consortium for Dermatological Research), the German Society of Allergy and Clinical Immunology (DGAKI), the German Society of Immunology (DGfI), the American Association of Immunologists (AAI), the International Psoriasis Council (IPC) and the European Hidradenitis Suppurativa Foundation (EHSF), and is the spokesman of the ADF Psoriasis Group. R.S. has received research grants and scientific awards or honoraria for participation on advisory boards, clinical trials or as speaker for one or more of the following: AbbVie Inc., AbbVie Deutschland GmbH & Co. KG, Bayer Schering Pharma AG, Biogen IDEC GmbH, Boehringer Ingelheim Pharma GmbH & Co. KG, Celgene GmbH, Celgene International II Sàrl, Charité Research Organization GmbH, Dr. Willmar Schwabe GmbH & Co. KG, Flexopharm GmbH & Co. KG, Generon Corporation Ltd., Janssen-Cilag GmbH, La Roche-Posay Laboratoire Dermatologique Deutschland, Novartis Pharma GmbH, Parexel International GmbH, Pfizer Deutschland GmbH, Sanofi-Aventis Deutschland GmbH, TFS Trial Form Support GmbH, UCB Pharma GmbH. G.B.E.J. is the vice president and a founding member of the EHSF, a board member of the Nordic Association of Dermatology and an honorary member of the British Association of Dermatologists. G.B.E.J. is also a member of the European Academy of Dermatology and Venereology, the American Academy of Dermatology, the Serbian Association of Dermatovenereologists, the Hungarian Society of Dermatology, the Baltic Association of Dermatovenerologists, the Finnish Society of Dermatology and the Latvian Society of Dermatology and Venereology. G.B.E.J. is the Editor-in-chief of Dermatology, commissioning editor of Clinical Problems in Dermatology, associate editor of Clinical Dermatology and Acta Dermatovenerologica Adriatica, Pannonica et Alpina, and an editorial board member of Acta Dermatovenerologica Croatica and Frontiers in Medicine. G.B.E.J. has received research grants and grants for participation as an investigator from Abbvie, Astra-Zeneca, Inflarx, Janssen-Cilag, Leo Pharma, Novartis, Regeneron and Sanofi. He has also received unrestricted departmental grants from Abbvie, Leo Pharma, and Novartis. G.B.E.J. has received honoraria from AbbVie, Chemocentryx, Coloplast, Incyte, Inflarx, Novartis, Pierre Fabre and UCB for participation on advisory boards, and has received speaker honoraria from AbbVie, Boehringer-Ingelheim, Galderma, MSD and Novartis. Ł.M. is a founding member of the EHSF and a member of the European Academy of Dermatology and Venereology and Polish Dermatological Society. Ł.M. has received research grants, travel grants, consulting honoraria or lecturer’s honoraria from AbbVie, Amgen, Behringer Ingelheim, InfraRX, Janssen, Leo Pharma, Medac, Menlo Therapeutics, Pfizer, Pierre Fabre, Polpharma, Regeneron, Novartis, Trevi, UCB and Valeant. A.B.K. is a consultant and investigator for Novartis, Abbvie, UCB, Pfizer, Lilly and Janssen. A.B.K. has received fellowship funding from Janssen and Abbvie. K.W. has received research grants, travel grants, consulting honoraria or lecturer’s honoraria from AbbVie, Bayer Schering Pharma, Biogen IDEC, Celgene, Dr. Willmar Schwabe GmbH & Co. KG, Flexopharm, Generon Corporation, Janssen-Cilag, Johnson & Johnson, Novartis, Pfizer, Sanofi-Aventis, TFS Trial Form Support and UCB. E.P. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Nodules
-
Firm swellings of the skin, mainly arising from the dermis and subcutis.
- Abscesses
-
Red, tender, pus-containing cavities in the skin or any organ, surrounded by inflammation.
- Sinus tracts
-
Linear, palpable, subcutaneous tunnels draining to the skin surface via an opening.
- Fistulas
-
Permanent, abnormal tunnels between two hollow organs or from a hollow organ to the skin surface.
- Plugging
-
The occlusion of a hair follicle or sweat gland pore by a keratin mass.
- Open comedones
-
Wide skin pores, plugged with a mass of black keratin debris.
- Double-ended pseudocomedones
-
Two interconnected dilated pores with a plug of black keratin debris at each end.
- Pilonidal sinus
-
A recurrent nodule or abscess draining via a sinus in the cleft between the buttocks near the tailbone.
- Furuncle
-
An abscess or nodule arising from a hair follicle, caused by infection with Staphylococcus aureus.
- Pyoderma gangrenosum
-
Rare skin disease characterized by growing painful ulcers with undermined borders.
- Acne conglobata
-
A rare and severe form of acne presenting with interconnected nodules, abscesses and sometimes sinus tracts, mostly in the neck, back and chest.
- Dissecting cellulitis of the scalp
-
A rare condition of the scalp associated with hidradenitis suppurativa, with pus-filled or indurated lumps, scarring and permanent hair loss.
- Pyogenic arthritis
-
Arthritis caused by invasion of a joint by an infectious agent resulting in painful joint inflammation.
Rights and permissions
About this article
Cite this article
Sabat, R., Jemec, G.B.E., Matusiak, Ł. et al. Hidradenitis suppurativa. Nat Rev Dis Primers 6, 18 (2020). https://doi.org/10.1038/s41572-020-0149-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0149-1
This article is cited by
-
Neutrophil extracellular traps and neutrophilic dermatosis: an update review
Cell Death Discovery (2024)
-
Hidradenitis suppurativa
Die Dermatologie (2024)
-
An Atlas of the Hidradenitis Suppurativa Transcriptome
Dermatology and Therapy (2024)
-
Burden of Hidradenitis Suppurativa: A Systematic Literature Review of Patient Reported Outcomes
Dermatology and Therapy (2024)
-
Autoimmune, Autoinflammatory Disease and Cutaneous Malignancy Associations with Hidradenitis Suppurativa: A Cross-Sectional Study
American Journal of Clinical Dermatology (2024)