Abstract
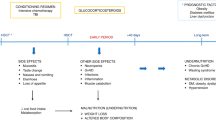
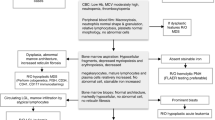
Hematopoietic stem cell transplantation (HSCT) is currently the standard of care for many malignant and nonmalignant blood diseases. As several treatment-emerging acute toxicities are expected, optimal supportive measurements critically affect HSCT outcomes. The paucity of good clinical studies in supportive practices gives rise to the establishment of heterogeneous guidelines across the different centers, which hampers direct clinical comparison in multicentric studies. Aiming to harmonize the supportive care provided during the pediatric HSCT in Europe, the Pediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT) promoted dedicated workshops during the years 2017 and 2018. The present paper describes the resulting consensus on the management of sinusoidal obstructive syndrome, mucositis, enteral and parenteral nutrition, iron overload, and emesis during HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
20 February 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41409-020-0838-0
References
Bras G, Hill KR. Veno-occlusive disease of the liver; essential pathology. Lancet. 1956;271:161–3.
Corbacioglu S, Carreras E, Ansari M, Balduzzi A, Cesaro S, Dalle JH, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53:138–45.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transplant. 2019;25:1271–80.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906–12.
Faraci M, Bertaina A, Luksch R, Calore E, Lanino E, Saglio F, et al. Sinusoidal obstruction syndrome/veno-occlusive disease after autologous or allogeneic hematopoietic stem cell transplantation in children: a retrospective study of the Italian Hematology-Oncology Association-Hematopoietic Stem Cell Transplantation Group. Biol Blood Marrow Transplant. 2019;25:313–20.
Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, et al. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975–81.
Gharib MI, Bulley SR, Doyle JJ, Wynn RF. Venous occlusive disease in children. Thromb Res. 2006;118:27–38.
Ohashi K, Tanabe J, Watanabe R, Tanaka T, Sakamaki H, Maruta A, et al. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64:32–8.
Park SH, Lee MH, Lee H, Kim HS, Kim K, Kim WS, et al. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:137–43.
Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–83.
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9.
Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163:444–57.
Al Beihany A, Al Omar H, Sahovic E, Chaudhri N, Al Mohareb F, Al Sharif F, et al. Successful treatment of hepatic veno-occlusive disease after myeloablative allogeneic hematopoietic stem cell transplantation by early administration of a short course of methylprednisolone. Bone Marrow Transplant. 2008;41:287–91.
Myers KC, Lawrence J, Marsh RA, Davies SM, Jodele S. High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:500–3.
Qutob AF, Gue S, Revesz T, Logan RM, Keefe D. Prevention of oral mucositis in children receiving cancer therapy: a systematic review and evidence-based analysis. Oral Oncol. 2013;49:102–7.
Wallhult E, Quinn B. Early and acute complications and the principles of HSCT nursing care. In: Kenyon M, Babic A, editors. The european blood and marrow transplantation textbook for nurses: under the auspices of EBMT. Cham: Springer International Publishing; 2018. p. 163–95.
Tomlinson D, Judd P, Hendershot E, Maloney AM, Sung L. Measurement of oral mucositis in children: a review of the literature. Support Care Cancer. 2007;15:1251–8.
Quinn B, Potting CM, Stone R, Blijlevens NM, Fliedner M, Margulies A, et al. Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer. 2008;44:61–72.
Ethier MC, Regier DA, Tomlinson D, Judd P, Doyle J, Gassas A, et al. Perspectives toward oral mucositis prevention from parents and health care professionals in pediatric cancer. Support Care Cancer. 2012;20:1771–7.
Harris DJ, Eilers J, Harriman A, Cashavelly BJ, Maxwell C. Putting evidence into practice: evidence-based interventions for the management of oral mucositis. Clin J Oncol Nurs. 2008;12:141–52.
The modified would be:American Academy of Pediatric Dentistry (AAPD)". Guideline on dental management of pediatric patients receiving chemotherapy, hematopoietic cell transplantation and/or radiation therapy. The reference manual of pediatric dentistry; 37:422–30
Chaveli-Lopez B, Bagan-Sebastian JV. Treatment of oral mucositis due to chemotherapy. J Clin Exp Dent. 2016;8:e201–9.
Wang L, Gu Z, Zhai R, Zhao S, Luo L, Li D, et al. Efficacy of oral cryotherapy on oral mucositis prevention in patients with hematological malignancies undergoing hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. PLoS ONE. 2015;10:e0128763.
Treister N, Nieder M, Baggott C, Olson E, Chen L, Dang H, et al. Caphosol for prevention of oral mucositis in pediatric myeloablative haematopoietic cell transplantation. Br J Cancer. 2017;116:21–7.
Gobbo M, Verzegnassi F, Ronfani L, Zanon D, Melchionda F, Bagattoni S, et al. Multicenter randomized, double-blind controlled trial to evaluate the efficacy of laser therapy for the treatment of severe oral mucositis induced by chemotherapy in children: laMPO RCT. Pediatr Blood Cancer. 2018;65:e27098.
Vitale MC, Modaffari C, Decembrino N, Zhou FX, Zecca M, Defabianis P. Preliminary study in a new protocol for the treatment of oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation (HSCT) and chemotherapy (CT). Lasers Med Sci. 2017;32:1423–8.
Langdana A, Tully N, Molloy E, Bourke B, O’Meara A. Intensive enteral nutrition support in paediatric bone marrow transplantation. Bone Marrow Transplant. 2001;27:741–6.
Wilken M. The impact of child tube feeding on maternal emotional state and identity: a qualitative meta-analysis. J Pediatr Nurs. 2012;27:248–55.
