Abstract
Background
Salvage lymph node dissection (sLND) for nodal recurrence in prostate cancer (PCa) patients with biochemical recurrence (BCR) is still not recommended in current guidelines, because of the diagnostic inaccuracy of current conventional imaging. To assess the performance of [68Ga] Ga-prostate-specific membrane antigen conjugate 11 positron emission tomography (PSMA-PET) in detecting PCa lymph node metastasis using pathologic confirmation through sLND.
Methods
Literature search was conducted using the MEDLINE, SCOPUS, Web of Science, and Cochrane Library on November 11th, 2018 to identify the eligible studies. Studies were eligible if they investigated the diagnostic performance of PSMA-PET before sLND in PCa patients with BCR and reported the number of true positive, false positive, false negative, and true negative on a lesion-based and/or field-based analyses to compare with histopathologic findings in sLND specimens.
Results
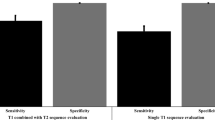
Fourteen studies published between 2015 and 2018 comprising 462 patients were selected in this systematic review and meta-analysis. The positive predictive value of PSMA-PET before sLND on a patient-based analysis ranged between 0.70 and 0.93. The pooled sensitivity using lesion-based and field-based analyses were 0.84 (95%CI: 0.61–0.95) and 0.82 (95%CI: 0.72–0.89), respectively. The pooled specificity using lesion-based and field-based analyses were 0.97 (95%CI: 0.95–0.99) and 0.95 (95%CI: 0.70–0.99), respectively. The diagnostic odds ratio using lesion-based and field-based analyses were 189 (95%CI: 39–920) and 82 (95%CI: 8–832), respectively.
Conclusions
PSMA-PET before sLND provided highly accurate performance with clinically relevant high positive and negative predictive values for detecting lymph node disease in patients with BCR after local treatment with curative intent for PCa. PSMA-PET can identify the patients who are likely to benefit from sLND and possibly direct to lesion or region-based dissection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869–75.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–29.
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. J Am Med Assoc. 2005;294:433–9.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Nini A, Gandaglia G, Fossati N, Suardi N, Cucchiara V, Dell’Oglio P, et al. Patterns of clinical recurrence of node-positive prostate cancer and impact on long-term survival. Eur Urol. 2015;68:777–84.
Heidenreich A, Moul JW, Shariat S, Karnes RJ. Role of salvage lymph node dissection in prostate cancer. Curr Opin Urol. 2016;26:581–9.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2019. pii: S0302–2838(19)30095–8. https://doi.org/10.1016/j.eururo.2019.01.049. [Epub ahead of print].
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37.
Pfister D, Porres D, Heidenreich A, Heidegger I, Knuechel R, Steib F, et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with 68Ga-PSMA-HBED-CC than with 18F-fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:1410–7.
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90.
Arends LR, Hamza TH, van Houwelingen JC, Heijenbrok-Kal MH, Hunink MG, Stijnen T. Bivariate random effects meta-analysis of ROC curves. Med Decis Mak. 2008;28:621–38.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate. 2015;75:1934–40.
Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–7.
Rauscher I, Maurer T, Beer A, Haller B, Gschwend J, Schwaiger M, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: retrospective comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–9.
Sahlmann CO, Meller B, Bouter C, Ritter CO, Strobel P, Lotz J, et al. Biphasic (6)(8)Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur J Nucl Med Mol Imaging. 2016;43:898–905.
Fossati N, Suardi N, Gandaglia G, Stabile A, Colicchia M, Karnes RJ, et al. MP77-01 11C-choline versus 68GA-PSMA PET/CT scan for the detection of nodal recurrence from prostate cancer: results from a large, multi-institutional salvage lymph node dissection series. J Urol. 2017;197:e1021.
Herlemann A, Kretschmer A, Buchner A, Karl A, Tritschler S, El-Malazi L, et al. Salvage lymph node dissection after68Ga-PSMA or18F-FEC PET/CT for nodal recurrence in prostate cancer patients. Oncotarget. 2017;8:84180–92.
Jilg CA, Drendel V, Rischke C, Beck T, Vach W, Schaal K, et al. Diagnostic accuracy of Ga-68-HBED-CC-PSMA-ligand-PET/CT before salvage lymph node dissection for recurrent prostate cancer. Theranostics. 2017;7:1770–80.
Siriwardana A, Thompson J, van Leeuwen PJ, Doig S, Kalsbeek A, Emmett L, et al. Initial multicentre experience of68gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int. 2017;120:673–81.
Dundee P, Gross T, Moran D, Ryan A, Ballok Z, Peters J, et al. Ga-labeled prostate-specific membrane antigen ligand-positron-emission tomography: still just the tip of the iceberg. Urology. 2018;120:187–91.
Linxweiler J, Saar M, Al-Kailani Z, Janssen M, Ezziddin S, Stockle M, et al. Robotic salvage lymph node dissection for nodal-only recurrences after radical prostatectomy: perioperative and early oncological outcomes. Surg Oncol. 2018;27:138–45.
Mandel P, Tilki D, Chun FK, Pristupa E, Graefen M, Klutmann S, et al. Accuracy of (68)Ga-prostate-specific membrane antigen positron emission tomography for the detection of lymph node metastases before salvage lymphadenectomy. Eur Urol Focus. 2018. pii: S2405–4569(18)30191–3. https://doi.org/10.1016/j.euf.2018.07.025. [Epub ahead of print].
Pfister D, Kohl T, Porres-Knoblauch D, Haidl F, Heidenreich A. 68-Ga-PSMA-PET in lymph node staging in PSA recurrent prostate cancer-does the primary influence the result? J Clin Oncol. 2018;36(6_suppl):210–210.
Savio L, Mota FHA, Santos R, da Cruz JA, Passerotti C. Salvage robot-assisted retroperitoneal lymphadenectomy for prostate cancer nodal recurrence only detected by 68GA-PSMA PET CT: technical aspects and results? J Urol. 2018;199:E821–E821.
Evangelista L, Zattoni F, Karnes RJ, Novara G, Lowe V. Radiolabeled choline PET/CT before salvage lymphadenectomy dissection: a systematic review and meta-analysis. Nucl Med Commun. 2016;37:1223–31.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Yaxley JW, Raveenthiran S, Nouhaud FX, Samartunga H, Yaxley AJ, Coughlin G, et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology: can preoperative 68Ga-PSMA positron emission tomography/computerized tomography replace pelvic lymph node dissection for prostate cancer staging? J Urol. 2019;201:815–20.
Chade DC, Eastham J, Graefen M, Hu JC, Karnes RJ, Klotz L, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: a systematic review of the literature. Eur Urol. 2012;61:961–71.
Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A, et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol. 2015;68:530–4.
Rauscher I, Düwel C, Wirtz M, Schottelius M, Wester HJ, Schwamborn K, et al. Value of111In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: correlation with histopathology and clinical follow-up. BJU Int. 2017;120:40–47.
Hofman MS, Murphy DG, Williams SG, Nzenza T, Herschtal A, Lourenco RA, et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int. 2018;122:783–93.
Author information
Authors and Affiliations
Contributions
Project development: SK, AB, SE, MH, and SFS. Data collection: SK, MA, FJ, TI, MK, BF, and NF. Data analysis: SK, MA, FJ, TI, MK, and BF. Manuscript writing/editing: SK, MA, FJ, TI, MK, BF, NF, AB, SE, MH, and SFS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, S., Abufaraj, M., Janisch, F. et al. Performance of [68Ga] Ga-PSMA 11 PET for detecting prostate cancer in the lymph nodes before salvage lymph node dissection: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 23, 1–10 (2020). https://doi.org/10.1038/s41391-019-0156-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-019-0156-z
This article is cited by
-
PSMA PET imaging in the diagnosis and management of prostate cancer
Abdominal Radiology (2023)
-
Falsch-positive – falsch-negative Befunde – Stellenwert der PSMA-PET/CT beim Staging von Patienten mit Prostatakarzinom
Journal für Urologie und Urogynäkologie/Österreich (2023)
-
The prognostic significance of the clinical T stage and Grade Group in patients with locally advanced prostate cancer treated via high-dose-rate brachytherapy and external beam radiation
International Journal of Clinical Oncology (2023)
-
How long is long enough to secure disease control after low-dose-rate brachytherapy in combination with other modalities in intermediate-risk, localized prostate cancer?
International Journal of Clinical Oncology (2022)
-
99mTc-PSMA targeted robot-assisted radioguided surgery during radical prostatectomy and extended lymph node dissection of prostate cancer patients
Annals of Nuclear Medicine (2022)