Azarnoush S, Bruno B, Beghin L, Guimber D, Nelken B, Yakoub-Agha I, et al. Enteral nutrition: a first option for nutritional support of children following allo-SCT? Bone Marrow Transplant. 2012;47:1191–5.
Lemal R, Cabrespine A, Pereira B, Combal C, Ravinet A, Hermet E, et al. Could enteral nutrition improve the outcome of patients with haematological malignancies undergoing allogeneic haematopoietic stem cell transplantation? A study protocol for a randomized controlled trial (the NEPHA study). Trials. 2015;16:136.
Gonzales F, Bruno B, Alarcon Fuentes M, De Berranger E, Guimber D, Behal H, et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin Nutr. 2018;37:2113–21.
Rzepecki P, Barzal J, Oborska S. Blood and marrow transplantation and nutritional support. Support Care Cancer. 2010;18:S57–65.
Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012;94:287–94.
Heubi JE. Whenever possible, use the gut! J Pediatr Hematol Oncol. 1999;21:88–90.
Angelucci E, Pilo F. Management of iron overload before, during, and after hematopoietic stem cell transplantation for thalassemia major. Ann N Y Acad Sci. 2016;1368:115–21.
Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008;41:997–1003.
Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood 2002;100:17–21.
Jastaniah W, Harmatz P, Pakbaz Z, Fischer R, Vichinsky E, Walters MC. Transfusional iron burden and liver toxicity after bone marrow transplantation for acute myelogenous leukemia and hemoglobinopathies. Pediatr Blood Cancer. 2008;50:319–24.
Taher AT, Saliba AN. Iron overload in thalassemia: different organs at different rates. Hematol Am Soc Hematol Educ Program. 2017;2017:265–71.
Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–31.
Wood JC. Use of magnetic resonance imaging to monitor iron overload. Hematol Oncol Clin North Am. 2014;28:747–64.
Wood JC. Guidelines for quantifying iron overload. Hematol Am Soc Hematol Educ Program. 2014;2014:210–5.
Yesilipek MA, Karasu G, Kaya Z, Kuskonmaz BB, Uygun V, Dag I, et al. A phase II, multicenter, single-arm study to evaluate the safety and efficacy of deferasirox after hematopoietic stem cell transplantation in children with beta-thalassemia major. Biol Blood Marrow Transplant. 2018;24:613–8.
Mariotti E, Angelucci E, Agostini A, Baronciani D, Sgarbi E, Lucarelli G. Evaluation of cardiac status in iron-loaded thalassaemia patients following bone marrow transplantation: improvement in cardiac function during reduction in body iron burden. Br J Haematol. 1998;103:916–21.
Jarisch A, Salzmann-Manrique E, Cario H, Grosse R, Soerensen J, Fischer R, et al. Serum ferritin is not a reliable predictor to determine iron overload in thalassemia major patients post-hematopoietic stem cell transplantation. Eur J Haematol. 2018;101:791–7.
Fischer R, Harmatz PR. Non-invasive assessment of tissue iron overload. Hematology Am Soc Hematol Educ Program. 2009;1:215–21.
Navari RM. 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim Biophys Acta. 2015;1848:2738–46.
Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Canc Netw. 2017;15:883–93.
Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8:187–98.
Dupuis LL, Boodhan S, Holdsworth M, Robinson PD, Hain R, Portwine C, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2013;60:1073–82.
Dupuis LL, Robinson PD, Boodhan S, Holdsworth M, Portwine C, Gibson P, et al. Guideline for the prevention and treatment of anticipatory nausea and vomiting due to chemotherapy in pediatric cancer patients. Pediatr Blood Cancer. 2014;61:1506–12.
Dupuis LL, Roscoe JA, Olver I, Aapro M, Molassiotis A. 2016 updated MASCC/ESMO consensus recommendations: anticipatory nausea and vomiting in children and adults receiving chemotherapy. Support Care Cancer. 2017;25:317–21. 2017
Einhorn LH, Rapoport B, Navari RM, Herrstedt J, Brames MJ. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer. 2017;25:303–8. 2017
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3240–61.
Patel P, Robinson PD, Thackray J, Flank J, Holdsworth MT, Gibson P, et al. Guideline for the prevention of acute chemotherapy-induced nausea and vomiting in pediatric cancer patients: a focused update. Pediatr Blood Cancer. 2017;64:1–12.
Dupuis LL, Boodhan S, Sung L, Portwine C, Hain R, McCarthy P, et al. Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2011;57:191–8.
Morrow GR. Effect of the cognitive hierarchy in the systematic desensitization treatment of anticipatory nausea in cancer patients: a component comparison with relaxation only, counseling, and no treatment. Cogn Ther Res. 1986;10:421–46.
Flank J, Robinson PD, Holdsworth M, Phillips R, Portwine C, Gibson P, et al. Guideline for the treatment of breakthrough and the prevention of refractory chemotherapy-induced nausea and vomiting in children with cancer. Pediatr Blood Cancer. 2016;63:1144–51.
Patel P, Leeder JS, Piquette-Miller M, Dupuis LL. Aprepitant and fosaprepitant drug interactions: a systematic review. Br J Clin Pharmacol. 2017;83:2148–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nava, T., Ansari, M., Dalle, JH. et al. Supportive care during pediatric hematopoietic stem cell transplantation: beyond infectious diseases. A report from workshops on supportive care of the Pediatric Diseases Working Party (PDWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 55, 1126–1136 (2020). https://doi.org/10.1038/s41409-020-0818-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0818-4
This article is cited by
-
Early vascular toxicity after pediatric allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2022)
-
Health-related quality of life predicts length of hospital stay and survival rates for pediatric patients receiving allogeneic hematopoietic cell transplantation
Quality of Life Research (2021)